血清IL-6和TNF-α对重症急性胰腺炎的早期诊断价值分析
DOI: 10.3969/j.issn.1001-5256.2023.07.020
Value of serum interleukin-6 and tumor necrosis factor-α in early diagnosis of severe acute pancreatitis
-
摘要:
目的 评估血清细胞因子在重症急性胰腺炎(SAP)早期诊断中的价值, 并利用LASSO算法构建复合指标的数理模型以提高对SAP诊断的准确性。 方法 纳入2019年1月-2022年6月在常熟市第一人民医院就诊的130例急性胰腺炎患者, 其中SAP患者73例, 非SAP患者57例。收集所有患者的外周血清样本并通过Luminex xMAP液相芯片技术完成13种血清细胞因子的精准检测。同时, 所有患者均进行APACHE Ⅱ、BISAP和CTSI评分。使用Kolmogorov-Smirnov法进行正态性检验, 对符合正态分布的计量资料两组间比较采用成组t检验; 对非正态分布的计量资料两组间比较采用Mann-Whitney U检验。计数资料两组间比较采用χ2检验。此外, 通过二元Logistic回归分析评估细胞因子对SAP的影响, 应用线性回归分析评估细胞因子与SAP疾病的严重程度之间的关联。偏相关分析在校正协变量(年龄、性别、BMI、高血压、糖尿病病史)后分析细胞因子与SAP疾病的严重程度评分的关联性。利用LASSO算法构建复合指标的数理模型, 并采用受试者工作特征曲线(ROC曲线)分析血清细胞因子对SAP临床诊断的效能, 计算曲线下面积(AUC)。 结果 非SAP组APACHE Ⅱ、BISAP和CTSI评分、改良Marshall评分均低于SAP组, 差异均有统计学意义(P值均 < 0.001)。SAP组患者IFN-γ、IL-1β、IL-6、IL-8、TNF-α水平均高于非SAP组, IL-12水平明显低于非SAP组, 差异均有统计学意义(P值均 < 0.05)。Logistic回归分析结果显示, IFN-γ(OR=1.190, 95%CI: 1.036~1.367, P=0.014)、IL-6(OR=1.148, 95%CI: 1.070~1.231, P < 0.001)和TNF-α(OR=1.100, 95%CI: 1.048~1.155, P < 0.001)为SAP的独立影响因素。偏相关分析提示, 在校正了性别、年龄、BMI、慢性疾病史(糖尿病、高血压)后, SAP患者IL-6和TNF-α的水平与APACHE Ⅱ评分均呈显著正相关(IL-6:r=0.503, P < 0.001;TNF-α: r=0.557, P < 0.001)。线性回归分析显示, SAP患者中IL-6和TNF-α水平均与APACHE Ⅱ评分有关(IL-6:β=0.049, P=0.044;TNF-α: β=0.054, P=0.046), 且IL-6和TNF-α存在交互作用, 影响APACHE Ⅱ评分。ROC曲线分析显示, LASSO算法联合IL-6和TNF-α构建的风险评分区分SAP和非SAP的AUC值最大(AUC=0.925), 而IL-6和TNF-α的AUC分别为0.885、0.878;偏相关分析发现, 在校正性别、年龄、BMI、慢性疾病史(糖尿病、高血压)后, SAP患者风险评分与APACHE Ⅱ评分呈显著正相关(r=0.565, P < 0.001)。 结论 血清IL-6和TNF-α水平可反映AP疾病严重程度。联合血清IL-6和TNF-α构建的风险评分可显著提高SAP早期诊断的准确性, 对SAP的临床诊疗具有重要的临床价值。 Abstract:Objective To investigate the value of serum cytokines in the early diagnosis of severe acute pancreatitis (SAP), and to improve the accuracy of the diagnosis of SAP by establishing a mathematical model with composite indices based on LASSO algorithm. Methods A total of 130 patients with acute pancreatitis (AP) who attended Changshu First People's Hospital from January 2019 to June 2022 were enrolled, among whom there were 73 SAP patients and 57 non-SAP patients.Peripheral serum samples were collected from all patients, and Luminex xMAP liquid chip technique was used to measure 13 serum cytokines.Meanwhile, Acute Physiology and Chronic Health Evaluation Ⅱ(APACHE Ⅱ), Bedside Index for Severity in Acute Pancreatitis (BISAP), and Computed Tomography Severity Index (CTSI) scores were determined for all patients.The Kolmogorov-Smirnov method was used for normality test; the independent-samples t test was used for comparison of normally distributed continuous data between two groups, and the Mann-Whitney U test was used for comparison of non-normally distributed continuous data between two groups; the chi-square test was used for comparison of categorical data between two groups.Furthermore, the binary logistic regression analysis was used to evaluate the effect of cytokines on SAP, and the linear regression analysis was used to investigate the correlation between cytokines and SAP severity.The partial correlation analysis was used to evaluate the correlation between cytokines and SAP severity score after adjustment for covariates[age, sex, body mass index (BMI), and history of hypertension and diabetes].The LASSO algorithm was used to establish a mathematical model with composite indices; the receiver operating characteristic (ROC) curve was used to assess the performance of serum cytokines in the clinical diagnosis of SAP, and the area under the ROC curve (AUC) was calculated. Results Compared with the SAP group, the non-SAP group had significantly lower APACHE Ⅱ, BISAP, CTSI, and modified Marshall scores (all P < 0.001).Compared with the non-SAP group, the SAP group had significantly higher levels of interferon-γ(IFN-γ), interleukin-6(IL-6), interleukin-8, and tumor necrosis factor-α(TNF-α) and a significantly lower level of interleukin-12(all P < 0.05).The logistic regression analysis showed that IFN-γ(odds ratio[OR]=1.190, 95% confidence interval[CI]: 1.036-1.367, P=0.014), IL-6 (OR=1.148, 95%CI: 1.070-1.231, P < 0.001), and TNF-α (OR=1.100, 95%CI: 1.048-1.155, P < 0.001) were independent influencing factors for SAP.The partial correlation analysis showed that after adjustment for sex, age, BMI, and history of chronic diseases (diabetes and hypertension), the levels of IL-6 and TNF-α were positively correlated with APACHE Ⅱ score in SAP patients (IL-6:r=0.503, P < 0.001;TNF-α: r=0.557, P < 0.001).The linear regression analysis showed that the levels of IL-6 and TNF-α were associated with APACHE Ⅱ score in SAP patients (IL-6:β=0.049, P=0.044;TNF-α: β=0.054, P=0.046), and there was an interaction between IL-6 and TNF-α, which affected APACHE Ⅱ score.The ROC curve analysis showed that the risk score based on IL-6 and TNF-α using LASSO algorithm had the largest AUC of 0.925 in distinguishing SAP from non-SAP, while IL-6 or TNF-α alone had an AUC of 0.885 and 0.878, respectively.The partial correlation analysis showed that after adjustment for sex, age, BMI, and history of chronic diseases (diabetes and hypertension), the risk score was positively correlated with APACHE Ⅱ score in SAP patients (r=0.565, P < 0.001). Conclusion The serum levels of IL-6 and TNF-α can reflect the severity of AP.The risk score combining serum IL-6 and TNF-α can significantly improve the accuracy of the early diagnosis of SAP, which has an important clinical value in the clinical diagnosis and treatment of SAP. -
Key words:
- Pancreatitis /
- Interleukin-6 /
- Tumor Necrosis Factor-alpha /
- LASSO Algorithm
-
急性胰腺炎(acute pancreatitis,AP)是由多种因素引起的胰腺内胰酶异常激活后发生局部炎症和全身性炎症反应综合征[1]。AP的病理机制复杂,尽管约有80%患者为轻症AP(MAP)且经过积极治疗后可痊愈,但仍有约20%的患者表现为重型AP(SAP),出现全身炎症反应和持续性器官衰竭,甚至发生死亡[2]。近年来,AP的发病率呈上升趋势,SAP的发生更需要高度重视[3]。因此,早期识别潜在的SAP患者对于临床选择合适的治疗方法和判断预后具有重要价值。血清脂肪酶、淀粉酶可用于明确AP的诊断,C反应蛋白水平与疾病严重程度存在一定相关性,但这些指标对SAP的早期诊断效果不佳[4-5]。此外,相关研究[6-7]表明炎症细胞及其部分细胞因子的过度激活是AP发生的潜在机制,特别是细胞因子诱导的全身炎症反应综合征与SAP的进展加重以及相关并发症的出现有重要关联。本研究的目的是评估多种血清细胞因子在SAP早期诊断中的价值,并分析这些血清细胞因子与疾病严重程度的内在关联性。
1. 资料与方法
1.1 研究对象
纳入本院2019年1月—2022年6月收治的AP患者,收集所有患者的一般资料,包括性别、年龄、BMI、合并症(高血压、糖尿病)。AP的严重程度根据2012年修订的亚特兰大国际共识中的定义,MAP:没有器官功能衰竭、局部或全身并发症;中度SAP(MSAP):以一过性器官衰竭为特征,或伴有局部或全身并发症,但无持续性器官衰竭(<48 h);SAP:伴持续性(>48 h)器官功能障碍(单个或多个器官)。基于完整的住院病历,纳入的MSAP患者仅伴有一过性器官衰竭,且均未转变为SAP,故将MAP和MSAP患者合并为非SAP组,其余SAP患者为SAP组。
1.2 纳入与排除标准
纳入标准:(1)所有患者均符合《中国急性胰腺炎诊治指南(2021)》[8]中AP的诊断标准:上腹部持续性疼痛;血清淀粉酶和/或脂肪酶水平高于3倍正常值上限;腹部影像学检查结果显示符合AP影像学改变。上述3项标准中符合2项即可诊断为AP。(2)发病至就诊时间在48 h以内。排除标准:(1)肝、肾、心脏等重要脏器功能异常者;(2)有严重感染性疾病、全身性免疫功能异常者;(3)近3个月使用免疫抑制剂或激素者;(4)慢性胰腺炎;(5)有恶性肿瘤病史;(6)处于妊娠前后的患者。
1.3 观察指标
所有患者于入院第2天清晨抽取空腹外周静脉血2 mL,置于EDTA抗凝管中。血样本处理:在4 ℃条件下以3 500 r/min离心10 min,分离获得的血清分装至0.5 mL离心管中保存于超低温冰箱(-80 ℃)备用。
利用Luminex xMAP液相芯片技术,选择13细胞因子试剂盒(Cat. No. HSTCMAG28SPMX13)检测血清中粒细胞-巨噬细胞集落刺激因子(GM-CSF)、IFN-γ、IL-1β、IL-2、IL-4、IL-5、IL-6、IL-7、IL-8、IL-10、IL-12、IL-13和TNF-α水平。所有样本重复检测3次。
为了较好地评估AP的疾病严重程度,所有患者均接受了AP相关的临床评估,包括急性生理学和慢性健康评分Ⅱ(APACHE Ⅱ)评分、急性胰腺炎严重程度床边指数(BISAP)和CT严重指数(CTSI);改良Marshall评分作为评判脏器功能的标准。所有患者的诊断和量表评分均由经验丰富的专科医师完成。
1.4 统计学方法
所有数据采用SPSS 22.0软件进行统计学分析。使用Kolmogorov- Smirnov法进行正态性检验,对符合正态分布的计量资料以x±s表示,两组间比较采用成组t检验;对非正态分布的计量资料以M (P25~P75)表示,两组间比较采用Mann-Whitney U检验。计数资料两组间比较采用χ2检验。此外,通过二元Logistic回归分析评估细胞因子对SAP的影响,而应用线性回归分析评估细胞因子与SAP疾病的严重程度之间的关联。偏相关分析在校正协变量(年龄、性别、BMI、高血压、糖尿病病史)后分析细胞因子与SAP疾病的严重程度评分的关联性。最后,利用受试者工作特征曲线(ROC曲线)评估细胞因子对识别SAP的临床价值,计算曲线下面积(AUC),并通过约登指数确定最佳截断值、敏感度和特异度。此外,通过R语言软件,基于LASSO数理模型[9]构建SAP诊断的风险评分模型。P<0.05为差异有统计学意义。
2. 结果
2.1 一般资料
共纳入AP患者130例,非SAP组57例,SAP组73例。所有患者在住院期间均无死亡,其中有89例(68.46%)患者存在并发症,包括休克、胰腺脓肿、腹腔出血、急性呼吸窘迫综合征、腹腔室隔综合征、假性囊肿、急性肾损伤、肝功能不全。非SAP组APACHE Ⅱ、BISAP和CTSI评分、改良Marshall评分均低于SAP组,差异均有统计学意义(P值均<0.001)(表 1)。
表 1 两组AP患者临床资料比较Table 1. Comparison of clinical data between two groups of patients with AP项目 非SAP组(n=57) SAP组(n=73) 统计值 P值 年龄(岁) 56.04±15.70 57.25±16.90 t=-0.418 0.676 男[例(%)] 28(49.12) 40(54.79) χ2=0.413 0.521 BMI(kg/m2) 22.44±3.56 23.31±3.14 t=-1.474 0.143 糖尿病[例(%)] 7(12.28) 15(20.55) χ2=1.556 0.212 高血压[例(%)] 16(28.07) 23(31.51) χ2=0.180 0.671 AP病因[例(%)] 胆源性 45(78.95) 51(69.86) χ2=1.368 0.242 高脂血症性 4(7.02) 11(15.07) χ2=2.033 0.154 酒精性 6(10.53) 7(9.59) χ2=0.031 0.860 病因不明 2(3.51) 4(5.48) χ2=0.282 0.595 APACHE Ⅱ评分 4(4~5) 8(6~12) Z=-7.503 <0.001 BISAP评分 2(1~2) 4(3~4) Z=-9.818 <0.001 CTSI评分 2(2~3) 6(5~7) Z=-9.797 <0.001 改良Marshall评分 1(0~2) 3(3~4) Z=-8.555 <0.001 循环衰竭[例(%)] 4(7.02) 25(34.25) 呼吸衰竭[例(%)] 10(17.54) 47(63.38) 肾衰竭[例(%)] 2(3.51) 8(10.96) 2.2 非SAP组与SAP组患者入院时血清细胞因子水平比较
SAP组患者IFN-γ、IL-1β、IL-6、IL-8、TNF-α水平均高于非SAP组,差异均有统计学意义(P值均<0.05);但SAP组的IL-12水平明显低于非SAP,差异亦有统计学意义(P<0.05)(表 2)。
表 2 两组AP患者细胞因子水平的比较Table 2. Comparison of cytokines levels in patients with SAP group and non-SAP group细胞因子 非SAP(n=57) SAP(n=73) 统计值 P值 GM-CSF(pg/mL) 36.45±5.01 38.39±7.79 t=-1.638 0.104 IFN-γ(pg/mL) 38.69±7.11 42.26±11.96 t=-2.114 0.037 IL-1β(pg/mL) 12.93(10.09~16.60) 15.85(12.96~19.67) Z=-3.207 0.001 IL-2(pg/mL) 1.13(0.80~1.72) 1.20(0.98~1.79) Z=-1.185 0.236 IL-4(pg/mL) 38.76±7.22 40.12±5.65 t=-1.174 0.243 IL-5(pg/mL) 0.56(0.43~0.72) 0.59(0.47~0.76) Z=-1.159 0.246 IL-6(pg/mL) 69.12±24.01 110.81±27.78 t=-9.004 <0.001 IL-7(pg/mL) 5.16±2.55 5.33±2.39 t=-0.383 0.702 IL-8(pg/mL) 62.48±15.04 74.90±11.78 t=-5.279 <0.001 IL-10(pg/mL) 31.29(28.21~34.41) 31.28(30.03~33.84) Z=-1.370 0.171 IL-12(pg/mL) 14.66(11.78~18.06) 13.60(10.37~15.52) Z=-1.985 0.047 IL-13(pg/mL) 0.86 (0.80~0.91) 0.89 (0.75~1.04) Z=-1.234 0.217 TNF-α(pg/mL) 77.64±31.64 128.48±25.33 t=-10.177 <0.001 将上述细胞因子及性别、年龄、BMI、慢性疾病史(糖尿病、高血压)等指标纳入多因素Logistic回归分析,结果提示,IFN-γ、IL-6和TNF-α为SAP的独立影响因素(P值均<0.05)(表 3)。
表 3 多因素Logistic回归分析SAP的影响因素Table 3. Multivariate Logistic regression analysis of independent risk factors of SAP patients因素 β值 SE P值 OR值 95% CI 性别 0.037 1.046 0.972 1.038 0.134~1.228 年龄 0.117 0.045 0.880 1.170 0.184~10.110 BMI -0.269 0.140 0.054 0.764 0.581~1.005 饮酒史 0.576 1.532 0.707 1.778 0.088~35.804 吸烟史 0.165 1.148 0.886 1.179 0.124~11.180 糖尿病 0.185 1.205 0.878 1.203 0.113~12.763 高血压 -0.598 1.133 0.598 0.550 0.060~5.070 GM-CSF -0.023 0.100 0.818 0.977 0.803~1.189 IFN-γ 0.174 0.071 0.014 1.190 1.036~1.367 IL-1β 0.071 0.068 0.298 1.074 0.939~1.227 IL-2 0.393 0.540 0.466 1.482 0.514~4.271 IL-4 0.042 0.061 0.493 1.043 0.925~1.175 IL-5 4.655 2.411 0.054 105.074 0.932~11 851.669 IL-6 0.138 0.036 <0.001 1.148 1.070~1.231 IL-7 -0.099 0.205 0.629 0.906 0.606~1.353 IL-8 -0.054 0.038 0.161 0.948 0.879~1.022 IL-10 0.097 0.051 0.057 1.101 0.997~1.216 IL-12 -0.099 0.111 0.373 0.906 0.729~1.126 IL-13 1.232 1.592 0.439 3.427 0.151~77.664 TNF-α 0.096 0.025 <0.001 1.100 1.048~1.155 2.3 SAP组中IFN-γ、IL-6和TNF-α水平与APACHE Ⅱ评分的关联分析
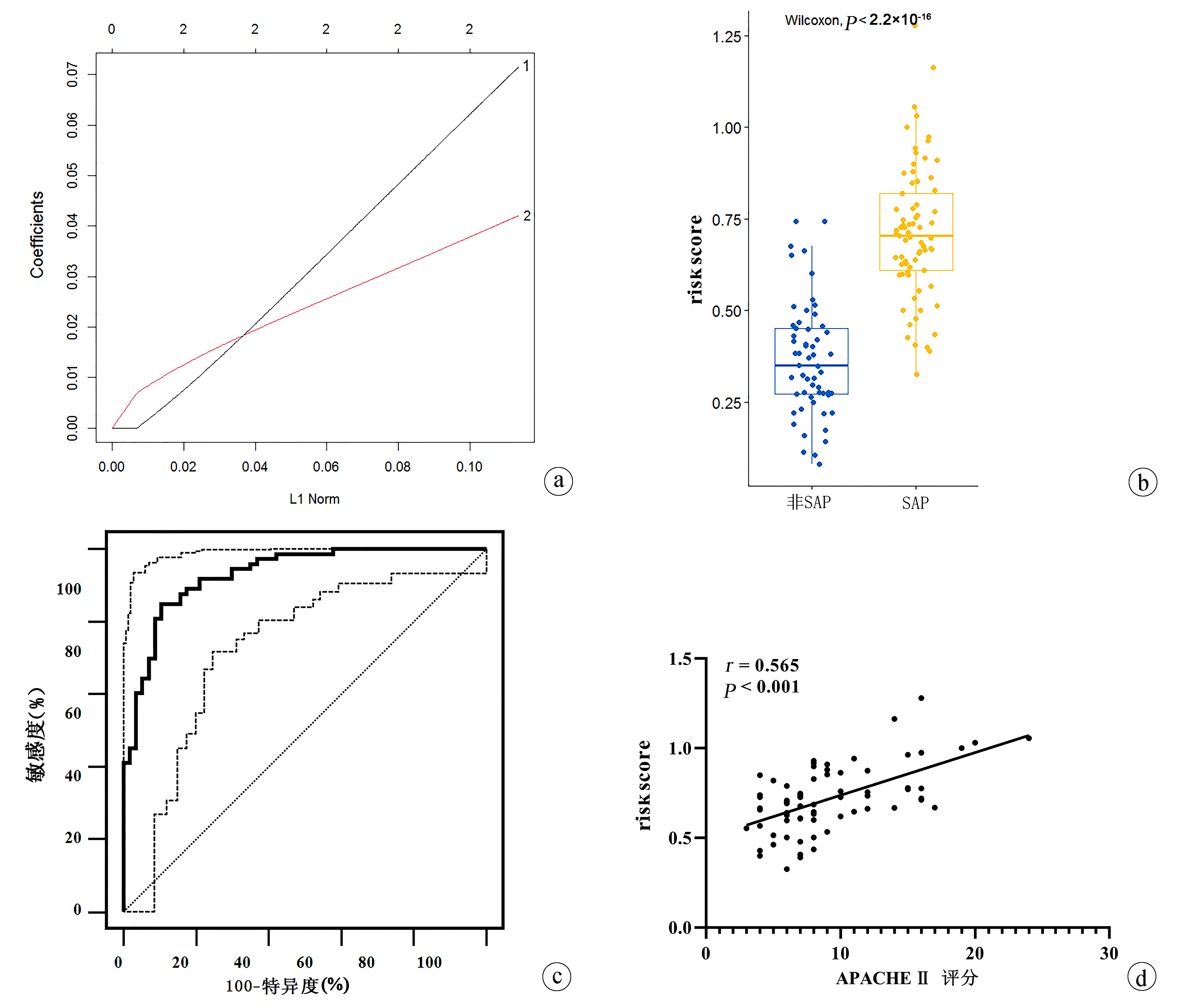
偏相关分析提示,在校正了性别、年龄、BMI、慢性疾病史(糖尿病、高血压)后,SAP患者IL-6和TNF-α的水平与APACHE Ⅱ评分均呈显著正相关(IL-6:r=0.503,P<0.001;TNF-α:r=0.557,P<0.001)(图 1a、b);但IFN-γ与APACHE Ⅱ评分之间均无关联(P值均>0.05)。
此外,线性回归分析显示,SAP患者中IL-6和TNF-α水平均与APACHE Ⅱ评分有关(IL-6:β=0.049,P=0.044;TNF-α:β=0.054,P=0.046);提示IL-6和TNF-α存在交互作用影响APACHE Ⅱ评分(图 1c)。
2.4 ROC曲线分析
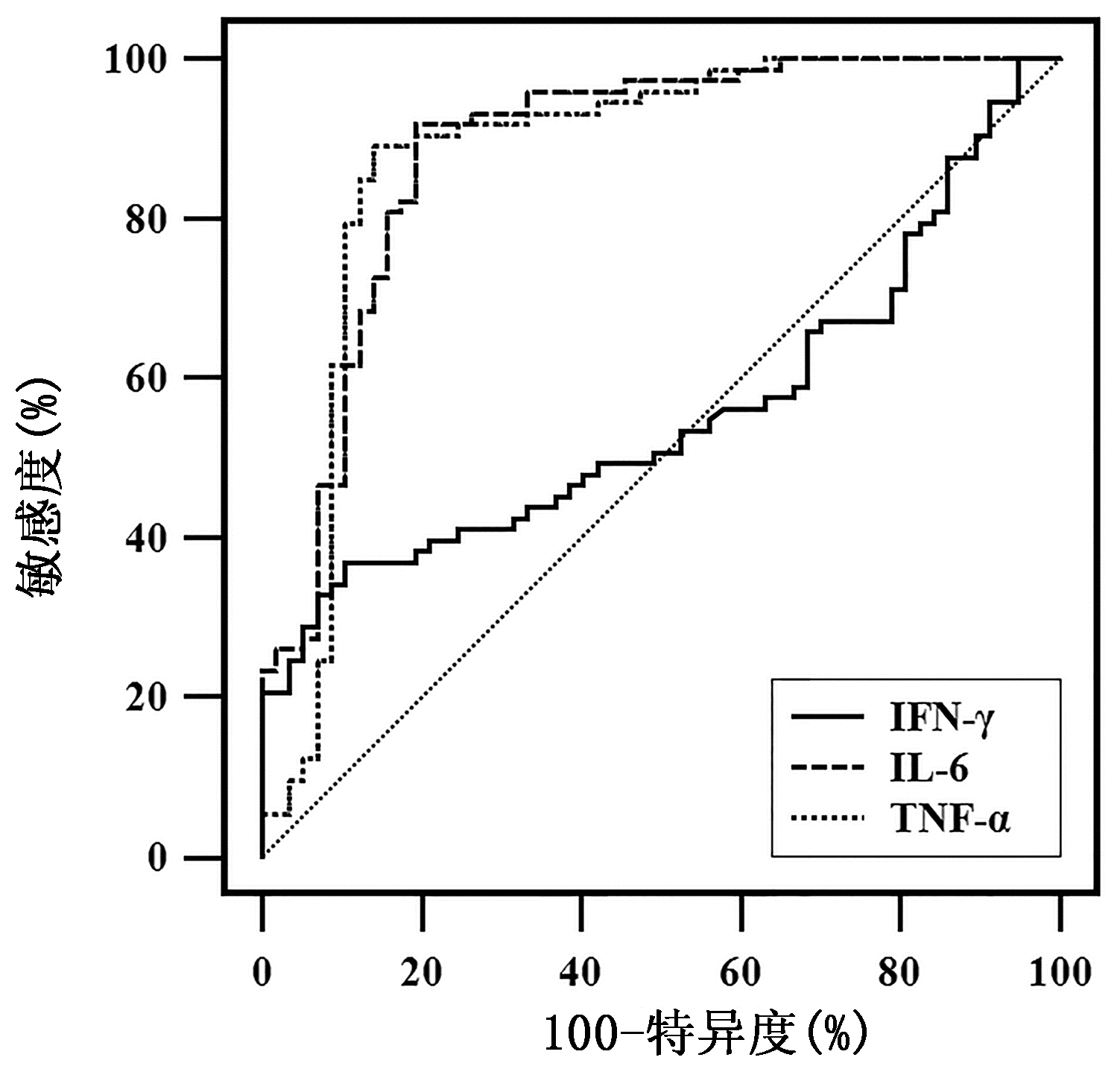
ROC曲线分析IFN-γ、IL-6和TNF-α对SAP和非SAP的鉴别诊断效能(图 2)。结果发现,IL-6和TNF-α的AUC分别为0.885(95%CI:0.818~0.935,P<0.001)和0.878(95%CI:0.809~0.929,P<0.001)。通过约登指数计算,IL-6的截断值为86.93 pg/mL,此时敏感度为91.78%,特异度为80.70%;TNF-α的截断值为97.64 pg/mL,此时敏感度为89.04%,特异度为85.96%。而IFN-γ的AUC为0.556(95%CI:0.466~0.643,P=0.272)。
2.5 SAP诊断的风险评分模型构建
为进一步提高SAP的诊断准确性,应用LASSO模型构建SAP诊断的风险评分模型为:0.004 735×IL-6+ 0.005 892× TNF-α-0.502 154(图 3a)。通过模型计算出每个受试者的风险评分,相比非SAP患者,SAP患者风险评分显著升高(P<0.001)(图 3b)。此外,ROC曲线分析该风险评分模型在SAP患者和非SAP患者间的鉴别诊断效能,结果提示AUC为0.925(95%CI:0.865~0.964),截断值为0.530,敏感度为84.93%,特异度为89.47%(图 3c)。偏相关分析发现,在校正性别、年龄、BMI、慢性疾病史(糖尿病、高血压)后,SAP患者风险评分与APACHE Ⅱ评分呈显著正相关(r=0.565,P<0.001)(图 3d)。
3. 讨论
AP是临床上最常见的胰腺疾病之一,随着疾病研究的进展,AP患者的临床预后逐渐改善,病死率较低,但SAP患者病死率可高达40%[10]。尽早发现潜在的SAP人群对提高患者生存率至关重要。本研究首次利用Luminex xMAP液相芯片技术精准检测了AP患者13种血清细胞因子的水平,充分评估各细胞因子对SAP早期诊断及疾病严重程度的关联性;通过LASSO算法进一步构建了风险评分模型,为SAP的准确诊断提供重要方法。
最近,Sternby等[11]通过检测7种血清细胞因子发现IL-1β、IL-6、IL-8和IL-10的水平随着AP疾病的进展而改变,其中IL-6识别SAP具有较高的临床诊断效能。此外,一项荟萃分析[12]表明入院时的IL-6水平对于SAP预测的敏感度为81%~84%,而特异度为76%~85%。虽然细胞因子水平的异常改变可能是SAP发生的独立危险因素,且对SAP早期诊断有重要的临床价值,但血清细胞因子与AP严重程度之间的关联性仍不明确,特别是缺少可用于SAP早期诊断的客观生物标志物。本研究比较了非SAP与SAP患者之间13种不同血清细胞因子水平的差异,确定了血清IFN-γ、IL-6和TNF-α是SAP的独立影响因素;进一步的ROC曲线分析证实血清IL-6和TNF-α在早期识别SAP方面具有更出色的临床潜力。尽管之前有研究[13]发现联合利用IL-6和TNF-α可提高SAP的诊断准确性,但尚未有利用数理模型进一步分析,本研究利用LASSO算法构建的风险评分模型将IL-6和TNF-α拟合成新的复合标志物,其早期诊断SAP的效能要明显优于单一的细胞因子。
IL是炎症重要的启动因子,尤其是IL-6作为炎症早期阶段的重要细胞因子,其刺激肝脏细胞合成急性期蛋白进而调节炎症级联反应的发生[10, 14-15]。此外,单核细胞与巨噬细胞分泌产生的TNF-α可诱导腺泡细胞的损伤反应,是AP发病中促炎反应的关键调节因子。此外,抗IL-6和抗TNF-α治疗对SAP发生过程中的炎症进行抑制,有助于提高SAP的疗效。地塞米松作为一种抗炎药物,有研究[16]通过大鼠模型证实其可通过抑制IL-6和TNF-α的产生减轻胰腺损伤和改善预后的作用。最近,Huang等[17]开展的一项临床试验中环氧合酶-2抑制剂通过减轻血清中IL-6和TNF-α水平显著降低了SAP的发生率并显著减少了晚期局部并发症的发生。因此,利用血清IL-6和TNF-α确立SAP诊断的客观生物标志物具有明确的病理生理学基础。
APACHE Ⅱ评分、BISAP评分和CTSI评分是反映AP严重程度的重要评分方法,且对于SAP和死亡风险具有重要预测价值,其中APACHE Ⅱ评分预测SAP和死亡风险的准确性最高[18]。本研究相关分析指出血清IL-6和TNF-α与APACHE Ⅱ评分之间存在显著相关性,且风险评分模型与APACHE Ⅱ评分之间仍存在稳定的相关性,提示血清IL-6和TNF-α可反映SAP的病理损伤,且可用于提示SAP的严重程度。此外,血清IL-6和TNF-α之间的交互作用影响SAP患者的APACHE Ⅱ评分,进一步支持血清IL-6和TNF-α联合构建的复合标志物可用于SAP的临床预测。因此,与操作复杂的AP临床量表评分相比,基于血清细胞因子的检测可实现快速、有效的早期诊断SAP。
本研究存在一定的局限性:(1)本研究为单中心研究,纳入的AP患者样本量较小,无法更进一步地分析MAP、MSAP和SAP之间细胞因子水平的差异。在随后的研究中,将开展多中心研究以验证当前的结果,并进一步探究AP不同阶段细胞因子水平的差异。(2)当前的研究结果需要在纵向队列中进一步验证,特别是本研究提出的风险评分与SAP预后和死亡风险之间的关系,以及在轻重转换型患者中的变化特征。(3)血钙和C反应蛋白是预测SAP严重程度的重要指标,但由于本研究中有23例AP患者的血钙和C反应蛋白数据缺失,因而未分析血钙和C反应蛋白与风险评分的关系。
综上所述,血清IL-6和TNF-α与SAP发生的病理机制密切相关,其水平的变化可反映SAP疾病严重程度。此外,联合血清IL-6和TNF-α构建的风险评分对实现SAP早期准确诊断具有重要的临床价值。
-
表 1 两组AP患者临床资料比较
Table 1. Comparison of clinical data between two groups of patients with AP
项目 非SAP组(n=57) SAP组(n=73) 统计值 P值 年龄(岁) 56.04±15.70 57.25±16.90 t=-0.418 0.676 男[例(%)] 28(49.12) 40(54.79) χ2=0.413 0.521 BMI(kg/m2) 22.44±3.56 23.31±3.14 t=-1.474 0.143 糖尿病[例(%)] 7(12.28) 15(20.55) χ2=1.556 0.212 高血压[例(%)] 16(28.07) 23(31.51) χ2=0.180 0.671 AP病因[例(%)] 胆源性 45(78.95) 51(69.86) χ2=1.368 0.242 高脂血症性 4(7.02) 11(15.07) χ2=2.033 0.154 酒精性 6(10.53) 7(9.59) χ2=0.031 0.860 病因不明 2(3.51) 4(5.48) χ2=0.282 0.595 APACHE Ⅱ评分 4(4~5) 8(6~12) Z=-7.503 <0.001 BISAP评分 2(1~2) 4(3~4) Z=-9.818 <0.001 CTSI评分 2(2~3) 6(5~7) Z=-9.797 <0.001 改良Marshall评分 1(0~2) 3(3~4) Z=-8.555 <0.001 循环衰竭[例(%)] 4(7.02) 25(34.25) 呼吸衰竭[例(%)] 10(17.54) 47(63.38) 肾衰竭[例(%)] 2(3.51) 8(10.96) 表 2 两组AP患者细胞因子水平的比较
Table 2. Comparison of cytokines levels in patients with SAP group and non-SAP group
细胞因子 非SAP(n=57) SAP(n=73) 统计值 P值 GM-CSF(pg/mL) 36.45±5.01 38.39±7.79 t=-1.638 0.104 IFN-γ(pg/mL) 38.69±7.11 42.26±11.96 t=-2.114 0.037 IL-1β(pg/mL) 12.93(10.09~16.60) 15.85(12.96~19.67) Z=-3.207 0.001 IL-2(pg/mL) 1.13(0.80~1.72) 1.20(0.98~1.79) Z=-1.185 0.236 IL-4(pg/mL) 38.76±7.22 40.12±5.65 t=-1.174 0.243 IL-5(pg/mL) 0.56(0.43~0.72) 0.59(0.47~0.76) Z=-1.159 0.246 IL-6(pg/mL) 69.12±24.01 110.81±27.78 t=-9.004 <0.001 IL-7(pg/mL) 5.16±2.55 5.33±2.39 t=-0.383 0.702 IL-8(pg/mL) 62.48±15.04 74.90±11.78 t=-5.279 <0.001 IL-10(pg/mL) 31.29(28.21~34.41) 31.28(30.03~33.84) Z=-1.370 0.171 IL-12(pg/mL) 14.66(11.78~18.06) 13.60(10.37~15.52) Z=-1.985 0.047 IL-13(pg/mL) 0.86 (0.80~0.91) 0.89 (0.75~1.04) Z=-1.234 0.217 TNF-α(pg/mL) 77.64±31.64 128.48±25.33 t=-10.177 <0.001 表 3 多因素Logistic回归分析SAP的影响因素
Table 3. Multivariate Logistic regression analysis of independent risk factors of SAP patients
因素 β值 SE P值 OR值 95% CI 性别 0.037 1.046 0.972 1.038 0.134~1.228 年龄 0.117 0.045 0.880 1.170 0.184~10.110 BMI -0.269 0.140 0.054 0.764 0.581~1.005 饮酒史 0.576 1.532 0.707 1.778 0.088~35.804 吸烟史 0.165 1.148 0.886 1.179 0.124~11.180 糖尿病 0.185 1.205 0.878 1.203 0.113~12.763 高血压 -0.598 1.133 0.598 0.550 0.060~5.070 GM-CSF -0.023 0.100 0.818 0.977 0.803~1.189 IFN-γ 0.174 0.071 0.014 1.190 1.036~1.367 IL-1β 0.071 0.068 0.298 1.074 0.939~1.227 IL-2 0.393 0.540 0.466 1.482 0.514~4.271 IL-4 0.042 0.061 0.493 1.043 0.925~1.175 IL-5 4.655 2.411 0.054 105.074 0.932~11 851.669 IL-6 0.138 0.036 <0.001 1.148 1.070~1.231 IL-7 -0.099 0.205 0.629 0.906 0.606~1.353 IL-8 -0.054 0.038 0.161 0.948 0.879~1.022 IL-10 0.097 0.051 0.057 1.101 0.997~1.216 IL-12 -0.099 0.111 0.373 0.906 0.729~1.126 IL-13 1.232 1.592 0.439 3.427 0.151~77.664 TNF-α 0.096 0.025 <0.001 1.100 1.048~1.155 -
[1] BANKS PA, BOLLEN TL, DERVENIS C, et al. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus[J]. Gut, 2013, 62(1): 102-111. DOI: 10.1136/gutjnl-2012-302779. [2] LANKISCH PG, APTE M, BANKS PA. Acute pancreatitis[J]. Lancet, 2015, 386(9988): 85-96. DOI: 10.1016/S0140-6736(14)60649-8. [3] BOXHOORN L, VOERMANS RP, BOUWENSE SA, et al. Acute pancreatitis[J]. Lancet, 2020, 396(10252): 726-734. DOI: 10.1016/S0140-6736(20)31310-6. [4] STAUBLI S M, OERTLI D, NEBIKER CA. Laboratory markers predicting severity of acute pancreatitis[J]. Crit Rev Clin Lab Sci, 2015, 52(6): 273-283. DOI: 10.3109/10408363.2015.1-051659. [5] LUO XP, WANG J, WU Q, et al. Research advances in acute pancreatitis scoring system[J]. J Clin Hepatol, 2022, 38(9): 2188-2192. DOI: 10.3969/j.issn.1001-5256.2022.09.046.罗秀平, 王洁, 吴青, 等. 急性胰腺炎评分系统的研究进展[J]. 临床肝胆病杂志, 2022, 38(9): 2188-2192. DOI: 10.3969/j.issn.1001-5256.2022.09.046. [6] KENEZ J. Charles Richet and the development of immuno-allergology[J]. Orv Hetil, 1975, 116(42): 2489-2492. DOI: 10.1016/S0140-6736(08)60107-5. [7] BHATIA M, WONG FL, CAO Y, et al. Pathophysiology of acute pancreatitis[J]. Pancreatology, 2005, 5(2-3): 132-144. DOI: 10.1159/000085265. [8] Pancreatic Surgery Group, Chinese Society of Surgery, Chinese Medical Association, Guidelines for diagnosis and treatment of acute pancreatitis in China (2021)[J]. Chin J Dig Surg, 2021, 20(7): 730-739. DOI: 10.3760/cma.j.cn112139-20210416-00172.中华医学会外科学分会胰腺外科学组. 中国急性胰腺炎诊治指南(2021)[J]. 中华消化外科杂志, 2021, 20(7): 730-739. DOI: 10.3760/cma.j.cn112139-20210416-00172. [9] ZHUANG J, ZHU WW, ZHANG C. Establishment and validation of a noninvasive diagnostic model for chronic hepatitis B liver fibrosis based on LASSO regression[J]. J Clin Hepatol, 2022, 38(8): 1790-1795. DOI: 10.3969/j.issn.1001-5256.2022.08.014.壮健, 朱韦文, 张超. 基于LASSO回归的慢性乙型肝炎肝纤维化无创诊断模型的构建及验证[J]. 临床肝胆病杂志, 2022, 38(8): 1790-1795. DOI: 10.3969/j.issn.1001-5256.2022.08.014. [10] GIBOR U, PERRY Z, NETZ U, et al. Circulating cell-free DNA in patients with acute biliary pancreatitis: association with disease markers and prolonged hospitalization time-A prospective cohort study[J]. Ann Surg, 2020, 2(3): 77-78. DOI: 10.1097/SLA.0000000000004679. [11] STERNBY H, HARTMAN H, THORLACIUS H, et al. The initial course of IL1β, IL-6, IL-8, IL-10, IL-12, IFN-γ and TNF-α with regard to severity grade in acute pancreatitis[J]. Biomolecules, 2021, 11(4). DOI: 10.3390/biom11040591. [12] AOUN E, CHEN J, REIGHARD D, et al. Diagnostic accuracy of interleukin-6 and interleuk- in-8 in predicting severe acute pancreatitis: a meta-analysis[J]. Pancreatology, 2009, 9(6): 777 -785. DOI: 10.1159/000214191. [13] LIANG ZX, PAN WC, MAI JW, et al. Application value of serum hs-CRP, IL-6 and TNF-α in the evaluation of acute pancreatitis[J]. Chin J Mod Drug Appl, 2020, 14(22): 48-50. DOI: 10.14164/j.cnki.cn11-5581/r.2020.22.021.梁灼星, 潘伟才, 麦静雯, 等. 血清hs-CRP、IL-6、TNF-α在急性胰腺炎病情评估中的应用价值研究[J]. 中国现代药物应用, 2020, 14(22): 48-50. DOI: 10.14164/j.cnki.cn11-5581/r.2020.22.021. [14] DI GIOIA M, SPREAFICO R, SPRINGSTEAD JR, et al. Endogenous oxidized phospholipids reprogram cellular metabolism and boost hyperinflammation[J]. Nat Immunol, 2020, 21(1): 42-53. DOI: 10.1038/s41590-019-0539-2. [15] YE M, JOOSSE M E, LIU L, et al. Deletion of IL-6 exacerbates colitis and induces systemic inflammation in IL-10-deficient mice[J]. J Crohns Colitis, 2020, 14(6): 831-840. DOI: 10.1093/ecco-jcc/jjz176. [16] ZHANG XP, CHEN L, HU QF, et al. Effects of large dose of dexamethasone on inflammatory mediators and pancreatic cell apoptosis of rats with severe acute pancreatitis[J]. World J Gastroenterol, 2007, 13(41): 5506-5511. DOI: 10.3748/wjg.v13.i41.5506. [17] HUANG Z, MA X, JIA X, et al. Prevention of severe acute pancreatitis with cyclooxygenase-2 inhibitors: A randomized controlled clinical trial[J]. Am J Gastroenterol, 2020, 115(3): 473-480. DOI: 10.14309/ajg.0000000000000529. [18] HE WH, ZHENG X, ZHU Y, et al. Comparison of APACHEⅡ, Ranson, BISAP and CTSI scores in early prediction of the severity of acute pancreatitis based on large sample database[J]. Chin J Pancreatol, 2019, 19(3): 172-176. DOI: 10.3760/cma.j.issn.1674-1935.2019.03.004.何文华, 郑西, 祝荫, 等. 基于大样本数据库比较APACHEⅡ、Ranson、BISAP和CTSI评分在早期预测急性胰腺炎病情严重程度的价值[J]. 中华胰腺病杂志, 2019, 19(3): 172-176. DOI: 10.3760/cma.j.issn.1674-1935.2019.03.004. 期刊类型引用(14)
1. 张子弋,王洪建. CD64及Th1/Th2细胞因子对SAP继发细菌感染的诊断价值. 新疆医学. 2025(01): 56-59 .  百度学术
百度学术2. 葛志文,聂时南,张炜,孙兆瑞,冯靖. 急性胰腺炎病情严重程度及预后的预测指标. 中国临床研究. 2025(02): 320-324 .  百度学术
百度学术3. 林梵,谭晓宇,刘骏. 急性胰腺炎患者miR-365a-3p、miR-10a-5p的表达变化及与疾病严重程度的关系. 国际检验医学杂志. 2025(05): 563-567+574 .  百度学术
百度学术4. 葛凯杰,孟佳,王化强. 炎症因子对重症急性胰腺炎的预测价值评估. 南通大学学报(医学版). 2025(01): 30-34 .  百度学术
百度学术5. 冯敏超,秦百君,罗芳,李凯,王宁,陈国忠,唐曦平. 清解化攻方调控NLRP3/TLR4/NF-κB信号通路对重症急性胰腺炎小鼠模型胰腺组织的保护作用. 临床肝胆病杂志. 2024(02): 343-350 .  本站查看
本站查看6. 张啸,王慧,余志宏,蓝海兵,龚园其. BISAP评分、白细胞介素-6、降钙素原与重症急性胰腺炎预后的相关性研究. 青岛医药卫生. 2024(01): 6-9 .  百度学术
百度学术7. 徐娇阳,张林,马春燕. 急性胰腺炎患者血清VEGF、IL-6及血浆D-D水平变化及其与病情严重程度的相关性. 中国实用医刊. 2024(02): 34-37 .  百度学术
百度学术8. 鲁庆欣,杨白婧. 清胰利胆颗粒联合艾司奥美拉唑对急性胰腺炎预后的影响. 现代医学与健康研究电子杂志. 2024(05): 88-90 .  百度学术
百度学术9. 陈斌,胡炜,刘东丽. 基于机器学习的重症急性胰腺炎的辅助诊断模型. 中国数字医学. 2024(03): 26-30 .  百度学术
百度学术10. 盛名,郭爽,王敬文. 血清铁蛋白、IL-6、TNF-α表达水平与高炎症表型急性呼吸窘迫综合征患者病情严重程度及早期预后的相关性. 临床和实验医学杂志. 2024(11): 1138-1141 .  百度学术
百度学术11. 秦耐宇,陈恳,田巍巍. 血清脂蛋白(a)、降钙素原、红细胞分布宽度水平与急性胰腺炎严重程度关系及其预后评估价值. 创伤与急危重病医学. 2024(01): 19-23 .  百度学术
百度学术12. 邝春玲,何六六,黄梨华,游晓燕,徐奕胜. 外周血前白蛋白、hs-CRP、SAA、IL-6联合检测在小儿上呼吸道感染中的应用价值. 中国医学创新. 2024(21): 128-131 .  百度学术
百度学术13. 赵东颖,王清华,于艳,姚旭. 大柴胡汤含药血清通过JAK2/STAT3信号通路抑制AR42J细胞炎症反应. 吉林中医药. 2024(08): 960-964 .  百度学术
百度学术14. 范远华,马彩霞,张敏,李明. 三黄泻心汤加减联合中药灌肠治疗重症急性胰腺炎合并脓毒血症临床观察. 中国中医急症. 2024(08): 1409-1412 .  百度学术
百度学术其他类型引用(3)
-




 PDF下载 ( 2570 KB)
PDF下载 ( 2570 KB)

 下载:
下载:




 下载:
下载:
 百度学术
百度学术




