沙棘熊果酸通过调节线粒体-细胞色素c抑制酒精性肝病大鼠模型肝细胞凋亡的作用分析
DOI: 10.3969/j.issn.1001-5256.2023.07.016
Ursolic acid in Hippophae rhamnoides L. inhibits hepatocyte apoptosis in rats with alcoholic liver disease by regulating mitochondria-cytochrome c
-
摘要:
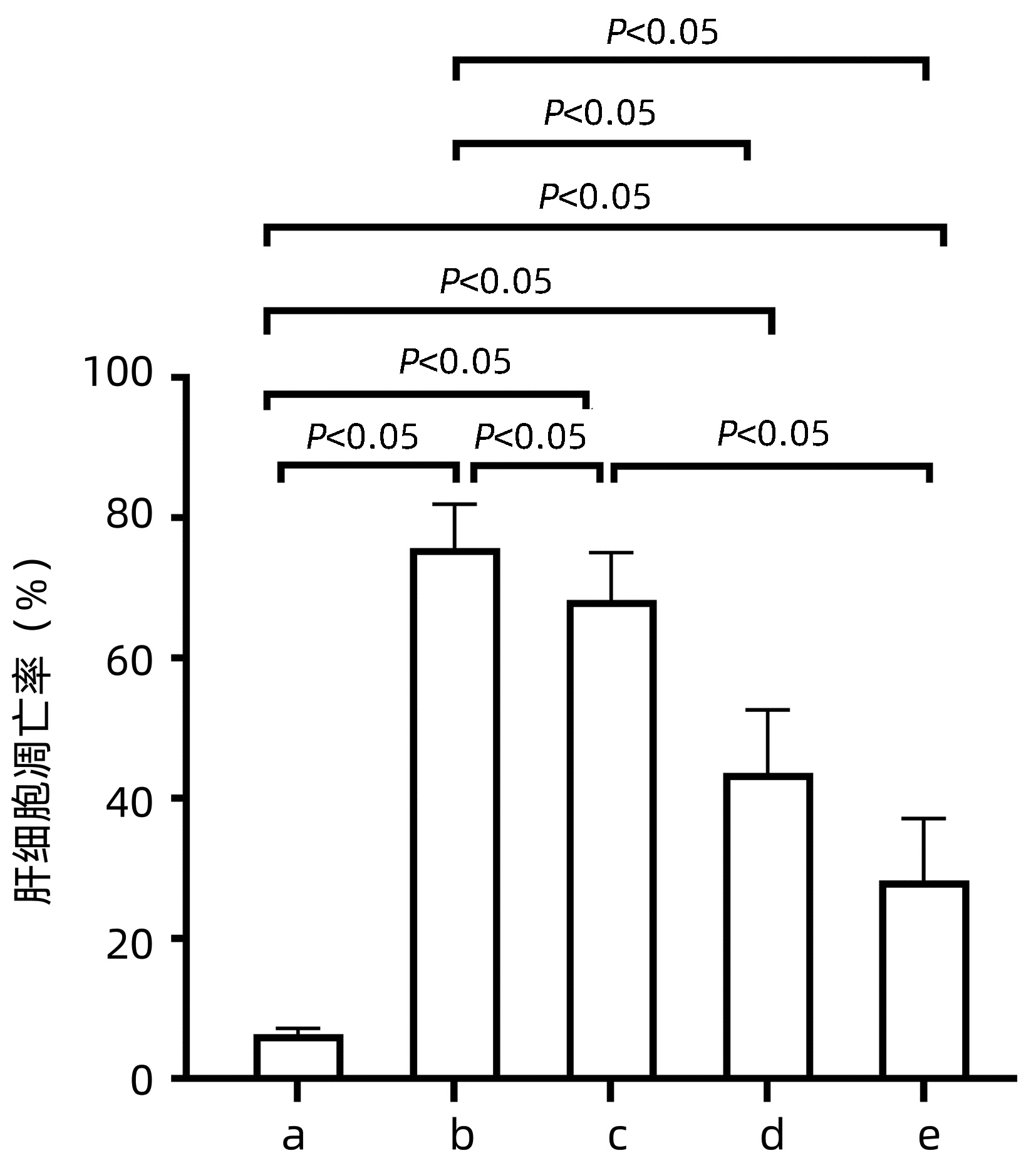
目的 基于线粒体-细胞色素c途径探讨沙棘熊果酸对酒精性肝病大鼠肝细胞凋亡的抑制作用。 方法 根据随机数字表将50只SPF级雄性Wistar大鼠进行完全随机分组,分为正常对照组、酒精模型组、沙棘熊果酸低、中和高剂量组,每组10只。正常对照组给予每日1次生理盐水灌胃8周;酒精模型组用阶梯式浓度酒精灌胃的方法持续灌胃8周;沙棘熊果酸组分别按50 mg/kg、100 mg/kg和150 mg/kg灌胃,1 h后再灌喂模型组同等剂量酒精。测定各组大鼠血清肝功能指标;HE染色观察肝组织病理情况;电镜下观察大鼠肝细胞超微结构;TUNEL法检测大鼠肝细胞凋亡情况;Western Blot法检测肝细胞线粒体和胞浆细胞色素c和活化caspase-3蛋白表达水平。计量资料多组间比较采用单因素方差分析,进一步两两比较采用LSD-t检验。 结果 与酒精模型组相比,沙棘熊果酸中、高剂量组大鼠血清ALT、AST和胆碱酯酶水平均下降(P值均<0.05);酒精模型组大鼠肝细胞索排列紊乱,肝细胞水肿、脂肪变性明显,而沙棘熊果酸中、高剂量组大鼠肝细胞索排列逐渐趋于正常、肝脂肪变性明显改善;肝细胞线粒体数目增加、形态明显改善;肝细胞凋亡率、胞浆细胞色素c和活化caspase-3蛋白的表达均低于酒精模型组(P值均<0.05)。 结论 沙棘熊果酸可改善酒精性肝病大鼠肝功能和肝组织形态,可能与抑制肝细胞线粒体细胞色素c的释放及caspase-3蛋白的活化,通过线粒体-细胞色素c途径抑制肝细胞凋亡有关。 Abstract:Objective To investigate the inhibitory effect of ursolic acid in Hippophae rhamnoides L. on hepatocyte apoptosis in rats with alcoholic liver disease based on the mitochondria-cytochrome c pathway. Methods A total of 50 specific pathogen-free male Wistar rats were divided into normal control group, alcohol model group, and low-, middle-, and high-dose ursolic acid groups using a random number table, with 10 rats in each group. The rats in the normal control group were given normal saline by gavage once a day for 8 weeks; the rats in the alcohol model group were given alcohol at increasing concentrations by gavage for 8 consecutive weeks; the rats in the low-, middle-, and high-dose ursolic acid groups were given ursolic acid at a dose of 50, 100, and 150 mg/kg, respectively, followed by an equal volume of alcohol as the model group 1 hour later. Serum liver function parameters were measured for each group; HE staining was used to observe liver histopathology; an electron microscope was used to observe hepatocyte ultrastructure; the TUNEL method was used to measure hepatocyte apoptosis; Western Blotting was used to measure the protein expression levels of cytochrome c and activated caspase-3 in hepatocyte mitochondria and cytoplasm. A one-way analysis of variance was used for comparison of continuous data between multiple groups, and the least significant difference t-test was used for further comparison between two groups. Results Compared with the alcohol model group, the middle- and high-dose ursolic acid groups had significant reductions in the serum level of alanine aminotransferase, aspartate aminotransferase, and cholinesterase (all P < 0.05). The rats in the alcohol model group had disordered arrangement of hepatic cords with marked hepatocyte edema and fatty degeneration, while those in the middle- and high- dose ursolic acid groups had basically normal arrangement of hepatic cords and a significant improvement in hepatocyte fatty degeneration, as well as a significant increase in the number of hepatocyte mitochondria and a significant improvement in morphology. Compared with the alcohol model group, the middle- and high-dose ursolic acid groups had significantly lower hepatocyte apoptosis rate and protein expression levels of cytochrome c and caspase-3 in cytoplasm (all P < 0.05). Conclusion Ursolic acid in Hippophae rhamnoides L. can improve the liver function and histomorphology of rats with alcoholic liver disease, possibly by inhibiting the release of cytochrome c in hepatocyte mitochondria, the activation of caspase-3, and the apoptosis of hepatocytes via the mitochondria-cytochrome c pathway. -
Key words:
- Liver Diseases, Alcoholic /
- Hippophae Fructus /
- Apoptosis /
- Mitochondria /
- Cytochromes c
-
表 1 沙棘UA对ALD大鼠血清ALT、AST和ChE水平的影响
Table 1. Effects of UA on level of ALT、AST、ChE in ALD rats serum
组别 动物数(只) ALT (U/L) AST (U/L) ChE (U/L) 正常对照组 10 45.88±6.73 88.14±11.89 291.69±65.95 酒精模型组 10 77.75±8.701) 125.14±16.421) 435.44±82.171) UA低剂量组 10 77.88±13.211) 141.00±14.041) 382.85±66.391) UA中剂量组 10 62.75±11.652)3) 102.86±5.872)3) 323.69±63.092) UA高剂量组 10 60.25±8.612)3) 91.28±12.292)3) 319.52±95.582) F值 11.050 10.316 4.095 P值 <0.001 <0.001 0.009 注:与正常对照组比较,1)P<0.05;与酒精模型组比较,2)P<0.05;与UA低剂量组比较,3)P<0.05。 -
[1] GONG Y, ZHANG X, HE L, et al. Optimization of subcritical water extraction parameters of antioxidant polyphenols from sea buckthorn (Hippophaë rhamnoides L. ) seed residue[J]. J Food Sci Technol, 2015, 52(3): 1534-1542. DOI: 10.1007/s13197-013-1115-7. [2] TURAN MI, AKTAŞ M, GUNDOGDU B, et al. The effect of Hippophae rhamnoides L. extract on acrylamideinduced brain injury in rats[J]. Acta Cir Bras, 2021, 36(10): e361005. DOI: 10.1590/ACB361005. [3] WEI ZC, TONG D, YANG J, et al. Action mechanism of total flavonoids of Hippophae rhamnoides in treatment of myocardial ischemia based on network pharmacology[J]. China J Chin Mater Med, 2017, 42(7): 1238-1244. DOI: 10.19540/j.cnki.cjcmm.20161222.077.魏志成, 童东, 杨娟, 等. 基于网络药理学的沙棘总黄酮治疗心肌缺血的作用机制研究[J]. 中国中药杂志, 2017, 42(7): 1238-1244. DOI: 10.19540/j.cnki.cjcmm.20161222.077. [4] PUNDIR S, GARG P, DVIWEDI A, et al. Ethnomedicinal uses, phytochemistry and dermatological effects of Hippophae rhamnoides L. : A review[J]. J Ethnopharmacol, 2021, 266: 113434. DOI: 10.1016/j.jep.2020.113434. [5] KWON EY, LEE J, KIM YJ, et al. Seabuckthorn leaves extract and flavonoid glycosides extract from seabuckthorn leaves ameliorates adiposity, hepatic steatosis, insulin resistance, and inflammation in diet-induced obesity[J]. Nutrients, 2017, 9(6): 569. DOI: 10.3390/nu9060569. [6] TAN J, HUANG W, CHEN SL, et al. Synthesis and anti-inflammatory activity of ursolic acid derivative-chalcone conjugate[J]. Acta Pharm Sin, 2016, 51(6): 938-946. DOI: 10.16438/j.0513-4870.2015-1162.谭娟, 黄微, 陈善龙, 等. 熊果酸衍生物与查耳酮缀合物的合成及抗炎活性[J]. 药学学报, 2016, 51(6): 938-946. DOI: 10.16438/j.0513-4870.2015-1162. [7] GAO JG. Study on the protective effect of ursolic acid on nonalcoholic fatty liver in rats[J]. Pharmocol Clin Chin Mater Med, 2016, 32(2): 27-31. DOI: 10.13412/j.cnki.zyyl.2016.02.009.高敬国. 熊果酸对非酒精性脂肪肝大鼠肝脏的保护作用及机制[J]. 中药药理与临床, 2016, 32(2): 27-31. DOI: 10.13412/j.cnki.zyyl.2016.02.009. [8] KALINOWSKA M, BIELAWSKA A, LEWANDOWSKA-SIWKIEWICZ H, et al. Apples: content of phenolic compounds vs. variety, part of apple and cultivation model, extraction of phenolic compounds, biological properties[J]. Plant Physiol Biochem, 2014, 84: 169-188. DOI: 10.1016/j.plaphy.2014.09.006. [9] IQBAL J, ABBASI BA, AHMAD R, et al. Ursolic acid a promising candidate in the therapeutics of breast cancer: Current status and future implications[J]. Biomed Pharmacother, 2018, 108: 752-756. DOI: 10.1016/j.biopha.2018.09.096. [10] LIANG KY, CHU X. Effects of ursolic acid on cholesterol metabolism in hepatic cells[J]. Herald Med, 2017, 36(1) : 9-12. DOI: 10.3870/j.issn.1004-0781.2017.01.002.梁奎英, 初霞. 熊果酸对肝细胞胆固醇代谢的影响[J]. 医药导报, 2017, 36(01): 9-12. DOI: 10.3870/j.issn.1004-0781.2017.01.002. [11] YANG XL. The effects of urosolic acid on blood lipid and hemorheology in rats with atherosclerosis[J]. Chin J Integr Med Cardio-Cereb Dis, 2018, 16(11): 1509-1512. DOI: 10.12102/j.issn.1672-1349.2018,11.011.杨晓龙. 熊果酸对动脉粥样硬化大鼠血脂和血液流变学的影响[J]. 中西医结合心脑血管病杂志, 2018, 16(11): 1509-1512. DOI: 10.12102/j.issn.1672-1349.2018,11.011. [12] NIE Y, LIU Q, ZHANG W, et al. Ursolic acid reverses liver fibrosis by inhibiting NOX4/NLRP3 inflammasome pathways and bacterial dysbiosis[J]. Gut Microbes, 2021, 13(1): 1972746. DOI: 10.1080/19490976.2021.1972746. [13] MA XY, ZHANG M, FANG G, et al. Ursolic acid reduces hepatocellular apoptosis and alleviates alcohol-induced liver injury via irreversible inhibition of CASP3 in vivo[J]. Acta Pharmacol Sin, 2021, 42(7): 1101-1110. DOI: 10.1038/s41401-020-00534-y. [14] ZHANG NN, GE N. Research progress on the protective effect of ursolic acid on experimental liver injury[J]. Acta Med Sin, 2018, 31(4): 173-177. DOI: 10.19296/j.cnki.1008-2409.2018-04-056.张男男, 戈娜. 熊果酸对实验性肝损伤的保护作用研究进展[J]. 华夏医学, 2018, 31(4): 173-177. DOI: 10.19296/j.cnki.1008-2409.2018-04-056. [15] TORRUELLAS C, FRENCH SW, MEDICI V. Diagnosis of alcoholic liver disease[J]. World J Gastroenterol, 2014, 20(33): 11684-11699. DOI: 10.3748/wjg.v20.i33.11684. [16] CHENG J, LIU Y, LIU Y, et al. Ursolic acid alleviates lipid accumulation by activating the AMPK signaling pathway in vivo and in vitro[J]. J Food Sci, 2020, 85(11): 3998-4008. DOI: 10.1111/1750-3841.15475. [17] QIAO JY, ZANG YC, MIAO YY, et al. Protective effects and mechanisms of ursolic acid on acute alcohol-induced liver injury in mice[J]. Pharmocol Clin Chin Mater Med, 2017, 33(4): 14-17. DOI: 10.13412/j.cnki.zyyl.2017.04.004.乔靖怡, 臧云彩, 苗艳艳, 等. 熊果酸对小鼠急性酒精性肝损伤的保护作用及机制[J]. 中药药理与临床, 2017, 33(4): 14-17. DOI: 10.13412/j.cnki.zyyl.2017.04.004. [18] YE QY, LIN HJ, CHEN JH, et al. Protective effect and mechanism of ursolic acid on rats with alcoholic liver injury[J]. Shandong Med J, 2018, 58(41): 14-17. DOI: 10.3969/j.issn.1002-266X.2018.41.004.叶泉英, 林浩佳, 陈金慧, 等. 熊果酸对酒精性肝损伤大鼠的保护作用及机制[J]. 山东医药, 2018, 58(41): 14-17. DOI: 10.3969/j.issn.1002-266X.2018.41.004. [19] GE N, LIANG H, LIU Y, et al. Effects of Aplysin on ultrastructure, NO and iNOS in rats with chronic alcoholic liver injury[J]. Chin J Marine Drugs, 2014, 33(3): 63-68. DOI: 10.13400/j.cnki.cjmd.2014.03.010.戈娜, 梁惠, 刘颖, 等. 海兔素对慢性酒精性肝损伤大鼠肝超微结构及NO和iNOS的影响[J]. 中国海洋药物, 2014, 33(3): 63-68. DOI: 10.13400/j.cnki.cjmd.2014.03.010. [20] XIE YD. People with liver disease have many drinkers and are harmful[J]. Liver Doctor, 2021, 20(1): 47-48. https://www.cnki.com.cn/Article/CJFDTOTAL-GBSH202101021.htm谢艳迪. 肝病患者的饮酒者多危害大[J]. 肝博士, 2021, 20(1): 47-48. https://www.cnki.com.cn/Article/CJFDTOTAL-GBSH202101021.htm [21] ZHANG Y, HAN C, WANG ZX, et al. Protective effects of Tamarix chinensis Lour on mice with alcoholic liver injury and its mechanism[J]. J Shandong Univ(Health Sciences), 2017, 55(2): 61-67. DOI: 10.6040/j.issn.1671-7554.0.2016.995.张钰, 韩琛, 王朝霞, 等. 柽柳对小鼠酒精性肝损伤的保护作用及机制[J]. 山东大学学报(医学版), 2017, 55(2): 61-67. DOI: 10.6040/j.issn.1671-7554.0.2016.995. [22] PI JT, WANG C, ZHANG JM, et al. Epidemiologic investigation of alcohol consumption and alcoholic liver disease among residents in the Tongzhou District of Beijing[J]. Chron Pathematol J, 2022, 23(5): 712-716. DOI: 10.16440/J.CNKI.1674-8166.2022.05.20.邳建庭, 王晨, 张建明, 等. 北京市通州区常住居民饮酒与酒精性肝病流行病学调查[J]. 慢性病学杂志, 2022, 23(5): 712-716. DOI: 10.16440/J.CNKI.1674-8166.2022.05.20. [23] YAN H, ZHANG FL, GAO YQ, et al. Epidemiological study on alcohol consumption and alcoholic liver disease[J]. Shaanxi Med J, 2015, 44(7): 917-918, 920. DOI: 10.3969/j.issn.1000-7377.2015.07.066.延华, 张粉利, 高艳琼, 等. 饮酒与酒精性肝病流行病学调查研究[J]. 陕西医学杂志, 2015, 44(7): 917-918, 920. DOI: 10.3969/j.issn.1000-7377.2015.07.066. [24] GUO YY, TAO MX, CHENG GY, et al. Protective effect of polysaccharides from boletus aereus on alcoholic liver injury in mice[J]. J Chin Insti Food Sci Technol, 2016, 16(1): 35-41. DOI: 10.16429/j.1009-7848.2016.01.005.郭永月, 陶眀煊, 程光宇, 等. 黑牛肝菌多糖对急性酒精肝损伤小鼠的保护作用[J]. 中国食品学报, 2016, 16(1): 35-41. DOI: 10.16429/j.1009-7848.2016.01.005. [25] KIM MH, KIM JN, HAN SN, et al. Ursolic acid isolated from guava leaves inhibits inflammatory mediators and reactive oxygen species in LPS-stimulated macrophages[J]. Immunopharmacol Immunotoxicol, 2015, 37(3): 228-235. DOI: 10.3109/08923973.2015.1021355. [26] SID B, VERRAX J, CALDERON PB. Role of oxidative stress in the pathogenesis of alcohol-induced liver disease[J]. Free Radic Res, 2013, 47(11): 894-904. DOI: 10.3109/10715762.2013.819428. [27] WU Y, LI YR, YANG JZ, et al. Research advances in the pathogenesis of alcoholic liver disease[J]. J Clin Hepatol, 2020, 36(12): 2822-2825. DOI: 10.3969/j.issn.1001-5256.2020.12.038.吴亚, 李艳茹, 杨寄镯, 等. 酒精性肝病发病机制研究现状[J]. 临床肝胆病杂志, 2020, 36(12): 2822-2825. DOI: 10.3969/j.issn.1001-5256.2020.12.038. [28] BJØRKHAUG ST, AANES H, NEUPANE SP, et al. Characterization of gut microbiota composition and functions in patients with chronic alcohol overconsumption[J]. Gut Microbes, 2019, 10(6): 663-675. DOI: 10.1080/19490976.2019.1580097. [29] POTZ BA, LAWANDY IJ, CLEMENTS RT, et al. Alcohol modulates autophagy and apoptosis in pig liver tissue[J]. J Surg Res, 2016, 203(1): 154-162. DOI: 10.1016/j.jss.2016.03.009. [30] LI SQ, LU HJ, WANG P, et al. Study on the time of cell apoptosis in alcoholic liver injury in mice[J]. Chin J Clin Pharmacol, 2017, 33(21): 2154-2157. DOI: 10.13699/j.cnki.1001-6821.2017.21.018.李三强, 卢华杰, 王萍, 等. 酒精性肝损伤小鼠在损伤过程中细胞凋亡时间点研究[J]. 中国临床药理学杂志, 2017, 33(21): 2154-2157. DOI: 10.13699/j.cnki.1001-6821.2017.21.018. [31] ZHANG Y, WANG C, YU B, et al. Gastrodin protects against ethanol-induced liver injury and apoptosis in HepG2 cells and animal models of alcoholic liver disease[J]. Biol Pharm Bull, 2018, 41(5): 670-679. DOI: 10.1248/bpb.b17-00825. [32] ZIOL M, TEPPER M, LOHEZ M, et al. Clinical and biological relevance of hepatocyte apoptosis in alcoholic hepatitis[J]. J Hepatol, 2001, 34(2): 254-260. DOI: 10.1016/s0168-8278(00)00047-7. [33] WANG J. A study on the mechanism of NINJ2 regulating the alcoholic liver disease[D]. Wuhan: Huazhong University of Science and Technology, 2021.王晶. NINJ2调控酒精性肝病机制研究[D]. 武汉: 华中科技大学, 2021. [34] LANG F, HOFFMANN EK. Role of ion transport in control of apoptotic cell death[J]. Compr Physiol, 2012, 2(3): 2037-2061. DOI: 10.1002/cphy.c110046. [35] GROSSINI E, POLLESELLO P, BELLOFATTO K, et al. Protective effects elicited by levosimendan against liver ischemia/reperfusion injury in anesthetized rats[J]. Liver Transpl, 2014, 20(3): 361-375. DOI: 10.1002/lt.23799. [36] KATAYAMA S, SHIMODA K, TAKENAGA Y. Loss of ADAR1 in human iPS cells promotes caspase3-mediated apoptotic cell death[J]. Genes Cells, 2015, 20(8): 675-680. DOI: 10.1111/gtc.12261. [37] PLAPP BV, LEIDAL KG, MURCH BP, et al. Contribution of liver alcohol dehydrogenase to metabolism of alcohols in rats[J]. Chem Biol Interact, 2015, 234: 85-95. DOI: 10.1016/j.cbi.2014.12.040. [38] HAN D, JOHNSON HS, RAO MP, et al. Mitochondrial remodeling in the liver following chronic alcohol feeding to rats[J]. Free Radic Biol Med, 2017, 102: 100-110. DOI: 10.1016/j.freeradbiomed.2016.11.020. [39] CHU C, ZHAO YY, ZHOU CY, et al. Protective effect of mangiferin on alcoholic hepatitis in rats[J]. Nat Prod Res Dev, 2018, 30(5): 753-760. DOI: 10.16333/j.1001-6880.2018.5.005.楚策, 赵燕燕, 周程艳, 等. 芒果苷对大鼠酒精性肝炎的保护作用研究[J]. 天然产物研究与开发, 2018, 30(5): 753-760. DOI: 10.16333/j.1001-6880.2018.5.005. [40] ZHU PS, JIAO YJ, FU SN, et al. Changes of serum biomarkers levels in early stage of alcohol-induced liver injury in rats[J]. Chin J Exp Med Formul, 2019, 25(2): 129-133. DOI: 10.13422/j.cnki.syfjx.20190223.朱平生, 焦炎杰, 付双楠, 等. 酒精致大鼠肝损伤早期血清生物标志物水平的变化规律[J]. 中国实验方剂学杂志, 2019, 25(2): 129-133. DOI: 10.13422/j.cnki.syfjx.20190223. [41] SHEWEITA SA, ABD EL-GABAR M, BASTAWY M. Carbon tetrachloride-induced changes in the activity of phase Ⅱ drug-metabolizing enzyme in the liver of male rats: role of antioxidants[J]. Toxicology, 2001, 165(2-3): 217-224. DOI: 10.1016/s0300-483x(01)00429-2. [42] XU B, LI Y, JI PY, et al. Study on mechanism of total flavonoids from Hemerocallis fulva on oxidative stress and hepatocyte apoptosis in alcoholic liver injury[J]. Chongqing Med, 2017, 46(10): 1304-1307. DOI: 10.3969/j.issn.1671-8348.2017.10.003.徐博, 李妍, 纪朋艳, 等. 萱草花黄酮对酒精性肝损伤氧化应激及肝细胞凋亡机制的探讨[J]. 重庆医学, 2017, 46(10): 1304-1307. DOI: 10.3969/j.issn.1671-8348.2017.10.003. [43] XU GF, WANG XY, GE GL, et al. Dynamic changes of capillarization and peri-sinusoid fibrosis in alcoholic liver diseases[J]. World J Gastroenterol, 2004, 10(2): 238-243. DOI: 10.3748/wjg.v10.i2.238. [44] AN WW, WANG MW, TASHIRO S, et al. Norcantharidin induces human melanoma A375-S2 cell apoptosis through mitochondrial and caspase pathways[J]. J Korean Med Sci, 2004, 19(4): 560-566. DOI: 10.3346/jkms.2004.19.4.560. [45] CAO XH, ZHAO SS, LIU DY, et al. ROS-Ca(2+) is associated with mitochondria permeability transition pore involved in surfactin-induced MCF-7 cells apoptosis[J]. Chem Biol Interact, 2011, 190(1): 16-27. DOI: 10.1016/j.cbi.2011.01.010. -



 PDF下载 ( 4153 KB)
PDF下载 ( 4153 KB)


 下载:
下载: