基于HALP评分的列线图模型对肝细胞癌患者肝切除术后预后的预测价值
DOI: 10.3969/j.issn.1001-5256.2023.07.014
Value of the nomogram based on HALP score in predicting the prognosis of patients with hepatocellular carcinoma after hepatectomy
-
摘要:
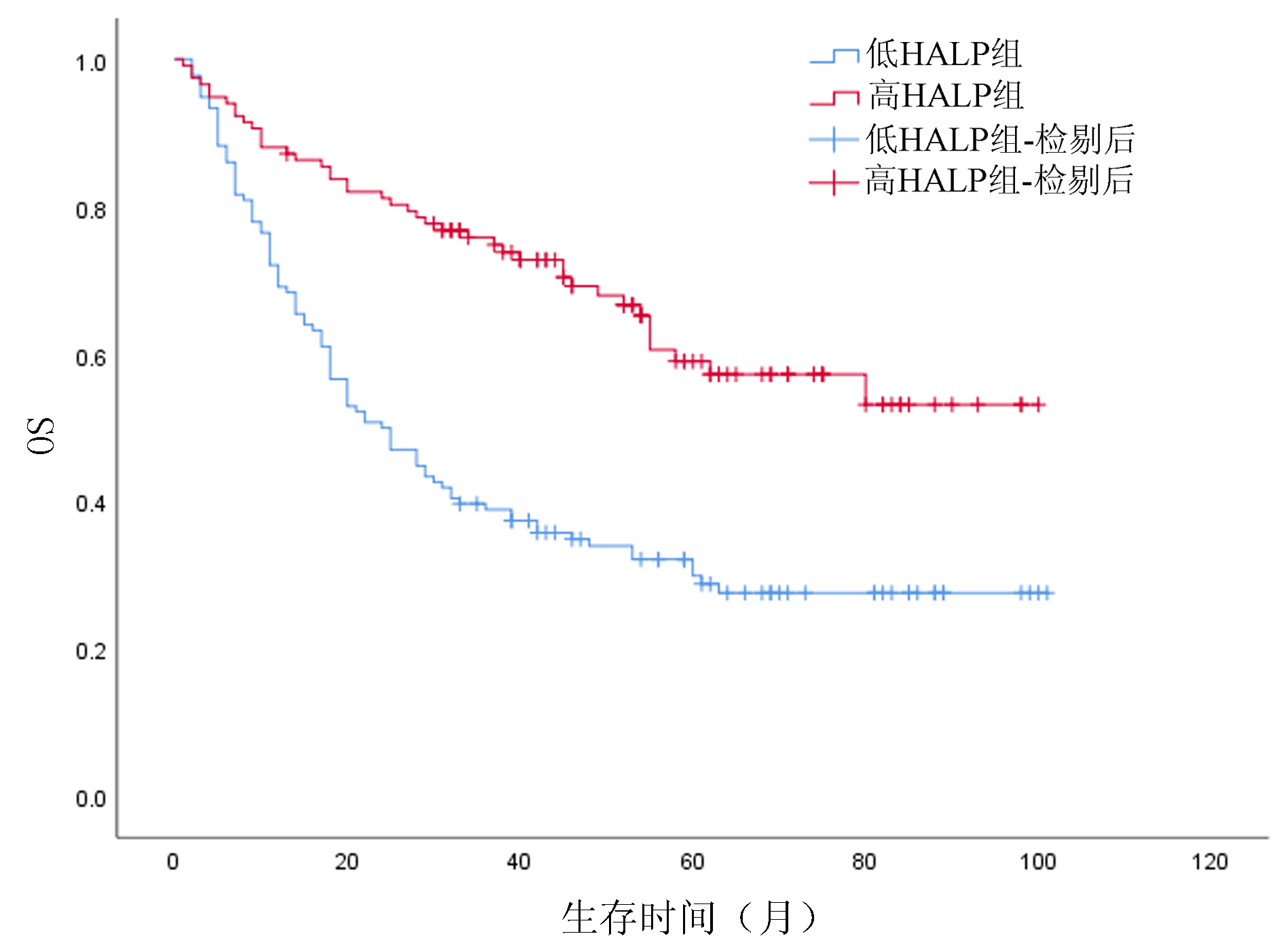
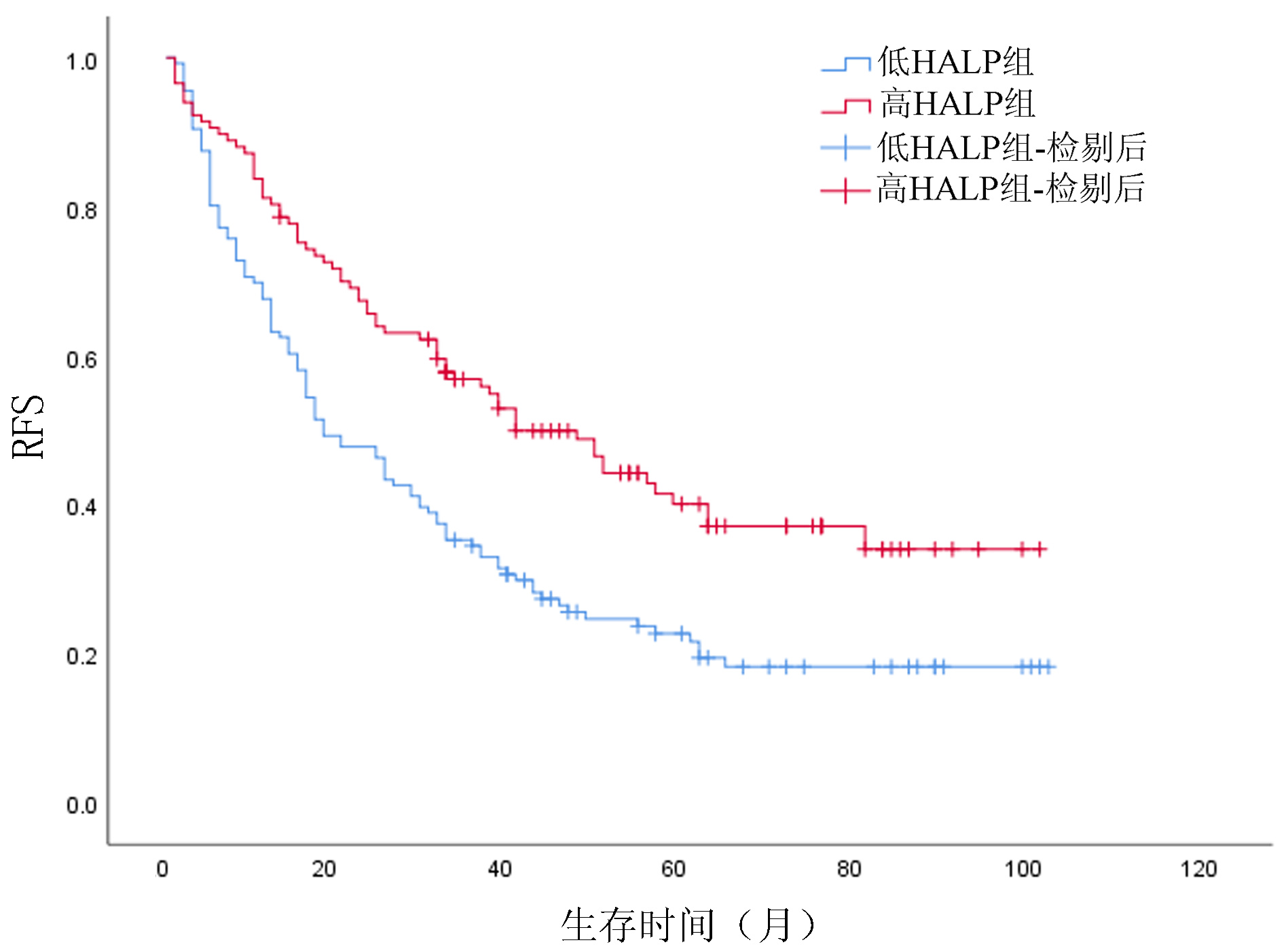
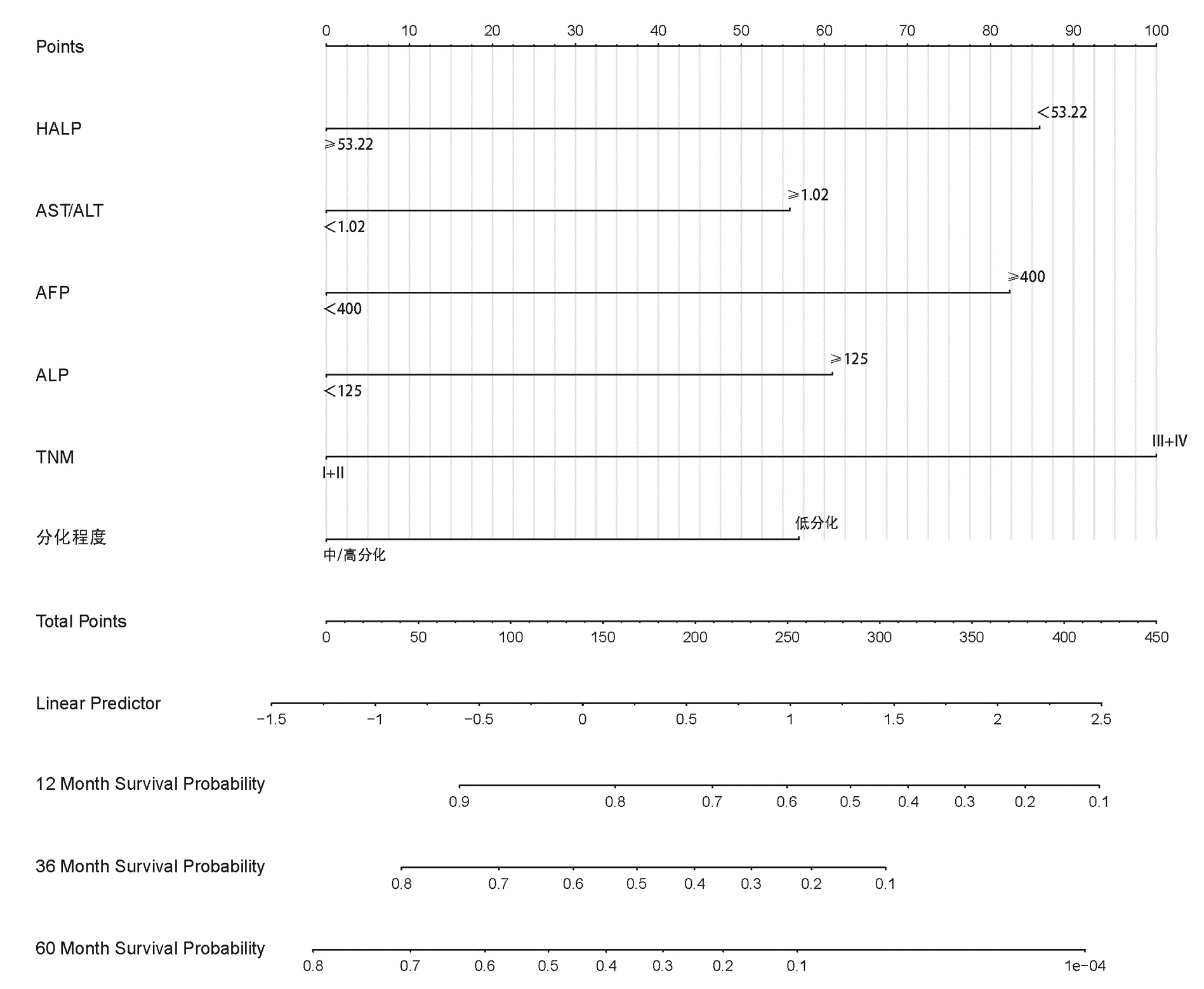
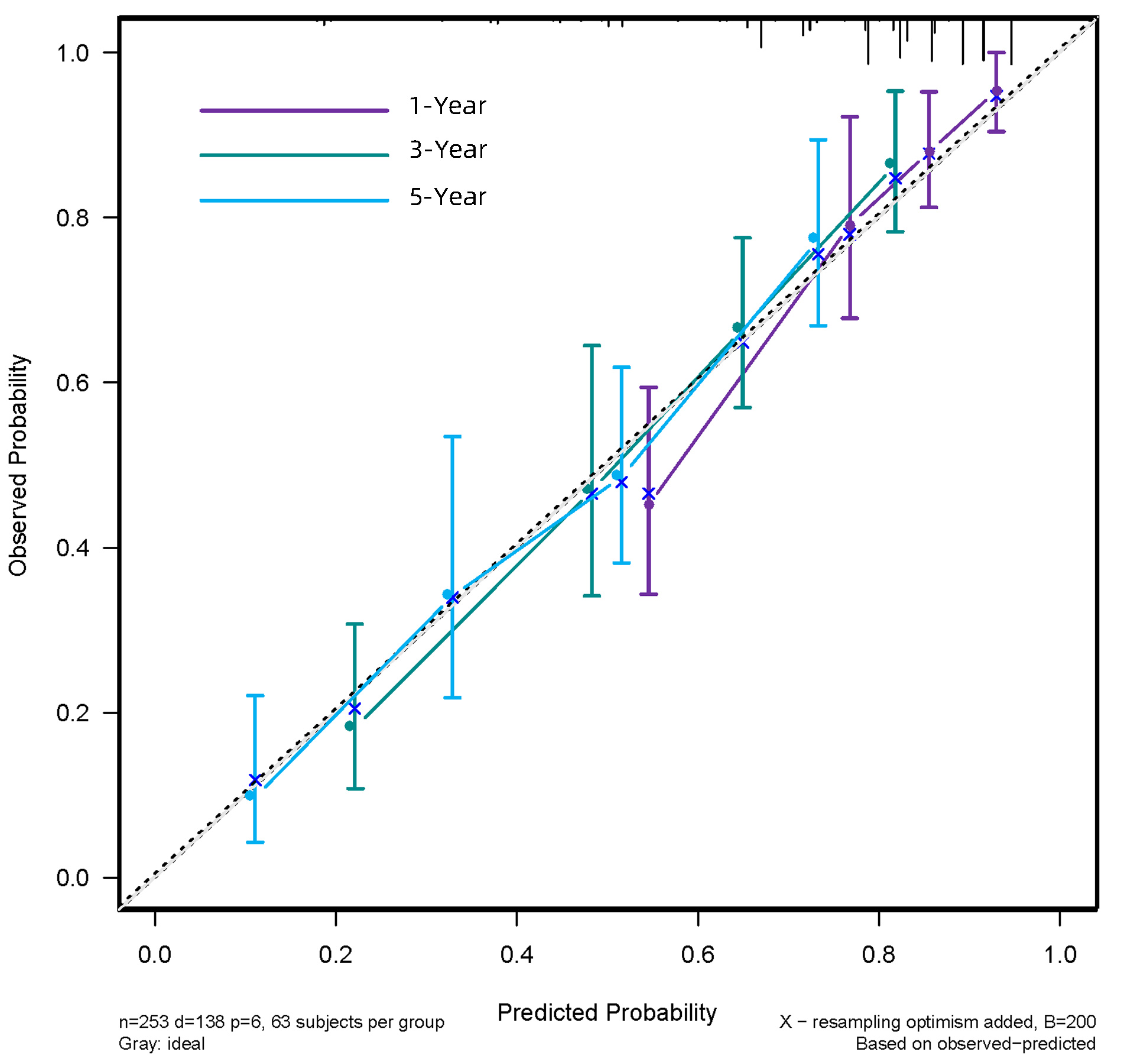
目的 探讨HALP评分在肝细胞癌(HCC)患者肝切除术后预后评估中的应用价值,分析基于HALP评分的列线图模型能否有效预测患者术后生存情况。 方法 回顾性分析2013年7月—2020年3月在西南医科大学附属医院肝胆外科手术治疗的253例HCC患者临床资料。通过绘制ROC曲线,计算出HALP和其他有关指标的最佳截断值。采用χ2检验分析HALP与临床病理特征之间的关系。使用Kaplan-Meier方法绘制生存曲线,并采用Log-rank检验进行比较。进行单因素分析和多因素Cox回归模型,分析HALP及其他临床参数与患者预后的关系。通过R 3.6软件构建列线图,使用C指数及校准图评价列线图的预测能力,通过净重新分类指数(NRI)、综合判别改善指数(IDI)比较列线图模型和传统模型的预测能力。 结果 Kaplan-Meier分析显示高HALP组患者OS、RFS优于低HALP组,差异均有统计学意义(P值均<0.001)。单因素Cox回归分析显示WBC、GGT、ALP、AFP、手术方式、微血管侵犯、TNM分期、分化程度、HALP、AST/ALT、NLR、MLR均与总生存期(OS)显著相关(P值均<0.05),将单因素Cox回归分析中有统计学意义的变量纳入多因素Cox回归分析,结果显示HALP、AST/ALT、ALP、AFP、分化程度、TNM分期是HCC患者术后OS的独立影响因素(P值均<0.05)。单因素Cox回归分析显示GGT、ALP、AFP、微血管侵犯、TNM分期、分化程度、HALP、AST/ALT、NLR、MLR均与无复发生存期(RFS)显著相关(P值均<0.05);多因素Cox回归分析,结果显示HALP、AST/ALT、NLR、ALP、AFP、TNM分期是HCC患者术后RFS的独立影响因素(P值均<0.05)。根据多因素分析结果分别构建HCC患者OS、RFS的列线图,计算预测OS列线图的C指数为0.732(0.691~0.774),预测1、3、5年生存率的AUC分别为0.795、0.791、0.775。预测RFS列线图的C指数为0.677(0.637~0.717),预测1、3、5年生存率的AUC分别为0.742、0.733、0.716;并且1、3、5年OS、RFS校准图表现出了较好的拟合度。 结论 术前低水平HALP是接受手术治疗的HCC患者长期预后不佳的预测因素,基于HALP评分的列线图模型优于BCLC分期模型,可以更好地预测HCC的预后情况。 Abstract:Objective To investigate the value of HALP score in evaluating the prognosis of patients with hepatocellular carcinoma (HCC) after hepatectomy and whether the nomogram based on HALP score could effectively predict the postoperative survival of patients. Methods A retrospective study was performed for the clinical data of 253 HCC patients who underwent surgical treatment in Department of Hepatobiliary Surgery, The Affiliated Hospital of Southwest Medical University, from July 2013 to March 2020. The receiver operating characteristic (ROC) curve was plotted to calculate the optimal cut-off values of HALP score and other related indicators; the chi-square test was used to investigate the association between HALP score and clinicopathological features; the Kaplan-Meier method was used to plot survival curves, and the Log-rank test method was used for comparison. The univariate and multivariate Cox regression analyses were used to investigate the association of HALP score and other clinical parameters with the prognosis of patients. R3.6 was used to establish a nomogram; C-index and calibration curve were used to evaluate the predictive ability of the nomogram, and net reclassification index (NRI) and integrated discrimination improvement (IDI) were used to compare predictive ability between the nomogram model and the conventional model. Results The Kaplan-Meier analysis showed that the high HALP group had significantly better overall survival (OS) and recurrence-free survival (RFS) than the low HALP group (P < 0.001). The univariate Cox regression analysis showed that white blood cell count, gamma-glutamyl transpeptidase (GGT), alkaline phosphatase (ALP), alpha-fetoprotein (AFP), surgical approach, microvascular invasion, TNM stage, degree of tumor differentiation, HALP, aspartate aminotransferase (AST)/alanine aminotransferase (ALT) ratio, neutrophil-to-lymphocyte ratio (NLR), and monocyte-to-lymphocyte ratio (MLR) were significantly associated with OS (all P < 0.05). The variables with statistical significance in the univariate Cox regression analysis were included in the multivariate Cox regression analysis, and the results showed that ALP, AST/ALT ratio, ALP, AFP, degree of tumor differentiation, and TNM stage were independent influencing factors for OS after surgery in HCC patients (all P < 0.05). The univariate Cox regression analysis showed that GGT, ALP, AFP, microvascular invasion, TNM stage, degree of tumor differentiation, HALP, AST/ALT ratio, NLR, and MLR were significantly associated with RFS (all P < 0.05), and the multivariate Cox regression analysis showed that HALP, AST/ALT ratio, NLR, ALP, AFP, and TNM stage were independent influencing factors for RFS after surgery in HCC patients (all P < 0.05). The nomograms for OS and RFS of HCC patients were established based on the multivariate analysis. The nomogram for OS had a C-index of 0.732 (95% confidence interval [CI]: 0.691-0.774) and an area under the ROC curve of 0.795, 0.791, and 0.775, respectively, in predicting 1-, 3-, and 5-year survival rates, and the nomogram for RFS had a C-index of 0.677 (95%CI: 0.637-0.717) and an area under the ROC curve of 0.742, 0.733, and 0.716, respectively, in predicting 1-, 3-, and 5-year survival rates. The calibration curves of 1-, 3-, and 5-year OS were well fitted to those of 1-, 3-, and 5-year RFS. Conclusion A low level of HALP before surgery is a predictive factor for poor long-term prognosis in HCC patients undergoing surgical treatment, and the nomogram model based on HALP score is superior to the BCLC staging model and can better predict the prognosis of HCC. -
Key words:
- Carcinoma, Hepatocellular /
- Prognosis /
- Risk Factors /
- Nomograms
-
表 1 MLR、NLR、AST/ALT、HALP的诊断指标
Table 1. Diagnostic indicators for MLR, NLR, AST/ALT, HALP
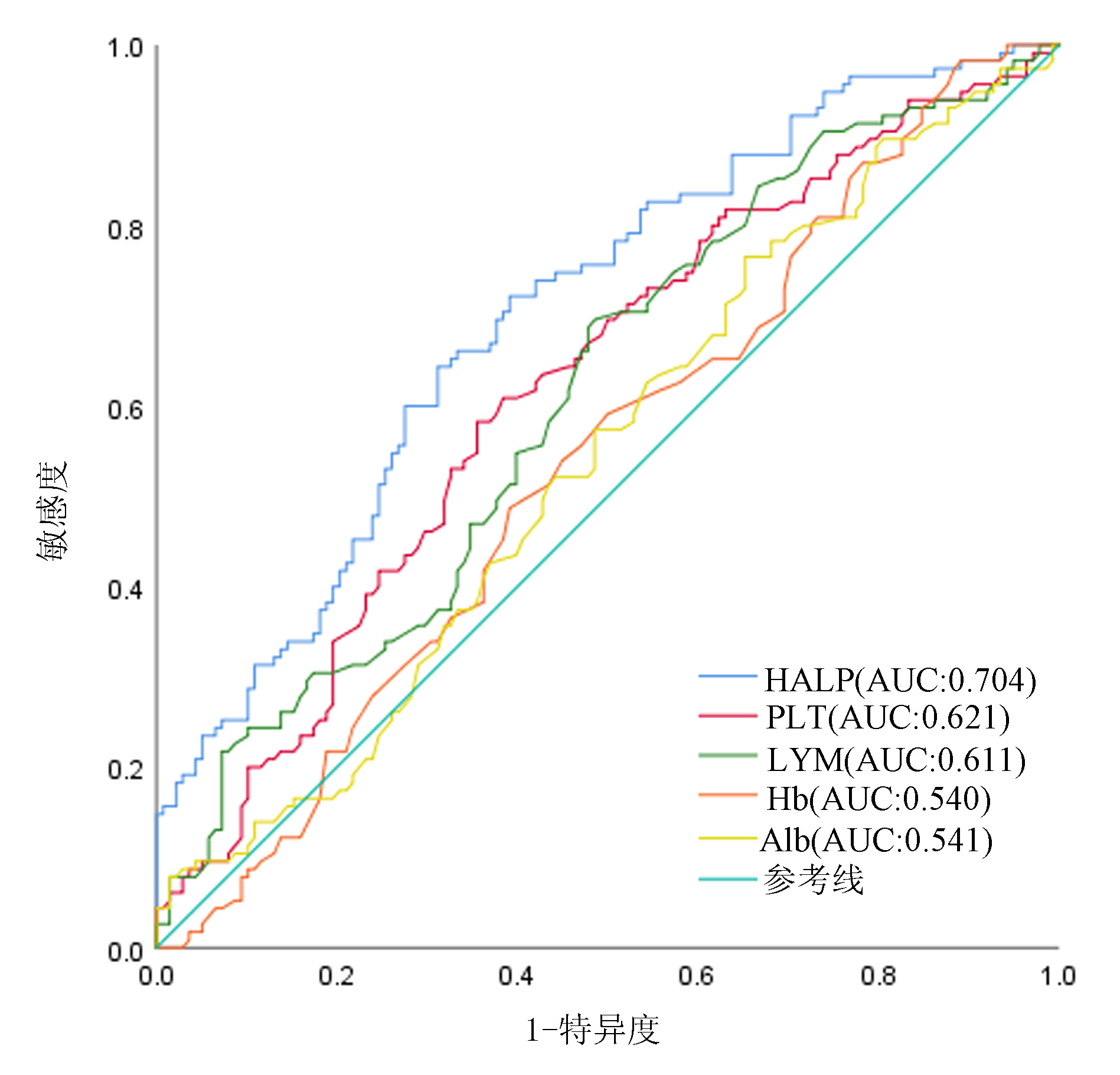
变量 约登指数 截断值 AUC(95%CI) 特异度 敏感度 MLR 0.283 0.39 0.663(0.597~0.729) 0.457 0.826 NLR 0.325 2.92 0.681(0.616~0.746) 0.551 0.774 AST/ALT 0.279 1.02 0.664(0.597~0.730) 0.670 0.609 HALP 0.331 53.22 0.704(0.640~0.767) 0.688 0.643 表 2 HCC患者的临床病理特征和HALP评分的关系
Table 2. Relationship between clinicopathological characteristics and HALP score of HCC patients
临床特征 低HALP组
(n=136)高HALP组
(n=117)χ2值 P值 性别(例) 3.326 0.068 男 111 105 女 25 12 年龄(例) 0.656 0.418 <60岁 107 87 ≥60岁 29 30 TBil(例) 0.168 0.682 <23 μmol/L 112 94 ≥23 μmol/L 24 23 WBC(例) 0.731 0.393 <10×109/L 124 110 ≥10×109/L 12 7 APTT(例) 0.380 0.538 <35 s 48 37 ≥35 s 88 80 GGT(例) 3.132 0.077 <60 U/L 49 55 ≥60 U/L 87 62 ALP(例) 2.925 0.087 <125 U/L 94 92 ≥125 U/L 42 25 AFP(例) 2.308 0.129 <400 ng/mL 78 78 ≥400 ng/mL 58 39 肿瘤大小(例) 19.834 <0.001 ≤5 cm 49 75 >5 cm 87 42 TNM分期(例) 2.878 0.090 Ⅰ+Ⅱ 121 111 Ⅲ+Ⅳ 15 6 分化程度(例) 4.160 0.041 低 28 13 中+高 108 104 手术方式(例) 8.846 0.003 开腹 124 91 腹腔镜 12 26 微血管侵犯(例) 0.127 0.721 无 102 90 有 34 27 HBsAg(例) 2.027 0.155 阴性 18 9 阳性 118 108 BCLC分期(例) 1.301 0.254 0+A 91 86 B+C 45 31 表 3 HCC患者OS的单因素和多因素分析
Table 3. Univariate and multivariate analysis of OS in HCC patients
临床特征 单因素分析 多因素分析 HR(95%CI) P值 HR(95%CI) P值 性别(男/女) 1.125(0.713~1.775) 0.612 年龄(<60岁/≥60岁) 0.809(0.537~1.219) 0.312 TBil(23 μmol/L/≥23 μmol/L) 1.247(0.832~1.869) 0.286 WBC(<10×109/L/≥10×109/L) 1.801(1.052~3.084) 0.032 APTT(<35 s/≥35 s) 1.047(0.733~1.495) 0.800 GGT(<60 U/L/≥60 U/L) 1.552(1.095~2.201) 0.014 ALP(<125 U/L/≥125 U/L) 2.015(1.421~2.857) <0.001 1.502(1.017~2.221) 0.041 AFP(<400 ng/mL/≥400 ng/mL) 2.328(1.665~3.253) <0.001 2.128(1.499~3.020) <0.001 手术方式(开腹/腔镜) 0.519(0.299~0.902) 0.020 术后住院时间(<12 d/≥12 d) 1.374(0.976~1.934) 0.069 HBsAg(阴性/阳性) 1.020(0.597~1.745) 0.941 微血管侵犯(无/有) 1.479(1.010~2.167) 0.045 TNM分期(Ⅰ+Ⅱ/Ⅲ+Ⅳ) 3.745(2.256~6.217) <0.001 2.346(1.318~4.175) 0.004 分化程度(低/中+高) 0.525(0.349~0.787) 0.002 0.643(0.418~0.990) 0.045 HALP(<53.22/≥53.22) 0.395(0.275~0.567) <0.001 0.555(0.373~0.827) 0.004 AST/ALT(<1.02/≥1.02) 1.827(1.297~2.572) 0.001 1.585(1.101~2.282) 0.013 NLR(<2.92/≥2.92) 2.561(1.827~3.590) <0.001 MLR(<0.39/≥0.39) 2.148(1.535~3.005) <0.001 表 4 HCC患者RFS的单因素和多因素分析
Table 4. Univariate and multivariate analysis of RFS in HCC patients
临床特征 单因素分析 多因素分析 HR(95%CI) P值 HR(95%CI) P值 性别(男/女) 0.830(0.535~1.287) 0.404 年龄(<60岁/≥60岁) 0.896(0.627~1.279) 0.544 TBil(23 μmol/L/≥23 μmol/L) 1.140(0.787~1.651) 0.489 WBC(<10×109/L/≥10×109/L) 1.601(0.955~2.683) 0.074 APTT(<35 s/≥35 s) 1.242(0.899~1.716) 0.189 GGT(<60 U/L/≥60 U/L) 1.433(1.054~1.947) 0.022 ALP(<125 U/L /≥125 U/L) 1.930(1.403~2.653) <0.001 1.535(1.070~2.202) 0.020 AFP(<400 ng/mL/≥400 ng/mL) 1.630(1.207~2.201) 0.001 1.498(1.101~2.038) 0.010 手术方式(开腹/腔镜) 0.672(0.426~1.060) 0.087 术后住院时间(<12 d/≥12 d) 1.105(0.819~1.492) 0.513 HBsAg(阴性/阳性) 0.955(0.605~1.507) 0.842 微血管侵犯(无/有) 1.510(1.074~2.121) 0.018 TNM分期(Ⅰ+Ⅱ/Ⅲ+Ⅳ) 2.839(1.732~4.656) <0.001 1.919(1.107~3.325) 0.020 分化程度(低/中+高) 0.652(0.446~0.953) 0.027 HALP(<53.22/≥53.22) 0.568(0.419~0.771) <0.001 0.704(0.503~0.985) 0.041 AST/ALT(<1.02/≥1.02) 1.465(1.087~1.974) 0.012 1.378(1.004~1.891) 0.047 NLR(<2.92/≥2.92) 1.955(1.451~2.634) <0.001 1.476(1.014~2.150) 0.042 MLR(<0.39/≥0.39) 1.696(1.253~2.296) 0.001 表 5 列线图与BCLC分期的预测价值比较
Table 5. Comparison of the predictive value of nomograms with BCLC staging
模型 NRI(95%CI) P值 IDI(95%CI) P值 BCLC分期 Reference Reference OS列线图 0.307(0.101~0.515) <0.000 1 0.307(0.139~0.415) <0.000 1 RFS列线图 0.754(0.557~0.885) <0.000 1 0.700(0.548~0.788) <0.000 1 -
[1] General Office of National Health Commission. Standard for diagnosis and treatment of primary liver cancer (2022 edition)[J]. J Clin Hepatol, 2022, 38(2): 288-303. DOI: 10.3969/j.issn.1001-5256.2022.02.009.国家卫生健康委办公厅. 原发性肝癌诊疗指南(2022年版)[J]. 临床肝胆病杂志, 2022, 38(2): 288-303. DOI: 10.3969/j.issn.1001-5256.2022.02.009. [2] XIAN LF, FANG LT, LIU WB, et al. Epidemic status, main pathogenesis, and prevention and control strategies of primary liver cancer[J]. Chin J Oncol Prev Treat, 2022, 14(3): 320-328. DOI: 10.3969/j.issn.1674-5671.2022.03.13.鲜林峰, 方乐天, 刘文斌, 等. 原发性肝癌流行现状、主要发病机制及防控策略[J]. 中国癌症防治杂志, 2022, 14(3): 320-328. DOI: 10.3969/j.issn.1674-5671.2022.03.13. [3] YUAN Y, YANG F, WANG Y, et al. Factors associated with liver cancer prognosis after hepatectomy: A retrospective cohort study[J]. Medicine (Baltimore), 2021, 100(42): e27378. DOI: 10.1097/MD.0000000000027378. [4] MCMILLAN DC. Systemic inflammation, nutritional status and survival in patients with cancer[J]. Curr Opin Clin Nutr Metab Care, 2009, 12(3): 223-226. DOI: 10.1097/MCO.0b013e32832a7902. [5] CHOI J, KIM SH, HAN S, et al. A simple and clinically applicable model to predict liver-related morbidity after hepatic resection for hepatocellular carcinoma[J]. PLoS One, 2020, 15(11): e0241808. DOI: 10.1371/journal.pone.0241808. [6] WAN S, LAI Y, MYERS RE, et al. Post-diagnosis hemoglobin change associates with overall survival of multiple malignancies - results from a 14-year hospital-based cohort of lung, breast, colorectal, and liver cancers[J]. BMC Cancer, 2013, 13: 340. DOI: 10.1186/1471-2407-13-340. [7] HONG YM, YOON KT, HWANG TH, et al. Pretreatment peripheral neutrophils, lymphocytes and monocytes predict long-term survival in hepatocellular carcinoma[J]. BMC Cancer, 2020, 20(1): 937. DOI: 10.1186/s12885-020-07105-8. [8] LU L, SU Z, ZHENG P, et al. Association between platelet count and hepatocellular carcinoma overall survival: a large retrospective cohort study[J]. BMJ Open, 2020, 10(11): e038172. DOI: 10.1136/bmjopen-2020-038172. [9] JOHNSON PJ, DHANARAJ S, BERHANE S, et al. The prognostic and diagnostic significance of the neutrophil-to-lymphocyte ratio in hepatocellular carcinoma: a prospective controlled study[J]. Br J Cancer, 2021, 125(5): 714-716. DOI: 10.1038/s41416-021-01445-3. [10] WU Y, TU C, SHAO C. Inflammatory indexes in preoperative blood routine to predict early recurrence of hepatocellular carcinoma after curative hepatectomy[J]. BMC Surg, 2021, 21(1): 178. DOI: 10.1186/s12893-021-01180-9. [11] ZHANG LX, LV Y, XU AM, et al. The prognostic significance of serum gamma-glutamyltransferase levels and AST/ALT in primary hepatic carcinoma[J]. BMC Cancer, 2019, 19(1): 841. DOI: 10.1186/s12885-019-6011-8. [12] FENG JF, WANG L, YANG X. The preoperative hemoglobin, albumin, lymphocyte and platelet (HALP) score is a useful predictor in patients with resectable esophageal squamous cell carcinoma[J]. Bosn J Basic Med Sci, 2021, 21(6): 773-781. DOI: 10.17305/bjbms.2021.5666. [13] XU SS, LI S, XU HX, et al. Haemoglobin, albumin, lymphocyte and platelet predicts postoperative survival in pancreatic cancer[J]. World J Gastroenterol, 2020, 26(8): 828-838. DOI: 10.3748/wjg.v26.i8.828. [14] CHEN XL, XUE L, WANG W, et al. Prognostic significance of the combination of preoperative hemoglobin, albumin, lymphocyte and platelet in patients with gastric carcinoma: a retrospective cohort study[J]. Oncotarget, 2015, 6(38): 41370-41382. DOI: 10.18632/oncotarget.5629. [15] VIRCHOW R. An address on the value of pathological experiments[J]. Br Med J, 1881, 2(1075): 198-203. DOI: 10.1136/bmj.2.1075.198. [16] QIAN S, GOLUBNITSCHAJA O, ZHAN X. Chronic inflammation: key player and biomarker-set to predict and prevent cancer development and progression based on individualized patient profiles[J]. EPMA J, 2019, 10(4): 365-381. DOI: 10.1007/s13167-019-00194-x. [17] GRIVENNIKOV SI, GRETEN FR, KARIN M. Immunity, inflammation, and cancer[J]. Cell, 2010, 140(6): 883-899. DOI: 10.1016/j.cell.2010.01.025. [18] MADEDDU C, GRAMIGNANO G, ASTARA G, et al. Pathogenesis and treatment options of cancer related anemia: perspective for a targeted mechanism-based approach[J]. Front Physiol, 2018, 9: 1294. DOI: 10.3389/fphys.2018.01294. [19] PERGIALIOTIS V, DASKALAKIS G, THOMAKOS N, et al. Prechemotherapy hemoglobin levels as a predictive factor of ovarian cancer survival: a systematic review and meta-analysis[J]. Am J Clin Oncol, 2019, 42(9): 725-731. DOI: 10.1097/COC.0000000000000570. [20] HUANG XZ, YANG YC, CHEN Y, et al. Preoperative anemia or low hemoglobin predicts poor prognosis in gastric cancer patients: a meta-analysis[J]. Dis Markers, 2019, 2019: 7606128. DOI: 10.1155/2019/7606128. [21] BANZET S, SANCHEZ H, CHAPOT R, et al. Interleukin-6 contributes to hepcidin mRNA increase in response to exercise[J]. Cytokine, 2012, 58(2): 158-161. DOI: 10.1016/j.cyto.2012.01.006. [22] VAUPEL P. The role of hypoxia-induced factors in tumor progression[J]. Oncologist, 2004, 9(Suppl 5): 10-17. DOI: 10.1634/theoncologist.9-90005-10. [23] WANG L, LI Q, ZHANG J, et al. A novel prognostic scoring model based on albumin and γ-glutamyltransferase for hepatocellular carcinoma prognosis[J]. Cancer Manag Res, 2019, 11: 10685-10694. DOI: 10.2147/CMAR.S232073. [24] GÜÇ ZG, ALACACıOǦLU A, KALENDER ME, et al. HALP score and GNRI: Simple and easily accessible indexes for predicting prognosis in advanced stage NSCLC patients. The İzmir oncology group (IZOG) study[J]. Front Nutr, 2022, 9: 905292. DOI: 10.3389/fnut.2022.905292. [25] JANSSEN EM, LEMMENS EE, WOLFE T, et al. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes[J]. Nature, 2003, 421(6925): 852-856. DOI: 10.1038/nature01441. [26] KENNEDY R, CELIS E. Multiple roles for CD4+ T cells in anti-tumor immune responses[J]. Immunol Rev, 2008, 222: 129-144. DOI: 10.1111/j.1600-065X.2008.00616.x. [27] GAY LJ, FELDING-HABERMANN B. Contribution of platelets to tumour metastasis[J]. Nat Rev Cancer, 2011, 11(2): 123-134. DOI: 10.1038/nrc3004. [28] LABELLE M, BEGUM S, HYNES RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis[J]. Cancer Cell, 2011, 20(5): 576-590. DOI: 10.1016/j.ccr.2011.09.009. [29] CICHOŻ -LACH H, CELŃSKI K, PROZOROW-KRÓL B, et al. The BARD score and the NAFLD fibrosis score in the assessment of advanced liver fibrosis in nonalcoholic fatty liver disease[J]. Med Sci Monit, 2012, 18(12): CR735-740. DOI: 10.12659/msm.883601. [30] XU XS, WAN Y, SONG SD, et al. Model based on γ-glutamyltransferase and alkaline phosphatase for hepatocellular carcinoma prognosis[J]. World J Gastroenterol, 2014, 20(31): 10944-10952. DOI: 10.3748/wjg.v20.i31.10944. [31] WU SJ, LIN YX, YE H, et al. Prognostic value of alkaline phosphatase, gamma-glutamyl transpeptidase and lactate dehydrogenase in hepatocellular carcinoma patients treated with liver resection[J]. Int J Surg, 2016, 36(Pt A): 143-151. DOI: 10.1016/j.ijsu.2016.10.033. [32] GUO C, LIANG H, YUAN W, et al. Analysis on the value of soluble intercellular adhesion molecule-1 (sICAM-1), alpha fetoprotein (AFP), and aspartate aminotransferase/platelet ratio index (APRI) in predicting the prognostic survival of patients with primary liver cancer after radiofrequency ablation[J]. Ann Palliat Med, 2021, 10(4): 4760-4767. DOI: 10.21037/apm-21-749. [33] CHUN YH, KIM SU, PARK JY, et al. Prognostic value of the 7th edition of the AJCC staging system as a clinical staging system in patients with hepatocellular carcinoma[J]. Eur J Cancer, 2011, 47(17): 2568-2575. DOI: 10.1016/j.ejca.2011.07.002. [34] MAI RY, BAI T, LUO XL, et al. Preoperative fibrinogen-to-albumin ratio predicts the prognosis of patients with hepatocellular carcinoma subjected to hepatectomy[J]. BMC Gastroenterol, 2022, 22(1): 261. DOI: 10.1186/s12876-022-02328-4. -



 PDF下载 ( 2967 KB)
PDF下载 ( 2967 KB)

 下载:
下载: