肝动脉灌注化疗及其综合治疗方案对中晚期肝细胞癌患者的临床疗效及预后因素分析
DOI: 10.3969/j.issn.1001-5256.2023.07.013
Efficacy of hepatic arterial infusion chemotherapy and its multimodality therapeutic regimens in treatment of patients with advanced hepatocellular carcinoma and related prognostic factors
-
摘要:
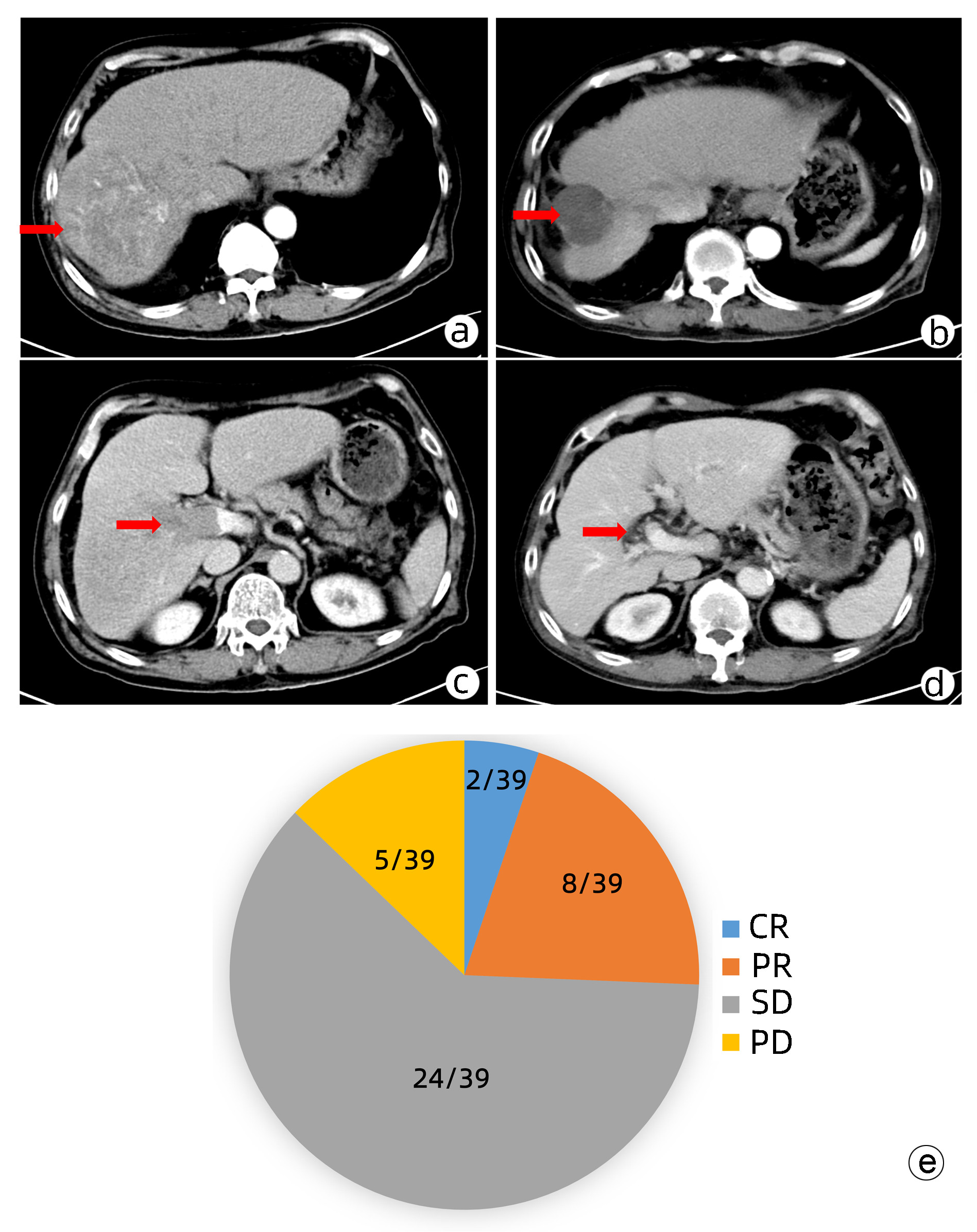
目的 本研究旨在观察FOLFOX方案持续肝动脉灌注化疗(HAIC)及其综合治疗方案对中晚期肝细胞癌患者的临床疗效并分析影响预后的因素。 方法 回顾性收集南方医科大学南方医院2018年9月—2021年11月行FOLFOX方案持续HAIC的66例中晚期肝细胞癌患者临床资料。观察治疗后患者的客观缓解率、疾病控制率、中位无疾病进展生存时间(mPFS)和中位生存时间(mOS)并记录治疗相关不良反应。针对伴有门静脉癌栓的患者,评价治疗对门静脉癌栓的疗效。采用Kaplan-Meier法进行生存分析。采用Cox回归分析影响预后的因素。 结果 按照RECIST1.1标准,FOLFOX-HAIC及其综合治疗方案治疗66例中晚期肝细胞癌患者的客观缓解率和疾病控制率分别为33.3%(22/66)、86.4%(57/66),mPFS和mOS分别为8.2个月和22.1个月。其中39例合并门静脉癌栓的肝癌患者中完全缓解2例,部分缓解8例,稳定24例,进展5例。客观缓解率为25.6%(10/39),疾病控制率为87.2%(34/39)。不良反应主要为消化道反应16.7%(11/66)、发热12.1%(8/66)、肝区疼痛10.6%(7/66)、骨髓抑制3.0%(2/66)和造影剂过敏3.0%(2/66)。无Ⅳ级以上的毒副反应。无并发症导致的死亡。Cox分析显示肝外转移(HR=2.668, 95% CI:1.357~5.245)和凝血酶原时间(HR=1.282, 95%CI:1.080~1.630)是影响患者PFS的独立危险因素(P值均<0.05), AST水平(HR=1.008, 95%CI:1.002~1.013)和凝血酶原时间(HR=1.303, 95%CI:1.046~1.630)是影响患者OS的独立危险因素(P值均<0.05)。 结论 FOLFOX-HAIC及其综合治疗方案治疗中晚期肝细胞癌有一定的疗效,不良反应可控。 Abstract:Objective To investigate the efficacy of continuous hepatic arterial infusion chemotherapy (HAIC) with the FOLFOX regimen and its multimodality therapeutic regimen in the treatment of patients with advanced hepatocellular carcinoma, as well as the influencing factors for prognosis. Methods A retrospective analysis was performed for the clinical data of 66 patients with advanced hepatocellular carcinoma who received continuous HAIC with FOLFOX regimen in Nanfang Hospital, Southern Medical University, from September 2018 to November 2021. The patients were observed in terms of objective response rate (ORR), disease control rate (DCR), median progression-free survival (mPFS), and median overall survival (mOS) after treatment, and treatment-related adverse reactions were recorded. For the patients with portal vein tumor thrombus, the effect of the treatment on portal vein tumor thrombus was assessed. The Kaplan-Meier method was used for survival analysis, and the Cox regression analysis was used to investigate the influencing factors for prognosis. Results According to the RECIST1.1 criteria, FOLFOX-HAIC and its multimodality therapeutic regimen achieved an ORR of 33.3% (22/66) and a DCR of 86.4% (57/66) in the treatment of 66 patients with advanced hepatocellular carcinoma, with an mPFS time of 8.2 months and an mOS time of 22.1 months. Among the 39 patients with portal vein tumor thrombus, 2 achieved complete remission, 8 achieved partial remission, 24 achieved stable disease, and 5 had disease progression, with an ORR of 25.6% (10/39) and a DCR of 87.2% (34/39). The main adverse reactions included gastrointestinal reactions (16.7%, 11/66), pyrexia (12.1%, 8/66), liver area pain (10.6%, 7/66), bone marrow suppression (3.0%, 2/66), and contrast agent allergy (3.0%, 2/66), and there were no grade > Ⅳ toxic or side effects or deaths caused by such complications. The Cox regression analysis showed that extrahepatic metastasis (hazard ratio [HR]=2.668, 95% confidence interval [CI]: 1.357-5.245, P < 0.05) and prothrombin time (PT) (HR=1.282, 95%CI: 1.080-1.630, P < 0.05) were independent risk factors for PFS, and aspartate aminotransferase level (HR=1.008, 95%CI: 1.002-1.013, P < 0.05) and PT (HR=1.303, 95%CI: 1.046-1.630, P < 0.05) were independent risk factors for OS. Conclusion FOLFOX-HAIC and its multimodality therapeutic regimen has a certain clinical effect with controllable adverse reactions in the treatment of advanced hepatocellular carcinoma. -
Key words:
- Carcinoma, Hepatocellular /
- Molecular Targeted Therapy /
- Prognosis
-
非酒精性脂肪性肝病(NAFLD)是一种常见的慢性肝病,包括非酒精性脂肪肝、非酒精性脂肪性肝炎(NASH)及其相关肝纤维化和肝硬化[1],部分患者甚至进展为肝癌,现在被称为代谢相关脂肪性肝病(metabolic associated fatty liver disease,MAFLD)[2-3],随着肥胖发病率逐年增高和低龄化趋势,NAFLD成为儿童慢性肝病的常见原因[4],肝硬化的三大主要原因之一。目前MAFLD的发病机制仍不清楚,临床上亦缺乏有效的药物治疗,因此MAFLD发病机制的研究是当前的热点。
黏膜相关恒定T(mucosal associated invariant T,MAIT)淋巴细胞是一类新的天然免疫T淋巴细胞,是最丰富的TCRαβ+T淋巴细胞,以1类主要组织相容性复合体相关分子和不依赖MR1的方式快速激活MAIT淋巴细胞[5],发挥生物学功能,释放多种细胞因子,如IFNγ、TNFα、IL-17和溶细胞产物穿孔素及颗粒素分泌并脱颗粒(将CD107a暴露于细胞表面)等杀伤性细胞因子[6],迅速诱导细胞溶解和靶细胞的死亡。国外相关报道[7-9]显示MAIT淋巴细胞在成人MAFLD发病中起到减少肝脏炎症及促肝纤维化作用,但在儿童MAFLD发病中作用的研究甚少。本研究通过分析MAFLD儿童外周血MAIT淋巴细胞的变化及其与临床指标的相关性,探讨MAIT淋巴细胞在儿童MAFLD发生发展中的作用。
1. 资料与方法
1.1 研究对象
收集2022年3月—2022年5月在本院肝病中心诊治的18例MAFLD患儿(MAFLD组)的外周血标本,根据年龄匹配的20例正常儿童作为对照(对照组),其外周血标本采集于本院健康管理中心。
1.2 纳入标准
MAFLD组纳入标准:脂肪肝合并超重/肥胖、2型糖尿病和代谢功能障碍中至少一项特征[1-2]。同时排除合并甲、乙、丙、丁、戊型肝炎病毒感染,自身免疫性肝病,人类免疫缺陷病毒等病毒感染。对照组纳入标准:肝功能正常,无慢性疾病,近期无感染史等。
1.3 方法
人外周血免疫细胞的制备:留取MAFLD组和对照组儿童外周血3 mL,将PBS加入3 mL新鲜全血中,吸取稀释后的外周血缓慢加到lymphoprep表面。全血在400×g室温下离心20 min,收集第二层外周血免疫细胞并转入含有PBS的离心管中,离心机800×g室温下离心10 min。离心结束后去上清,再加入10 mL PBS,离心机400×g室温下离心5 min,清洗外周血免疫细胞以彻底去除残留的lymphoprep。
应用流式细胞仪检测MAIT细胞,根据标准方案使用以下抗体:CD183(CXCR3)、CD279(PD-1), CD186(CXCR6)、CD3、TCRVα7.2、CD8、CD69、CD161、CD107a、CD196 (CCR6)、CD4和穿孔素抗体进行染色,上流式细胞仪(三激光八色流式细胞分析仪,型号:FacsCantoll,产地:美国)进行分析。外周血中MAIT细胞定义为CD3+CD161+TCRVα7.2+细胞。
收集MAFLD组和对照组儿童血常规及肝功能结果,使用瞬时弹性成像检测MAFLD组患儿的肝纤维化和脂肪含量,儿童MAFLD肝纤维化最佳临界值为6.65 kPa[10],区分有无脂肪变性的脂肪肝参数最佳临界值为222.5 dB/m[11]。
1.4 统计学方法
采用SPSS 23.0软件进行统计学分析。符合正态分布的计量资料以x±s表示,两组间比较采用独立样本t检验;非正态分布的计量资料以M(P25~P75)表示,两组间比较采用Mann-Whitney U检验。MAFLD组患儿外周血MAIT淋巴细胞频率与肝损伤、肝脏脂肪含量和纤维化相关性分析应用Spearman相关分析法。P<0.05为差异具有统计学意义。
2. 结果
2.1 一般资料
MAFLD组18例患儿年龄波动在6.17~13.08岁,对照组20例儿童年龄波动在5.83~13.00岁。MAFLD组的中性粒细胞、ALT、AST水平高于对照组(P值均<0.05)(表 1)。MAFLD组患儿肝纤维化弹性值为5.55(4.18~7.13)kPa,脂肪肝参数为(253.85±16.40) dB/m。
表 1 两组患儿临床资料比较Table 1. The comparation of clinical data between the two groups指标 MAFLD组(n=18) 对照组(n=20) 统计值 P值 男/女(例) 10/8 11/9 年龄(岁) 10.65(8.44~11.87) 9.00(7.40~11.25) Z=-1.083 0.279 ALT(U/L) 37.30(25.08~157.53) 13.65(11.75~17.00) Z=-4.474 <0.001 AST(U/L) 29.95(26.08~62.70) 20.95(18.90~24.23) Z=-3.304 0.001 WBC(×109/L) 7.31±2.56 7.00±1.50 t=0.937 0.355 中性粒细胞(×109/L) 4.47(3.36~5.18) 3.62(2.77~3.97) Z=-2.222 0.026 淋巴细胞(×109/L) 2.76(2.38~3.11) 2.44(1.82~2.91) Z=-1.608 0.112 2.2 儿童外周血MAIT淋巴细胞频率比较
与对照组相比,MAFLD组患儿外周血MAIT淋巴细胞占CD3+T淋巴细胞的比例明显升高(P<0.001),CD4+CD8-MAIT淋巴细胞、CD4+CD8+MAIT淋巴细胞所占MAIT淋巴细胞比例明显升高,CD4-CD8+MAIT淋巴细胞所占MAIT淋巴细胞比例降低(P值均<0.001),CD4-CD8-MAIT淋巴细胞所占MAIT淋巴细胞比例无变化(P>0.05)(表 2)。
表 2 两组患儿外周血MAIT淋巴细胞及各亚组MAIT淋巴细胞频率比较Table 2. The comparation of the peripheral blood MAIT lymphocytes and subtypes between the two groups指标 MAFLD组(n=18) 对照组(n=20) Z值 P值 MAIT淋巴细胞(%) 2.98(1.83~5.99) 0.29(0.09~0.69) -4.765 <0.001 CD4+CD8-MAIT淋巴细胞(%) 13.75(3.28~30.33) 0.38(0~1.55) -3.703 <0.001 CD4-CD8-MAIT淋巴细胞(%) 26.45(1.03~47.03) 31.25(24.05~50.33) 0.254 >0.05 CD4-CD8+MAIT淋巴细胞(%) 9.20(1.16~16.80) 43.65(29.65~64.98) -3.876 <0.001 CD4+CD8+MAIT淋巴细胞(%) 25.65(14.78~84.95) 9.36(6.04~22.45) -2.675 <0.001 2.3 外周血MAIT淋巴细胞表型和功能的差异
与对照组相比,MAFLD组患儿外周血表达PD-1、CD69、CD107α、CXCR3、CXCR6和CCR6的MAIT淋巴细胞比例均明显升高(P值均<0.05)(表 3)。
表 3 两组患儿外周血MAIT淋巴细胞表型和功能的差异Table 3. Differences in phenotype and function of peripheral blood MAIT lymphocytes between the two groups指标 MAFLD组(n=18) 对照组(n=20) Z值 P值 PD-1(%) 21.80(8.38~51.70) 4.43(2.07~11.29) -3.334 0.001 CD69(%) 56.00(28.93~68.35) 18.90(13.23~31.18) -2.646 0.008 穿孔素(%) 9.32(4.53~18.80) 4.16(1.35~9.53) -1.827 0.068 CD107α(%) 45.20(26.03~59.03) 10.95(6.80~17.18) -3.172 0.002 CXCR3(%) 87.35(80.08~96.03) 44.80(32.93~53.00) -4.678 <0.001 CXCR6(%) 30.40(8.24~54.90) 6.02(3.27~21.58) -2.939 0.003 CCR6(%) 26.80(13.03~77.70) 4.94(3.32~17.13) -2.749 0.006 2.4 MAFLD组患儿外周血MAIT淋巴细胞频率与肝脏炎症、脂肪含量和纤维化程度的关系
CD4+CD8+MAIT淋巴细胞和CD107α阳性MAIT淋巴细胞比例与ALT呈负相关(P值均<0.05);MAIT淋巴细胞、CD4+CD8-MAIT淋巴细胞、CD4-CD8+MAIT淋巴细胞比例与ALT无相关性(P值均>0.05);MAIT淋巴细胞、CD4+CD8-MAIT淋巴细胞、CD4-CD8+MAIT淋巴细胞、CD4+CD8+MAIT淋巴细胞和CD107α阳性MAIT淋巴细胞比例与AST、瞬时弹性成像中脂肪肝参数以及弹性值均无相关性(P值均>0.05)(表 4)。
表 4 MAFLD组患儿外周血MAIT细胞频率与肝脏炎症、脂肪含量和纤维化程度相关性分析Table 4. Correlation analysis between the frequency of MAIT lymphocytes in peripheral blood and the degree of liver inflammation, fat content, and fibrosis in children with MAFLD项目 ALT AST 弹性值 脂肪肝参数 MAIT淋巴细胞 r值 0.041 -1.050 0.064 0.112 P值 0.871 0.677 0.801 0.660 CD4+CD8-MAIT淋巴细胞 r值 -0.305 -0.330 -0.303 -0.272 P值 0.218 0.181 0.221 0.275 CD4-CD8+MAIT淋巴细胞 r值 0.140 -0.130 -0.047 0.228 P值 0.580 0.606 0.854 0.363 CD4+CD8+MAIT淋巴细胞 r值 -0.474 -0.436 -0.347 -0.115 P值 0.047 0.071 0.158 0.650 CD107α阳性MAIT淋巴细胞 r值 -0.550 -0.334 -0.201 -0.168 P值 0.018 0.176 0.432 0.504 3. 讨论
MAIT细胞与代谢功能障碍的发展有关, 肥胖儿童外周血MAIT细胞频率高于非肥胖儿童[12],其频率随着肥胖人群年龄的增长而下降,糖尿病和肥胖成人外周血MAIT细胞频率降低[13-15],肥胖成人和儿童中的高IL-17+表型在胰岛素抵抗发展中发挥着重要作用[12, 16]。目前MAIT淋巴细胞在MAFLD发病中作用机制的研究主要集中在成人,研究发现,MAFLD患者中循环MAIT淋巴细胞频率降低[7-9, 17],伴随着CXCR6表达的增加,而肝脏中MAIT淋巴细胞数量则明显增加,与MAFLD活动评分呈正相关[8]。本研究发现,MAFLD患儿外周血MAIT淋巴细胞频率明显升高的同时伴有趋化因子CCR6、CXCR6、CXCR3表达的增加,表明MAFLD患儿外周血MAIT淋巴细胞亦具有更强的迁移至肝脏的倾向[8],MAFLD患者外周血MAIT淋巴细胞频率的变化亦有着随着年龄增长而有下降的趋势,而这种下降的趋势可能与其迁移至肝脏有关。MAIT淋巴细胞可分为DP(CD4+CD8+)MAIT淋巴细胞、CD4+CD8-(CD4+)MAIT淋巴细胞、CD4-CD8+(CD8+)MAIT淋巴细胞和CD4-CD8-(DN)MAIT淋巴细胞。本研究发现健康儿童中以CD4-CD8-MAIT淋巴细胞和CD8+MAIT淋巴细胞为主[18],而MAFLD组患儿中以CD4-CD8-MAIT淋巴细胞和CD4+CD8+MAIT淋巴细胞为主,且与对照组相比,MAFLD组患儿CD4+MAIT淋巴细胞和CD4+CD8+MAIT淋巴细胞所占MAIT细胞比率升高,CD8+MAIT淋巴细胞所占MAIT淋巴细胞比率降低。
MAIT淋巴细胞在MAFLD中的作用为保护还是促进作用,与其所处疾病阶段有关。MAFLD成人患者中,外周血MAIT淋巴细胞降低的同时伴随着功能的改变,高表达CD69、PD-1及IL-4的产生增加,而IFNγ和TNFα的产生减少等。在蛋氨酸胆碱缺乏诱导的NASH模型中,MAIT细胞在肝脏中富集,并通过产生IL-4和IL-10以及诱导抗炎M2巨噬细胞来保护肝脏炎症[8]。随着病情的进展,肝内MAIT淋巴细胞的频率下降,在肝硬化和肝细胞癌的进展中起到促进作用[7, 17, 19]。本研究发现,MAFLD患儿外周血MAIT淋巴细胞水平明显升高的同时,伴有CD69、PD-1及CD107表达的增加,提示MAIT淋巴细胞被激活并功能性耗竭的同时,伴随着细胞毒性能力的增加。进一步研究显示CD4+CD8+MAIT淋巴细胞、CD107阳性MAIT淋巴细胞比例与ALT水平呈负相关,说明MAFLD患儿外周血MAIT细胞在肝脏炎症中具有保护作用,与文献[8]报道一致。
综上所述,MAIT淋巴细胞比例在MAFLD患儿外周血中明显升高,并在肝脏炎症中起着保护作用。本研究的局限性在于样本量偏少,进展期肝纤维化患儿病例数少,未检测肝脏组织MAIT淋巴细胞数量和其功能改变,MAIT淋巴细胞在儿童MAFLD发病中确切的免疫学机制及作用尚待进一步明确。
-
表 1 患者基线资料
Table 1. Baseline characteristics of HCC patients
指标 HAIC
(n=11)HAIC+TKI
(n=14)HAIC+TKI+PD-1/PD-L1
(n=41)合计 年龄(岁) 47.55±12.73 50.30±10.29 46.71±9.87 47.61±10.40 性别[例(%)] 男 10(90.9) 13(92.9) 39(95.1) 62(93.9) 女 1(9.1) 1(7.1) 2(4.9) 4(6.1) Child-Pugh分级[例(%)] A级 11(100.00) 9(64.3) 28(68.3) 48(72.7) B级 0(0.0) 5(35.7) 13(31.7) 18(27.3) BCLC分期[例(%)] B 4(36.4) 5(35.7) 9(22.0) 18(27.3) C 7(63.6) 9(64.3) 32(78.0) 48(72.7) AFP[例(%)] <400 ng/mL 8(72.7) 4(28.6) 12(29.3) 24(36.4) ≥400 ng/mL 3(27.3) 10(71.4) 29(70.7) 42(63.6) ALT(U/L) 47.00(24.00~96.00) 44.00(26.50~76.00) 36.00(26.00~55.50) 38.50(25.00~60.25) AST(U/L) 50.00(26.00~151.00) 54.00(43.00~98.25) 45.00(36.00~67.50) 48.50(35.00~74.25) Alb(g/L) 38.17±4.64 36.14±4.60 36.02±5.21 36.40±4.99 TBil(μmol/L) 13.20(8.10~21.00) 15.85(8.73~27.00) 15.40(12.10~23.45) 15.40(10.58~21.83) PLT(×109/L) 218.73±149.46 183.93±91.88 217.20±110.75 210.39±113.42 PT(s) 11.61±1.27 11.87±1.16 12.37±1.71 12.14±1.55 肿瘤特点[例(%)] 肝外转移 5(45.5) 6(42.9) 16(39.0) 27(40.9) 门静脉侵犯 5(45.5) 8(57.1) 26(63.4) 39(59.1) 单个肿瘤 0(0.0) 1(7.1) 4(9.8) 5(7.6) 多个肿瘤 11(100.0) 13(92.9) 37(90.2) 61(92.4) 肿瘤最大径<7 cm 5(45.5) 6(42.9) 11(26.8) 22(33.3) 肿瘤最大径≥7 cm 6(54.5) 8(57.1) 30(73.2) 44(66.7) 合并高血压或糖尿病 4(36.4) 2(14.3) 10(24.4) 16(24.2) HBV DNA[例(%)] 阳性 4(36.4) 6(42.9) 26(63.4) 36(54.5) 阴性 7(63.6) 8(57.1) 15(36.6) 30(45.5) 表 2 患者的最佳肿瘤疗效评价结果
Table 2. Best tumor response in patients
组别 例数 CR (例) PR (例) SD (例) PD (例) ORR (%) DCR (%) HAIC组 11 0 2 8 1 18.1 90.9 HAIC+TKI组 14 0 3 9 2 21.4 85.7 HAIC+TKI+PD-1/PD-L1组 41 0 17 18 6 41.5 85.4 合计 66 0 22 35 9 33.3 86.4 表 3 影响患者PFS的Cox单因素和多因素分析
Table 3. Univariate and multivariate Cox analysis of risk factors for progression-free survival
变量 单因素分析 多因素分析 HR 95%CI P值 HR 95%CI P值 肝外转移(有vs无) 2.907 1.536~5.500 0.001 2.668 1.357~5.245 0.004 HBV DNA (阳性vs阴性) 2.151 1.126~4.110 0.020 PT 1.254 1.024~1.535 0.028 1.282 1.080~1.630 0.042 表 4 影响患者OS的Cox单因素和多因素分析
Table 4. Univariate and multivariate Cox analysis of risk factors for overall survival
变量 单因素分析 多因素分析 HR 95%CI P值 HR 95%CI P值 年龄 0.964 0.931~0.999 0.042 AST 1.007 1.002~1.013 0.007 1.008 1.002~1.013 0.006 PT 1.276 1.036~1.572 0.022 1.303 1.046~1.630 0.018 -
[1] Global Burden of Disease Liver Cancer Collaboration, AKINYEMIJU T, ABERA S, et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: Results from the global burden of disease study 2015[J]. JAMA Oncol, 2017, 3(12): 1683-1691. DOI: 10.1001/jamaoncol.2017.3055. [2] BRAY F, FERLAY J, SOERJOMATARAM I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2018, 68(6): 394-424. DOI: 10.3322/caac.21492. [3] ZHENG R, ZENG H, ZHANG S, et al. Estimates of cancer incidence and mortality in China, 2013[J]. Chin J Cancer, 2017, 36(1): 66. DOI: 10.1186/s40880-017-0234-3. [4] WU T, CHEN L. New advances in the precision diagnosis and treatment of liver cancer[J]. J Clin Hepatol, 2022, 38(3): 497-498. DOI: 10.3969/j.issn.1001-5256.2022.03.001.吴彤, 陈磊. 肝癌精准诊疗新进展[J]. 临床肝胆病杂志, 2022, 38(3): 497-498. DOI: 10.3969/j.issn.1001-5256.2022.03.001. [5] LI Z, ZHU JY. Interpretation of Standard for diagnosis and treatment of primary liver cancer (2022 edition)[J]. J Clin Hepatol, 2022, 38(5): 1027-1029. DOI: 10.3969/j.issn.1001-5256.2022.05.010.李照, 朱继业. 《原发性肝癌诊疗指南(2022年版)》解读[J]. 临床肝胆病杂志, 2022, 38(5): 1027-1029. DOI: 10.3969/j.issn.1001-5256.2022.05.010. [6] CHENG AL, KANG YK, CHEN Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase Ⅲ randomised, double-blind, placebo- controlled trial[J]. Lancet Oncol, 2009, 10(1): 25-34. DOI: 10.1016/S1470-2045(08)70285-7. [7] KUDO M, FINN RS, QIN S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial[J]. Lancet, 2018, 391(10126): 1163-1173. DOI: 10.1016/S0140-6736(18)30207-1. [8] LYU N, KONG Y, PAN T, et al. Hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin in hepatocellular cancer with extrahepatic spread[J]. J Vasc Interv Radiol, 2019, 30(3): 349-357. e2. DOI: 10.1016/j.jvir.2018.09.004. [9] HE MK, LIANG RB, ZHAO Y, et al. Lenvatinib, toripalimab, plus hepatic arterial infusion chemotherapy versus lenvatinib alone for advanced hepatocellular carcinoma[J]. Ther Adv Med Oncol, 2021, 13: 17588359211002720. DOI: 10.1177/17588359211002720. [10] KOKUDO N, HASEGAWA K, AKAHANE M, et al. Evidence-based clinical practice guidelines for hepatocellular carcinoma: The Japan Society of Hepatology 2013 update (3rd JSH-HCC Guidelines)[J]. Hepatol Res, 2015, 45(2). DOI: 10.1111/hepr.12464. [11] IKEDA M, OKUSAKA T, FURUSE J, et al. A multi-institutional phase Ⅱ trial of hepatic arterial infusion chemotherapy with cisplatin for advanced hepatocellular carcinoma with portal vein tumor thrombosis[J]. Cancer Chemother Pharmacol, 2013, 72(2): 463-470. DOI: 10.1007/s00280-013-2222-x. [12] NOUSO K, MIYAHARA K, UCHIDA D, et al. Effect of hepatic arterial infusion chemotherapy of 5-fluorouracil and cisplatin for advanced hepatocellular carcinoma in the Nationwide Survey of Primary Liver Cancer in Japan[J]. Br J Cancer, 2013, 109(7): 1904-1907. DOI: 10.1038/bjc.2013.542. [13] Tumor Interventional Expert Committee of Chinese Anti-Cancer Association. Chinese tumor intervention expert consensus on the application principles of transcatheter arterial infusion chemotherapy[J]. J Intervent Radiol, 2017, 26(11): 963-970. DOI: 10.3969/j.issn.1008-794X.2017.11.001.中国抗癌协会肿瘤介入专家委员会. 经导管动脉灌注化疗药物应用原则——中国肿瘤介入专家共识[J]. 介入放射学杂志, 2017, 26(11): 963-970. DOI: 10.3969/j.issn.1008-794X.2017.11.001. [14] ZHAO M. Hepatic arterial infusion Chemotherapy in the Era of Precise[J]. J Sun Yat-Sen Univ(Medical Sciences), 2019, 40(5): 648-656. DOI: 1672-3554(2019)05-0648-09.赵明. 精准医疗时代背景下的肝动脉灌注化疗[J]. 中山大学学报(医学科学版), 2019, 40(5): 648-656. DOI: 1672-3554(2019)05-0648-09. [15] LI QJ, HE MK, CHEN HW, et al. Hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin versus transarterial chemoembolization for large hepatocellular carcinoma: A Randomized Phase Ⅲ Trial[J]. J Clin Oncol, 2022, 40(2): 150-160. DOI: 10.1200/JCO.21.00608. [16] SIDAWAY P. FOLFOX-HAIC active in large HCC[J]. Nat Rev Clin Oncol, 2022, 19(1): 5. DOI: 10.1038/s41571-021-00577-y. [17] General Office of National Health Commission. Standard for diagnosis and treatment of primary liver cancer (2022 edition)[J]. J Clin Hepatol, 2022, 38(2): 288-303. DOI: 10.3969/j.issn.1001-5256.2022.02.009.国家卫生健康委办公厅. 原发性肝癌诊疗指南(2022年版)[J]. 临床肝胆病杂志, 2022, 38(2): 288-303. DOI: 10.3969/j.issn.1001-5256.2022.02.009. [18] Chinese College of Transplant Doctors, Liver Transplantation Group, Chinese Society of Organ Transplantation, Chinese Medical Association. Chinese clinical practice guidelines on liver transplantation for hepatocellular carcinoma (2021edition)[J]. Chin J Dig Surg, 2022, 21(4): 433-443. DOI: 10.3760/cma.j.cn115610-20220316-00135.中国医师协会器官移植医师分会, 中华医学会器官移植学分会肝移植学组. 中国肝癌肝移植临床实践指南(2021版)[J]. 中华消化外科杂志, 2022, 21(4): 433-443. DOI: 10.3760/cma.j.cn115610-20220316-00135. [19] LIU YY. New progress of hepatocellular carcinoma treatment[J]. Chin J Dig Surg, 2022, 21(1): 15-18. DOI: 10.3760/cma.j.cn115610-20220107-00020.刘允怡. 肝细胞癌治疗的新发展[J]. 中华消化外科杂志, 2022, 21(1): 15-18. DOI: 10.3760/cma.j.cn115610-20220107-00020. [20] European Association for the Study of the Liver. EASL clinical practice guidelines: Management of hepatocellular carcinoma[J]. J Hepatol, 2018, 69(1): 182-236. DOI: 10.1016/j.jhep.2018.03.019. [21] Liver Cancer Professional Committee of Chinese Anti-Cancer Association. Chinese expert consensus on hepatic arterial infusion chemotherapy for hepatocellular carcinoma (2021 edition)[J]. J Chin J Dig Surg, 2021, 20(7): 754-759. DOI: 10.3760/cma.j.cn115610-20210618-00288.中国抗癌协会肝癌专业委员会. 肝动脉灌注化疗治疗肝细胞癌中国专家共识(2021版)[J]. 中华消化外科杂志, 2021, 20(7): 754-759. DOI: 10.3760/cma.j.cn115610-20210618-00288. [22] HE MK, LE Y, LI QJ, et al. Hepatic artery infusion chemotherapy using mFOLFOX versus transarterial chemoembolization for massive unresectable hepatocellular carcinoma: a prospective non-randomized study[J]. Chin J Cancer, 2017, 36(1): 83. DOI: 10.1186/s40880-017-0251-2. [23] HE M, LI Q, ZOU R, et al. Sorafenib plus hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin vs sorafenib alone for hepatocellular carcinoma with portal vein invasion: A randomized clinical trial[J]. JAMA Oncol, 2019, 5(7): 953-960. DOI: 10.1001/jamaoncol.2019.0250. [24] MEI J, LI SH, LI QJ, et al. Anti-PD-1 immunotherapy improves the efficacy of hepatic artery infusion chemotherapy in advanced hepatocellular carcinoma[J]. J Hepatocell Carcinoma, 2021, 8: 167-176. DOI: 10.2147/JHC.S298538. [25] MEI J, TANG YH, WEI W, et al. Hepatic arterial infusion chemotherapy combined With PD-1 inhibitors plus lenvatinib versus PD-1 inhibitors plus lenvatinib for advanced hepatocellular carcinoma[J]. Front Oncol, 2021, 11: 618206. DOI: 10.3389/fonc.2021.618206. [26] WU YB, WU ZQ, HUANG JL, et al. Hepatic arterial infusion chemotherapy for primary liver cancer effect of portal vein tumor thrombus[J]. China Health Standard Management, 2022, 13(12): 109-112. DOI: 10.3969/j.issn.1674-9316.2022.12.027.吴义波, 吴卓琼, 黄洁丽, 等. 肝动脉灌注化疗对原发性肝癌合并门静脉癌栓的效果[J]. 中国卫生标准管理, 2022, 13(12): 109-112. DOI: 10.3969/j.issn.1674-9316.2022.12.027. [27] KE YP, YE SG, LU SB. Short-term efficacy of transcatheter arterial embolization combined with FOLFOX4 regimen of continuous arterial infusion chemotherapy on hepatocellular carcinoma patients with portal vein tumor thrombus[J]. China Med Pharm, 2022, 12(16): 156-159. DOI: 10.3969/j.issn.2095-0616.2022.16.040.柯映平, 叶绍光, 卢舜彬. 肝动脉栓塞术联合FOLFOX4方案持续动脉灌注化疗对肝细胞癌门静脉癌栓患者的近期疗效[J]. 中国医药科学, 2022, 12(16): 156-159. DOI: 10.3969/j.issn.2095-0616.2022.16.040. -




 PDF下载 ( 2254 KB)
PDF下载 ( 2254 KB)

 下载:
下载:

 下载:
下载: