早期胸腔穿刺引流对重症急性胰腺炎急性肺损伤的影响
DOI: 10.3969/j.issn.1001-5256.2023.07.018
Effect of early thoracic paracentesis drainage on acute lung injury in severe acute pancreatitis
-
摘要:
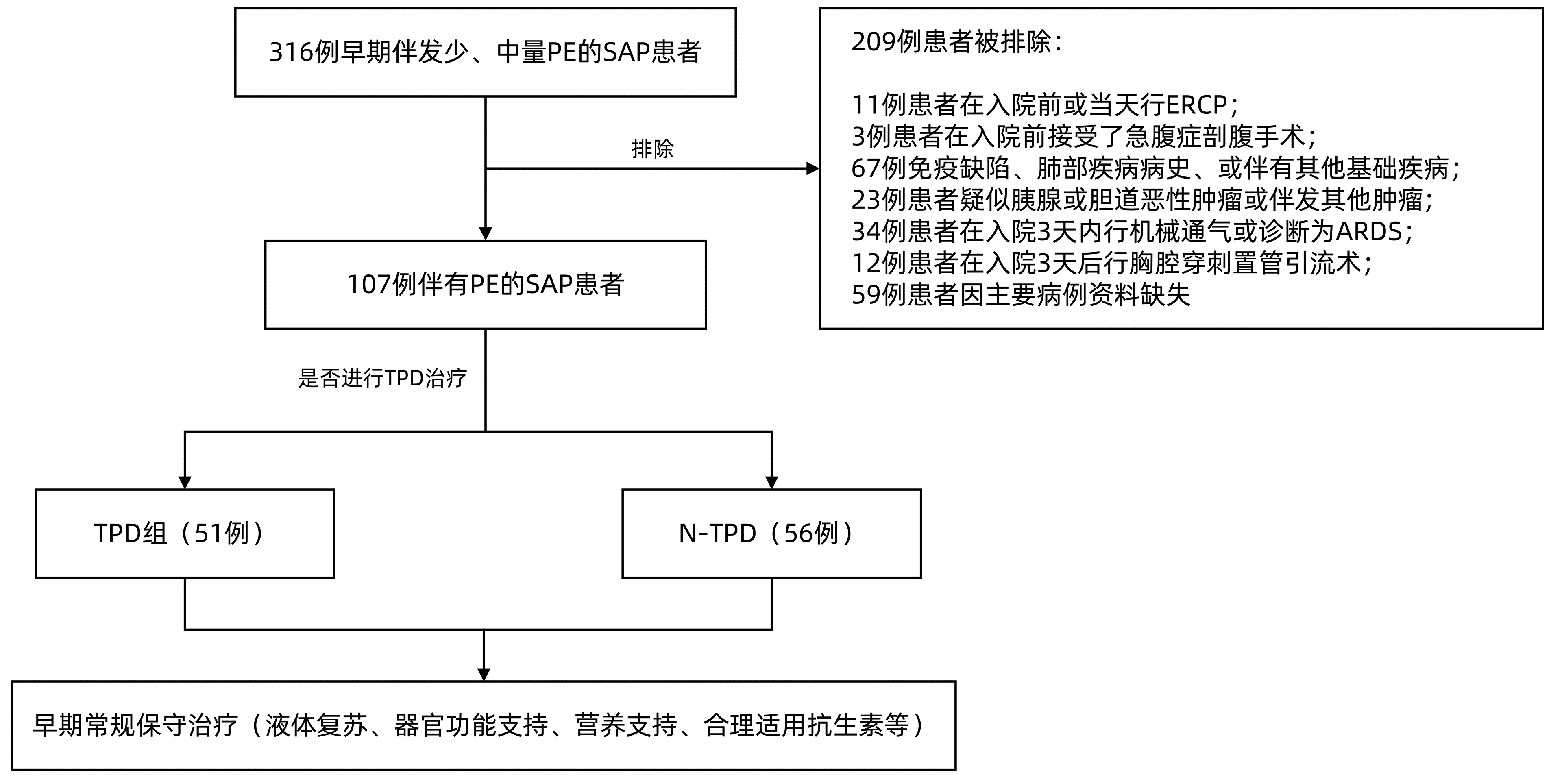
目的 探讨重症急性胰腺炎(SAP)伴发的少、中量胸腔积液早期穿刺引流对SAP患者急性肺损伤的影响。 方法 回顾性分析中国人民解放军西部战区总医院2015年1月-2021年12月收治的107例SAP患者临床资料, 根据入院后前3天是否进行胸腔穿刺置管引流, 分为胸腔穿刺置管引流组(TPD组, 51例)和非胸腔穿刺置管引流组(N-TPD组, 56例)。分别比较入院后第5天、第10天两组相应时间节点实验室指标及临床结局。正态分布或近似正态分布的计量资料两组间比较采用成组t检验, 非正态分布的计量资料两组间比较采用Mann-Whitney U检验, 计数资料两组间比较采用χ2检验。 结果 TPD组重症监护病房住院时间、总住院时间、住院费用明显少于N-TPD组(P值均 < 0.05);而TPD组与N-TPD组患者病死率(9.8% vs 14.3%, χ2=0.502, P=0.478)、脓毒症发生率(29.4% vs 44.6%, χ2=2.645, P=0.104)比较差异无统计学意义。TPD组急性呼吸窘迫综合征(ARDS)发生率明显降低(χ2=6.038, P=0.043), 且中度ARDS发生率显著低于N-TPD组, 差异有统计学意义(7.8% vs 21.4%, χ2=3.874, P=0.049)。TPD组患者机械通气使用率明显低于N-TPD组(35.3% vs 57.2%, χ2=6.735, P=0.034), 且有创机械通气患者, TPD组明显低于N-TPD组(9.8% vs 26.8%, χ2=5.065, P=0.024)。TPD组肺部感染发生率显著低于N-TPD组(23.5% vs 42.9%, χ2=4.466, P=0.035), TPD组全身炎症反应综合征持续时间缩短[(11.2±5.0) d vs (16.8±4.7) d, t=5.949, P < 0.001]。在入院后第5、10天, TPD组患者实验室指标(超敏C反应蛋白、IL-1、IL-6、IL-8和TNF-α、动脉血气氧分压、氧饱和度、氧合指数)、呼吸衰竭发生率均显著优于N-TPD组(P值均 < 0.05)。入院后第10天, TPD组APACHE Ⅱ评分、改良Mashall评分均显著优于N-TPD组(P值均 < 0.05)。 结论 对于SAP伴发少、中量胸腔积液患者, 早期进行胸腔穿刺引流, 可有效改善患者的急性肺损伤, 减轻全身炎症反应, 缩短住院时间, 减少住院费用。 Abstract:Objective To investigate the effect of early thoracic paracentesis drainage for pleural effusion with a small or moderate volume on acute lung injury in patients with severe acute pancreatitis (SAP). Methods A retrospective analysis was performed for the clinical data of 107 patients with SAP who were admitted to The General Hospital of Western Theater Command from January 2015 to December 2021, and according to whether thoracic paracentesis drainage was performed within the first three days after admission, the patients were divided into thoracic paracentesis drainage group (TPD group with 51 patients) and non-thoracic paracentesis drainage group (N-TPD group with 56 patients).The two groups were compared in terms of laboratory markers and clinical outcome on days 5 and 10 after admission.The independent-samples t test was used for comparison of normally distributed continuous data between two groups, and the Mann-Whitney U test was used for comparison of non-normally distributed continuous data between two groups; the chi-square test was used for comparison of categorical data between two groups. Results Compared with the N-TPD group, the TPD group had a significantly shorter length of stay in the intensive care unit, a significantly shorter length of hospital stay, and significantly lower hospital costs (all P < 0.05), while there were no significant differences between the TPD group and the N-TPD group in mortality rate (9.8% vs 14.3%, χ2=0.502, P=0.478) and the incidence rate of sepsis (29.4% vs 44.6%, χ2=2.645, P=0.104).The TPD group had a significant reduction in the incidence rate of acute respiratory distress syndrome (ARDS)(χ2=6.038, P=0.043), as well as a significantly lower incidence rate of moderate ARDS than the N-TPD group (7.8% vs 21.4%, χ2=3.874, P=0.049).Compared with the N-TPD group, the TPD group had a significantly lower rate of use of mechanical ventilation (35.3% vs 57.2%, χ2=6.735, P=0.034) and a significantly lower proportion of patients receiving invasive mechanical ventilation (9.8% vs 26.8%, χ2=5.065, P=0.024).Compared with the N-TPD group, the TPD group had a significantly lower incidence rate of pulmonary infection (23.5% vs 42.9%, χ2=4.466, P=0.035) and a significantly shorter duration of systemic inflammatory response syndrome (11.2±5.0 days vs 16.8±4.7 days, t=5.949, P < 0.001).Compared with the N-TPD group, the TPD group had significantly better laboratory markers (high-sensitivity C-reactive protein, interleukin-1, interleukin-6, interleukin-8, tumor necrosis factor-α, arterial partial pressure of oxygen, oxygen saturation, and oxygenation index) and incidence rate of respiratory failure on days 5 and 10 after admission (all P < 0.05).On day 10 after admission, the TPD group had significantly better APACHE Ⅱ score and modified Mashall score than the N-TPD group (both P < 0.05). Conclusion For SAP patients with a small or moderate volume of pleural effusion, early thoracic paracentesis drainage can effectively improve acute lung injury, alleviate systemic inflammatory response, shorten the length of hospital stay, and reduce hospital costs. -
Key words:
- Pancreatitis /
- Pleural Effusion /
- Drainage /
- Acute Lung Injury /
- Systemic Inflammatory Response Syndrome
-
表 1 两组患者基线资料比较
Table 1. Baseline patient characteristics between two groups
项目 TPD组(n=51) N-TPD组(n=56) 统计值 P值 年龄(岁) 52.3±14.0 51.2±11.3 t=0.417 0.678 性别[例(%)] χ2=0.117 0.846 男 29(56.9) 30(53.6) 女 22(43.1) 26(46.4) 病因[例(%)] χ2=0.140 0.932 胆源型 22(43.1) 25(44.6) 高脂血症型 19(37.3) 19(33.9) 其他 10(19.6) 12(21.4) 炎症指标 WBC(×109/L) 16.4±3.8 16.5±4.2 t=0.065 0.948 N%(%) 89.8±4.7 88.6±3.9 t=1.432 0.155 HS-CRP(mg/L) 235.9±52.6 241.3±47.5 t=0.550 0.583 血沉(mm/h) 55.3±20.7 52.1±21.6 t=0.592 0.439 PCT(ng/mL) 1.8(0.9~4.7) 2.3(1.0~4.2) Z=0.873 0.383 细胞因子(pg/mL) IL-1 15.1±6.6 15.9±5.6 t=0.645 0.515 IL-6 187.2±62.3 205.3±56.4 t=1.580 0.117 IL-8 79.9±63.9 89.4±59.9 t=0.791 0.431 TNF-α 22.9±3.6 21.7±5.1 t=1.378 0.171 血气指标 PaO2(mmHg) 68.2±8.3 69.8±8.1 t=1.042 0.300 PaCO2(mmHg) 36.3±4.2 36.2±5.4 t=0.077 0.939 氧饱和度(%) 87.6±3.7 86.8±4.0 t=1.127 0.262 OI 200.4±29.3 202.3±29.7 t=0.327 0.744 乳酸(mmol/L) 2.4±1.7 2.2±1.1 t=0.587 0.558 pH 7.38±0.58 7.37±0.59 t=0.628 0.532 RR(次/min) 29.9±3.6 30.1±3.7 t=0.164 0.870 APACHⅡ评分(分) 16.7±3.2 16.6±3.5 t=0.117 0.907 改良MarshaⅡ评分(分) 4.2±1.2 4.1±1.4 t=0.344 0.731 表 2 2组患者临床结局比较
Table 2. Clinical outcomes between two groups
项目 TPD组(n=51) N-TPD组(n=56) 统计值 P值 疾病特异病死率[例(%)] 5(9.8) 8(14.3) χ2=0.502 0.478 ARDS[例(%)] χ2=6.038 0.043 无 35(68.6) 26(46.4) 轻度 12(23.5) 18(32.1) 中度 4(7.8) 12(21.4) 机械通气[例(%)] χ2=6.735 0.034 无 33(64.7) 24(42.9) 无创通气 13(25.5) 17(30.4) 有创通气 5(9.8) 15(26.8) 肺部感染发生率[例(%)] 12(23.5) 24(42.9) χ2=4.466 0.035 脓毒症发生率[例(%)] 15(29.4) 25(44.6) χ2=2.645 0.104 机械通持续时间(d) 7.2±2.4 9.5±4.2 t=2.155 0.036 氧疗持续时间(d) 16.6±2.7 20.5±4.8 t=5.100 <0.001 SRIS持续时间(d) 11.2±5.0 16.8±4.7 t=5.949 <0.001 ICU住院时间(d) 9.2±4.6 11.7±6.1 t=2.384 0.019 总住院时间(d) 30.1±9.7 37.3±10.3 t=3.280 0.001 总费用(元) 72 247±27 314 85 735±34 186 t=2.207 0.029 表 3 入院后约5天和约10天两组实验室指标及临床评估比较
Table 3. Laboratory and clinical parameters between two groups 5 days and 10 days after admission
项目 入院后约5天 入院后约10天 TPD组
(n=51)N-TPD组
(n=56)统计值 P值 TPD组
(n=51)N-TPD组
(n=56)统计值 P值 炎症指标 WBC(×109/L) 15.7±2.9 16.3±4.1 t=0.873 0.384 12.6±6.1 14.8±5.4 t=2.046 0.040 N% 85.3±7.3 86.5±4.1 t=1.403 0.164 77.3±9.5 82.1±7.7 t=2.906 0.004 HS-CRP(mg/L) 173.8±51.1 225.4±54.8 t=5.018 <0.001 119.5±64.7 159.2±84.2 t=2.715 0.008 PCT(ng/mL) 3.9(2.4~7.3) 4.7(2.7~13.3) Z=0.986 0.324 1.5(0.5~4.1) 3.2(1.4~11.7) Z=2.532 0.011 细胞因子(pg/mL) IL-1 11.1±4.2 14.7±5.0 t=4.076 <0.001 10.7±7.0 14.5±6.5 t=2.889 0.005 IL-6 82.2±38.5 105.7±43.7 t=2.942 0.004 52.5±54.6 76.4±54.6 t=2.268 0.025 IL-8 40.1±24.2 64.0±26.2 t=4.884 <0.001 31.5±28.1 46.4±29.8 t=2.625 0.009 TNF-α 15.0±5.5 19.2±5.9 t=3.749 <0.001 14.0±6.2 17.1±6.4 t=2.533 0.010 血气指标 PaO2(mmHg) 84.9±10.2 80.8±9.7 t=2.104 0.038 89.9±9.3 84.84±11.4 t=2.509 0.014 PaCO2(mmHg) 38.3±3.7 37.2±3.8 t=1.505 0.135 37.6±2.7 37.1±2.9 t=1.006 0.317 氧饱和度(%) 95.1±2.3 90.5±4.2 t=6.967 <0.001 95.2±4.8 92.3±5.8 t=2.491 0.014 OI 269.1±47.8 246.3±49.5 t=2.421 0.017 299.0±47.7 270.9±51.4 t=2.918 0.004 乳酸(mmol/L) 1.4±0.7 1.5±0.6 t=0.311 0.756 1.3±0.5 1.4±0.7 t=0.985 0.327 pH 7.40±0.04 7.40±0.05 t=1.010 0.315 7.39±0.03 7.38±0.04 t=0.714 0.477 RR(次/min) 22.7±4.2 27.6±4.4 t=5.892 <0.001 19.6±4.3 23.5±5.8 t=3.933 <0.001 呼吸衰竭[例(%)] χ2=4.813 0.028 χ2=13.445 <0.001 无 22(43.1) 13(23.2) 38(74.5) 22(39.2) 有 29(59.6) 43(76.8) 13(25.5) 34(60.7) APACHEⅡ评分(分) 9.9±3.3 12.1±3.4 t=3.315 0.001 改良Marshall评分(分) 2.1±1.2 2.9±1.6 t=2.950 0.004 -
[1] GARDNER TB. Acute pancreatitis[J]. Ann Intern Med, 2021, 174(2): ITC17-ITC32. DOI: 10.7326/aitc202102160. [2] JIANG X, SHI JY, WANG XY, et al. The impacts of infectious complications on outcomes in acute pancreatitis: A retrospective study[J]. Mil Med Res, 2020, 7(1): 38. DOI: 10.1186/s40779-020-00265-5. [3] BANKS PA, BOLLEN TL, DERVENIS C, et al. Classification of acute pancreatitis—2012: Revision of the Atlanta classification and definitions by international consensus[J]. Gut, 2013, 62(1): 102-111. DOI: 10.1136/gutjnl-2012-302779. [4] CHELLIAH T, WERGE M, MERC AI, et al. Pulmonary dysfunction due to combination of extra-pulmonary causes and alveolar damage is present from first the day of hospital admission in the early phase of acute pancreatitis[J]. Pancreatology, 2019, 19(4): 519-523. DOI: 10.1016/j.pan.2019.04.009. [5] CAO JQ, TANG LJ. Research progress of systemic inflammatory response syndrome in acute pancreatitis-associated lung injury[J]. Chin J Dig Surg, 2015, 14(11): 975-979. DOI: 10.3760/cma.j.issn.1673-9752.2015.11.019.曹均强, 汤礼军. 全身炎症反应综合征在急性胰腺炎肺损伤中的研究进展[J]. 中华消化外科杂志, 2015, 14(11): 975-979. DOI: 10.3760/cma.j.issn.1673-9752.2015.11.019. [6] WAN ZH, ZENG L, ZHOU H, et al. Protective effect of polyphyllin Ⅶ on acute lung injury in rats with severe acute pancreatitis by inhibiting NF-κB signaling pathway[J]. J Jilin Univ(Med Edit), 2022, 48(3): 668-675. DOI: 10.13481/j.1671-587X.20220315.万朝辉, 曾良, 周辉, 等. 重楼皂苷Ⅶ通过抑制NF-κB信号通路对重症急性胰腺炎大鼠急性肺损伤的保护作用[J]. 吉林大学学报(医学版), 2022, 48(3): 668-675. DOI: 10.13481/j.1671-587X.20220315. [7] IYER H, ELHENCE A, MITTAL S, et al. Pulmonary complications of acute pancreatitis[J]. Expert Rev Respir Med, 2020, 14(2): 209-217. DOI: 10.1080/17476348.2020.1698951. [8] YAN GW, LI HW, BHETUWAL A, et al. Pleural effusion volume in patients with acute pancreatitis: A retrospective study from three acute pancreatitis centers[J]. Ann Med, 2021, 53(1): 2003-2018. DOI: 10.1080/07853890.2021.1998594. [9] CHOI HW, PARK HJ, CHOI SY, et al. Early prediction of the severity of acute pancreatitis using radiologic and clinical scoring systems with classification tree analysis[J]. AJR Am J Roentgenol, 2018, 211(5): 1035-1043. DOI: 10.2214/AJR.18.19545. [10] HUANG HL, CHEN WJ, TANG GD, et al. Optimal timing of contrast-enhanced computed tomography in an evaluation of severe acute pancreatitis-associated complications[J]. Exp Ther Med, 2019, 18(2): 1029-1038. DOI: 10.3892/etm.2019.7700. [11] BINTCLIFFE OJ, LEE GYC, RAHMAN NM, et al. The management of benign non-infective pleural effusions[J]. Eur Respir Rev, 2016, 25(141): 303-316. DOI: 10.1183/16000617.0026-2016. [12] LUIKEN I, EISENMANN S, GARBE J, et al. Pleuropulmonary pathologies in the early phase of acute pancreatitis correlate with disease severity[J]. PLoS One, 2022, 17(2): e0263739. DOI: 10.1371/journal.pone.0263739. [13] BROGI E, GARGANI L, BIGNAMI E, et al. Thoracic ultrasound for pleural effusion in the intensive care unit: A narrative review from diagnosis to treatment[J]. Crit Care, 2017, 21(1): 325. DOI: 10.1186/s13054-017-1897-5. [14] Pancreas Study Group, Chinese Society of Gastroenterology, Chinese Medical Association, Editorial Board of Chinese Journal of Pancreatology, Editorial Board of Chinese Journal of Digestion. Chinese guidelines for the management of acute pancreatitis (Shenyang, 2019)[J]. J Clin Hepatol, 2019, 35(12): 2706-2711. DOI: 10.3969/j.issn.1001-5256.2019.12.013.中华医学会消化病学分会胰腺疾病学组, 《中华胰腺病杂志》编委会, 《中华消化杂志》编委会. 中国急性胰腺炎诊治指南(2019年, 沈阳)[J]. 临床肝胆病杂志, 2019, 35(12): 2706-2711. DOI: 10.3969/j.issn.1001-5256.2019.12.013. [15] QI WQ, ZHANG B, ZHENG ZJ, et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock 2021[J]. Chin J Emerg Med, 2021, 30 (11): 1300-1304. DOI: 10.3760/cma.j.issn.1671-0282.2021.11.003.齐文旗, 张斌, 郑忠骏, 等. 拯救脓毒症运动: 2021年国际脓毒症和脓毒性休克管理指南[J]. 中华急诊医学杂志, 2021, 30(11): 1300-1304. DOI: 10.3760/cma.j.issn.1671-0282.2021.11.003. [16] Infection group, Chinese Society of Respiratory, Chinese Medical Association. Guidelines for the diagnosis and treatment of hospital-acquired pneumonia and ventilator-associated pneumonia in China (2018 edition)[J]. Chin J of Tuberc Respir Dis, 2018, 41 (4): 255-280. DOI: 10.3760/cma.j.issn.1001-0939.2018.04.006中华医学会呼吸病学分会感染学组. 中国成人医院获得性肺炎与呼吸机相关性肺炎诊断和治疗指南(2018年版)[J]. 中华结核和呼吸杂志, 2018, 41(4): 255-280. DOI: 10.3760/cma.j.issn.1001-0939.2018.04.006. [17] FERGUSON ND, FAN E, CAMPOROTA L, et al. The Berlin definition of ARDS: An expanded rationale, justification, and supplementary material[J]. Intensive Care Med, 2012, 38(10): 1573-1582. DOI: 10.1007/s00134-012-2682-1. [18] Respiratory Critical Care group, Chinese Society of Respiratory, Chinese Medical Association. Guidelines for mechanical ventilation for patients with acute respiratory distress syndrome (trial)[J]. Natl Med J China, 2016, 96(6): 404-424. DOI: 10.3760/cma.j.issn.0376-2491.2016.06.002.中华医学会呼吸病学分会呼吸危重症医学学组. 急性呼吸窘迫综合征患者机械通气指南(试行)[J]. 中华医学杂志, 2016, 96(6): 404-424. DOI: 10.3760/cma.j.issn.0376-2491.2016.06.002. [19] KUMAR P, GUPTA P, RANA S. Thoracic complications of pancreatitis[J]. JGH Open, 2018, 3(1): 71-79. DOI: 10.1002/jgh3.12099. [20] SU JL, HUANG Z, SUN HY, et al. Impact of timing of abdominal paracentesis drainage on treatment outcomes in patients with severe acute pancreatitis[J]. Chin J Hepatobiliary Surg, 2018, 24(10): 692-697. DOI: 10.3760/cma.j.issn.1007-8118.2018.10.009.苏江林, 黄竹, 孙红玉, 等. 腹腔引流穿刺时机对重症急性胰腺炎患者预后的影响[J]. 中华肝胆外科杂志, 2018, 24(10): 692-697. DOI: 10.3760/cma.j.issn.1007-8118.2018.10.009. [21] BAO ZG, XIAO B, TANG W, et al. Anatomical pathways of pleural effusion in acute pancreatitis[J]. Int J Med Radiol, 2012, 35(1): 14-16. DOI: 10.3784/j.issn.1674-1897.2012.01.Z0102.鲍志国, 肖波, 唐伟, 等. 急性胰腺炎胸腔积液的解剖通道[J]. 国际医学放射学杂志, 2012, 35(1): 14-16. DOI: 10.3784/j.issn.1674-1897.2012.01.Z0102. [22] XU HT, EBNER L, JIANG SM, et al. Retrocrural space involvement on computed tomography as a predictor of mortality and disease severity in acute pancreatitis[J]. PLoS One, 2014, 9(9): e107378. DOI: 10.1371/journal.pone.0107378. [23] LEE PJ, PAPACHRISTOU GI. New insights into acute pancreatitis[J]. Nat Rev Gastroenterol Hepatol, 2019, 16(8): 479-496. DOI: 10.1038/s41575-019-0158-2. [24] LIU WH, REN LN, CHEN T, et al. Abdominal paracentesis drainage ahead of percutaneous catheter drainage benefits patients attacked by acute pancreatitis with fluid collections: A retrospective clinical cohort study[J]. Crit Care Med, 2015, 43(1): 109-119. DOI: 10.1097/CCM.0000000000000606. [25] GARG PK, SINGH VP. Organ failure due to systemic injury in acute pancreatitis[J]. Gastroenterology, 2019, 156(7): 2008-2023. DOI: 10.1053/j.gastro.2018.12.041. [26] KOMARA NL, PARAGOMI P, GREER PJ, et al. Severe acute pancreatitis: Capillary permeability model linking systemic inflammation to multiorgan failure[J]. Am J Physiol Gastrointest Liver Physiol, 2020, 319(5): G573-G583. DOI: 10.1152/ajpgi.00285.2020. [27] ZHOU J, HUANG Z, LIN N, et al. Abdominal paracentesis drainage protects rats against severe acute pancreatitis-associated lung injury by reducing the mobilization of intestinal XDH/XOD[J]. Free Radic Biol Med, 2016, 99: 374-384. DOI: 10.1016/j.freeradbiomed.2016.08.029. [28] HUIDOBRO C, MARTÍN-VICENTE P, LÓPEZ-MARTÍNEZ C, et al. Cellular and molecular features of senescence in acute lung injury[J]. Mech Ageing Dev, 2021, 193: 111410. DOI: 10.1016/j.mad.2020.111410. [29] ZHOU JL, ZHOU PC, ZHANG YY, et al. Signal pathways and markers involved in acute lung injury induced by acute pancreatitis[J]. Dis Markers, 2021, 2021: 9947047. DOI: 10.1155/2021/9947047. [30] SHAH J, RANA SS. Acute respiratory distress syndrome in acute pancreatitis[J]. Indian J Gastroenterol, 2020, 39(2): 123-132. DOI: 10.1007/s12664-020-01016-z. [31] JIANG LL, GAO J. New advances in molecular mechanisms of ventilator induced lung injury[J]. Chin Crit Care Med, 2020, 32(7): 890-893. DOI: 10.3760/cma.j.cn121430-20200324-00099.蒋璐璐, 高巨. 呼吸机相关性肺损伤分子机制研究新进展[J]. 中华危重病急救医学, 2020, 32(7): 890-893. DOI: 10.3760/cma.j.cn121430-20200324-00099. [32] UMBRELLO M, MISTRALETTI G, GALIMBERTI A, et al. Drainage of pleural effusion improves diaphragmatic function in mechanically ventilated patients[J]. Crit Care Resusc, 2017, 19(1): 64-70. [33] MURUGANANDAN S, AZZOPARDI M, THOMAS R, et al. The Pleural Effusion And Symptom Evaluation (PLEASE) study of breathlessness in patients with a symptomatic pleural effusion[J]. Eur Respir J, 2020, 55(5): 1900980. DOI: 10.1183/13993003.00980-2019. [34] FITZGERALD DB, MURUGANANDAN S, PEDDLE-MCINTYRE CJ, et al. Ipsilateral and contralateral hemidiaphragm dynamics in symptomatic pleural effusion: The 2nd PLeural Effusion And Symptom Evaluation (PLEASE-2) Study[J]. Respirology, 2022, 27(10): 882-889. DOI: 10.1111/resp.14307. [35] DOMBERNOWSKY T, KRISTENSEN MØ, RYSGAARD S, et al. Risk factors for and impact of respiratory failure on mortality in the early phase of acute pancreatitis[J]. Pancreatology, 2016, 16(5): 756-760. DOI: 10.1016/j.pan.2016.06.664. [36] SHI YQ, CHEN M. Diagnosis and treatment strategy of lung injury caused by severe acute pancreatitis[J]. Chin J Dig, 2019, 39(5): 297-299. DOI: 10.3760/cma.j.issn.0254-1432.2019.05.004.时永全, 陈敏. 重症急性胰腺炎致肺损伤的诊治策略[J]. 中华消化杂志, 2019, 39(5): 297-299. DOI: 10.3760/cma.j.issn.0254-1432.2019.05.004. [37] ZHAO CS, YAO WJ, YUAN P, et al. Time distribution of risk factors for secondary pancreatic infection in acute pancreatitis[J]. J Clin Hepatol, 2022, 38(7): 1686-1690. DOI: 10.3969/j.issn.1001-5256.2022.07.044.赵成思, 姚维杰, 袁鹏, 等. 急性胰腺炎继发胰腺感染的危险因素及其时间分布[J]. 临床肝胆病杂志, 2022, 38(7): 1686-1690. DOI: 10.3969/j.issn.1001-5256.2022.07.044. [38] LU JD, CAO F, DING YX, et al. Timing, distribution, and microbiology of infectious complications after necrotizing pancreatitis[J]. World J Gastroenterol, 2019, 25(34): 5162-5173. DOI: 10.3748/wjg.v25.i34.5162. [39] TIAN H, LI FX, SONG SW. Features of infection secondary to severe acute pancreatitis and related control strategies[J]. J Clin Hepatol, 2019, 35(2): 451-456. DOI: 10.3969/j.issn.1001-5256.2019.02.048.田浩, 李富兴, 宋少伟. 重症急性胰腺炎继发感染的特点及防治进展[J]. 临床肝胆病杂志, 2019, 35(2): 451-456. DOI: 10.3969/j.issn.1001-5256.2019.02.048. [40] GRAJALES-FIGUEROA G, DÍAZ HERNÁNDEZ HA, CHACÓN PORTILLO MA, et al. Increased mortality from extrapancreatic infections in hospitalized patients with acute pancreatitis[J]. Gastroenterol Res Pract, 2019, 2019: 2789764. DOI: 10.1155/2019/2789764. [41] GUO F. Diagnosis and antibiotic use of severe acute pancreatitis complicated with infection[J]. Chin J Dig, 2020, 40(7): 444-447. DOI: 10.3760/cma.j.cn311367-20200506-00291.郭丰. 重症急性胰腺炎合并感染的诊断和抗生素使用[J]. 中华消化杂志, 2020, 40(7): 444-447. DOI: 10.3760/cma.j.cn311367-20200506-00291. [42] CHEN F, GAO Q. Analysis of pathogens and risk factors of severe acute pancreatitis complicated with infection[J]. Chin J Dig, 2019, 39(12): 846-849. DOI: 10.3760/cma.j.issn.0254-1432.2019.12.010.陈芳, 高青. 重症急性胰腺炎继发感染的病原菌及相关危险因素分析[J]. 中华消化杂志, 2019, 39(12): 846-849. DOI: 10.3760/cma.j.issn.0254-1432.2019.12.010. [43] XIE L, LIU H, SHEN Y, et al. Risk factors for severe acute pancreatitis complicated with infection and the effects on immune level[J]. Chin J Pancreatol, 2020, 20(4): 283-287. DOI: 10.3760/cma.j.cn115667-20190826-00076.谢蕾, 刘航, 申洋, 等. 重症急性胰腺炎并发感染的危险因素及对机体免疫水平的影响[J]. 中华胰腺病杂志, 2020, 20(4): 283-287. DOI: 10.3760/cma.j.cn115667-20190826-00076. -



 PDF下载 ( 2121 KB)
PDF下载 ( 2121 KB)

 下载:
下载:


