肠-肝轴:肠道微生物稳态与肝细胞癌
DOI: 10.3969/j.issn.1001-5256.2023.11.029
-
摘要: 肠道微生物群在维持肝脏代谢稳态中扮演重要角色,其通过参与胆汁酸代谢影响肝细胞癌的发生发展。“肠-肝轴”在肝脏疾病发病机制中占据举足轻重的地位,通过纠正肠道生态失衡以恢复正常胆汁酸水平,可能是阻止肝细胞癌进展的有效方法之一。本文总结了胆汁酸受体影响肝细胞癌的相关机制以及最新治疗靶点,旨在为早期防治肝癌提供参考依据。Abstract: Intestinal microbiota plays an important role in maintaining liver metabolic homeostasis and affects the development and progression of hepatocellular carcinoma by participating in bile acid metabolism. Gut-liver axis plays an important role in the pathogenesis of liver diseases, and it might be one of the effective methods to prevent the progression of hepatocellular carcinoma by correcting intestinal ecological imbalance to restore normal bile acid level. This article summarizes the mechanism of bile acid receptor affecting hepatocellular carcinoma and the latest therapeutic targets, in order to provide a reference for the early prevention and treatment of hepatocellular carcinoma.
-
肝细胞癌(HCC)由肝细胞异常转化引起,是临床最常见的肝恶性肿瘤,在原发性肝癌中占比达70%~90%[1],是全球第六大最常见癌症和第四大癌症死亡原因,2018年有超过84万例新发病例,超过78万人死亡[2]。与原发性HCC相关的危险因素包括慢性HBV/HCV感染、黄曲霉毒素摄入、酒精滥用、吸烟、肥胖等。在我国,由于HBV和HCV的高感染率,肝癌患病率和病死率逐步上升。
肠道和肝脏在解剖学和生理学上相连,两者之间的关系被称为“肠-肝轴”。肠道微生物群及其产物、营养物质等通过门静脉血液转移至肝脏,反之,肝脏通过胆道系统将胆汁分泌至肠腔。近年来越来越多的证据[3-5]表明,肠-肝轴在维持肝脏稳态和HCC发生发展中扮演重要角色。本文将针对肠-肝轴介导的HCC发病机制及治疗研究进展作一综述。
1. 肠-肝轴概述
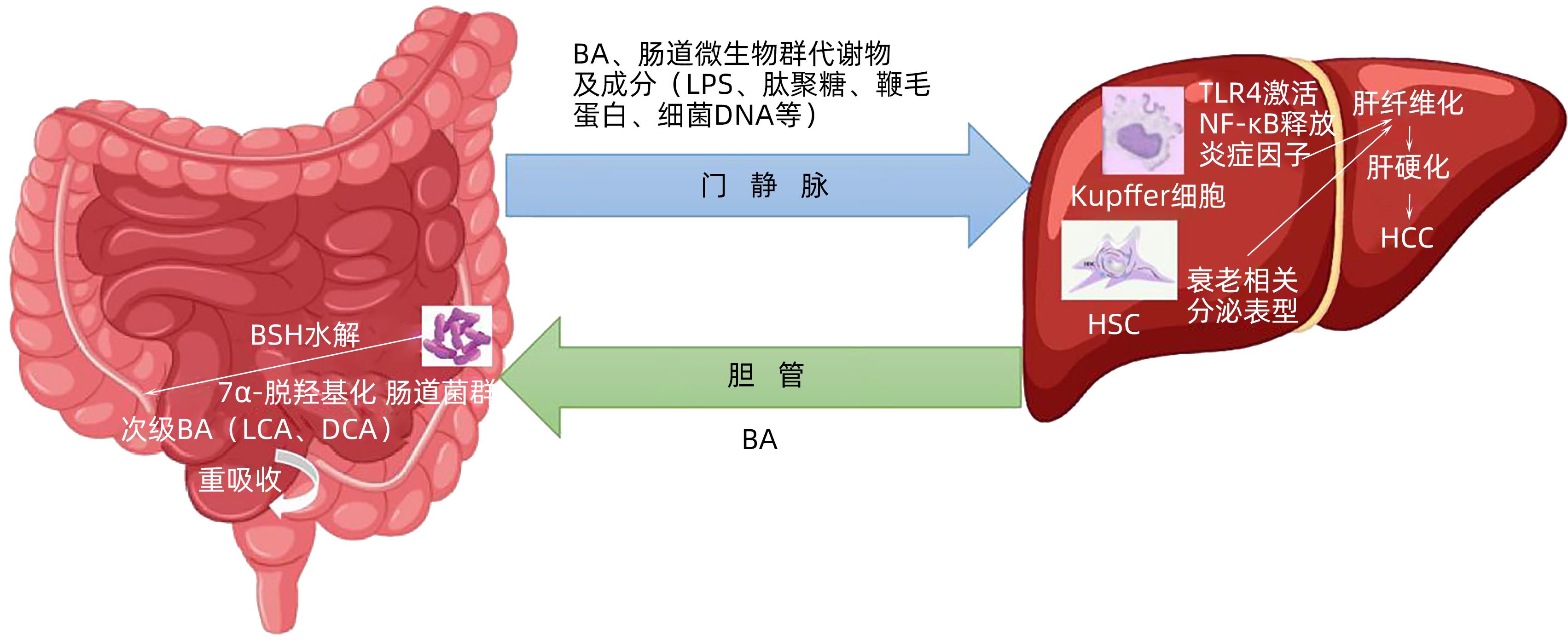
肠-肝轴是指肠道及其微生物群与肝脏之间的双向关系。肠道内75%的血液经肠系膜上、下腔静脉汇入门静脉从而进入肝脏,输送细菌及各种代谢产物,肠道内环境时刻影响着肝脏微环境[6]。与此同时,肝脏将胆汁的主要成分胆汁酸(bile acid,BA)运送至肠道,BA可促进肠道对膳食脂肪、类固醇、药物和亲脂性维生素的消化和吸收[7]。BA在肠道菌群的作用下,经水解、7α-脱羟基反应转变为次级BA,最终在回肠末端被重新吸收,运送回肝脏[8]。BA的生理变化及其所介导的信号通路影响着肠道屏障功能、菌群数量及组成。肠道屏障由黏液层和肠道上皮细胞组成[9],正常生理状况下,完整的肠道屏障能够阻止细菌、毒素等有害物质通过,同时允许营养物质进入循环并到达肝脏(图1)。
2. 肠-肝轴与HCC的关系
2.1 肠道微生物群与HCC
肠道微生物群的组分对于维持肠-肝轴的稳态至关重要,可影响BA代谢、肠道通透性和短链脂肪酸可利用性等[10],从而改变酒精、糖、脂质代谢。在不同个体之间可变性强,易被诸多因素影响,例如年龄、饮食、运动、使用抗生素频次和种类等。
2.1.1 参与BA代谢的肠道菌群失调
肝损伤与肠道生态失调的严重程度密切相关。参与BA代谢的肠道微生物群主要包括拟杆菌、梭杆菌、乳酸杆菌、双歧杆菌和李斯特菌,上述菌群通过生成胆盐水解酶(bile salt hydrolase,BSH)水解初级BA,HCC患者中检测到大肠杆菌、拟杆菌丰度升高,致使BSH生成增多[11],进一步增加循环中游离BA的含量,最终导致肝细胞线粒体功能障碍、细胞凋亡。
梭状芽孢杆菌属、真杆菌属、乳酸杆菌属和大肠杆菌属等通过7α-脱羟基化,将未结合的鹅去氧胆酸和胆酸转化为石胆酸(lithocholic acid,LCA)和脱氧胆酸(deoxy-cholate,DCA)[12]。疏水胆汁酸LCA、DCA具有细胞毒性,直接损害肝细胞膜、诱导细胞凋亡,释放炎症因子[13]。高水平的DCA诱导造血干细胞中的衰老相关分泌表型,进而在肝脏中分泌各种炎症因子和促癌因子,促进HCC发展[14]。
2.1.2 肠黏膜屏障破坏
肠道屏障的破坏与邻近肠道上皮细胞内的紧密连接受损相关,紧密连接的完整性对于防止病原体或外源性物质在肠道上皮细胞的易位至关重要[15]。当黏液相关防御被削弱,肠壁通透性增加,来自肠道微生物群的代谢物和成分将移位至肝脏,产生一系列毒副产物,又称为病原体相关分子模式(pathogen-associated molecular pattern,PAMP),如脂多糖(LPS)、肽聚糖、鞭毛蛋白、细菌DNA等[16]。Toll样受体(TLR)能够识别PAMP,在宿主防御中发挥重要作用[17]。TLR4为LPS受体,能够激活核因子κB(NF-κB)释放炎症因子,当炎症因子进入门静脉循环后便会对肝脏造成损伤[18]。此外,PAMP激活肝星状细胞(HSC),进一步促进肝纤维化[19],这种持续性损伤可导致严重肝纤维化最终发展为HCC。
酒精及高脂饮食已被证实可以改变肠道屏障的通透性,酒精使胃肠壁变薄,破坏其分层结构[20],使酒精更易吸收,导致血液中酒精水平升高,进一步促进后续肠道屏障损伤,这种恶性循环最终导致“肠瘘综合征”。高脂饮食直接下调紧密连接蛋白的表达,加剧肠道屏障损伤[21],还可使小肠细菌过度生长,增强炎症反应,并增加肠道微生物群中破坏黏膜屏障物种的数量[22]。此外,高脂饮食增强肝脏中胆固醇产生的BA代谢,可导致次级BA增多,过量的DCA产生活性氧,诱导人肝细胞、大鼠原代肝细胞凋亡[23]。
细菌易位及LPS的积累导致肠道微生物群组成发生改变,HCC患者中表现为高水平的大肠杆菌与低水平的乳酸杆菌、双歧杆菌和肠球菌[11]。研究[24]表明,肠道微生物群和TLR4的激活通过促进细胞增殖和抑制细胞凋亡而促进HCC的发生发展。
2.2 BA受体与HCC
肝脏和肠道中BA信号的改变与HCC相关,肠-肝轴稳态依赖于严格控制BA水平,以避免BA超载,当代谢稳态被打破,异常的BA水平将诱导细胞凋亡和炎症[25]。游离BA可激活BA信号受体,如法尼醇X受体(farnesoid X receptor,FXR)、G蛋白偶联胆汁酸受体5(G protein coupled bile acid receptor 5,TGR5)、孕烷X受体(pregnane X receptor,PXR)、鞘氨醇-1-磷酸受体2(sphingosine-1-phosphate receptor 2,S1PR2)、本构雄烷受体(constitutive androstane receptor,CAR)、维生素D受体(vitamin D receptor,VDR)等[3]。
2.2.1 FXR
FXR是主要的BA受体,通过调节肝脏和肠道中BA的摄取、合成、结合和运输,维持BA的稳态[26]。BA过度积累引起的慢性肝损伤被认为是HCC发病的原因之一,在肝脏中,FXR激活小异源二聚体伴侣受体(small heterodimer partner,SHP)表达,从而抑制限速酶胆固醇7α单加氧酶(cholesterol 7-alpha-monooxygenase,CYP7A1)的水平[27],该酶能够催化胆固醇合成BA;FXR还能够通过刺激成纤维细胞生长因子(fibroblast growth factor,FGF)15及其同源基因FGF19的合成,抑制CYP7A1的表达,负调控BA合成[28]。此外,FXR可通过增加胆盐输出泵的表达,促进胆汁分泌,抑制肝脏慢性炎症、肝细胞损伤[29]。
FXR不仅调节BA代谢和肠肝循环,还能够抑制炎症信号、增强组织修复。FXR的激活可降低肝脏促炎细胞因子IL-1β、IL-6和TNF-α的表达[30]。在肠道中,FXR的激活通过恢复肠道FXR-FGF15轴,保持肠上皮黏膜完整性,限制肝脏炎症和异常肝细胞的增殖[28]。
2.2.2 TGR5
TGR5是一种G蛋白偶联胆汁酸细胞表面受体,广泛分布于人体,在脾脏、胎盘、胃等多种组织中高表达,调节能量稳态、BA稳态和葡萄糖代谢[31],BA通过激活TGR5抑制LPS,从而降低促炎细胞因子水平,包括IL-1α、IL-1β、IL-6、TNF-α等[23]。最近一项研究[32]也表明,TGR5的激活极大地抑制了体外人肝癌细胞的增殖和迁移,而TGR5的缺失增强了化学诱导的肝癌发生,表明TGR5是一种新的抑癌基因。
据报道[33],TGR5可激活信号转导及转录激活蛋白3(signal transducer and activator of transcription 3,STAT3),STAT3是一种在肝脏炎症和HCC中起关键作用的转录因子,TGR5的激活抑制了STAT3的磷酸化,抑制其转录和DNA结合活性。与野生型小鼠相比,TGR5缺陷小鼠对酒精诱导的急性肝损伤和脂肪变性更为敏感,且增加促炎细胞因子的表达。研究[34]发现,基质金属蛋白酶在TGR5缺陷小鼠中的表达较高,而基质金属蛋白酶能够促进肿瘤细胞增殖、抑制凋亡、刺激侵袭转移、促进血管生成,这可能促进了HCC的发育和转移。
2.2.3 PXR
PXR是核受体蛋白家族成员,在肝脏和胃肠道组织中表达,通过下调NF-κB减少炎性细胞因子、趋化因子和黏附分子的产生[35]。当BA呈高水平时,PXR与FXR协同作用,调节和消除BA。肠上皮细胞内,PXR激活FGF19/FGF15,通过抑制肝脏中CYP7A1的转录,抑制BA的合成[36]。研究[37]表明,PXR激活剂可增加人肝细胞膜蛋白的表达,而人肝细胞膜蛋白能够降低肝脏BA水平、防止细胞坏死,在保护肝脏免受BA超载方面发挥突出作用。
2.2.4 S1PR2
S1PR2是鞘氨醇-1-磷酸的G蛋白偶联受体,表达于肝细胞、胆管细胞、HSC、肝窦内皮细胞和巨噬细胞中。S1PR2在胆汁淤积性肝小鼠中通过抑制巨噬细胞趋化因子诱导的细胞运动,促进巨噬细胞在炎症部位的募集[38],在肝细胞中,S1PR2激活细胞外信号调节激酶和蛋白激酶B磷酸化诱导环氧合酶2的表达,发挥促炎的作用[39]。
2.2.5 CAR
CAR被认为是BA的间接传感器,目前为止未发现主要的BA直接与之相结合,主要在肝脏中表达。CAR通过调节细胞色素酶CYP450介导BA代谢,催化BA的合成、氧化、葡萄糖醛酸化[40]。在人肝细胞中,CAR的激活同样抑制CYP7A1的表达,与FXR、PXR共同降低BA肝毒性[41]。
2.2.6 VDR
VDR的配体是维生素D的活性形式,即骨化三醇,在肾、肠、骨和肝细胞中高表达,参与细胞增殖、细胞分化和免疫,通过调节巨噬细胞、T淋巴细胞发挥抗炎作用。在肠道中,VDR通过细胞色素P450 3A4削弱BA毒性[42];在Kupffer细胞中,VDR的激活降低了饮食诱导的肥胖小鼠的炎症反应[43]。此外,LCA可激活VDR,诱导磺酸基转移酶2A1、基底侧多药耐药相关蛋白3和BA转运蛋白的表达,刺激BA排泄和转运[44]。综上研究所述,VDR的激活可能参与BA吸收,抑制BA的合成,并促进参与LCA解毒酶的表达。
3. 基于肠-肝轴在治疗HCC中的新探索
3.1 益生菌
益生菌是指活跃的、有益的微生物,定居在人类的肠道和生殖系统中,可改善宿主的微生态平衡,并发挥有益的作用。人和动物的益生菌主要包括丁酸梭菌、乳酸杆菌、双歧杆菌、放线菌和酵母。可通过改变肠道微生物群的类型和数量减少肠道炎症,维持肠道菌群平衡,改善肠道屏障功能,抑制HCC的发展[45]。
鼠李糖乳杆菌GG(lactobacillus rhamnosus GG,LGG)是一种益生菌菌株,已被广泛应用于肝脏疾病的预防和治疗。最近的一项研究[46]表明,LGG通过激活肠道FXR-FGF15信号通路,降低CYP7A1、SHP表达和肝脏BA的合成,显著减轻了胆管结扎小鼠的肝脏炎症、肝损伤和纤维化。此外,LGG可增强具有BSH活性的肠道微生物群,以增强BA的排泄,还可激活肠道FXR以抑制小鼠肝脏BA的合成,促进尿BA的排泄[47]。上述结果表明,LGG可用于预防及治疗与肝BA水平升高相关的肝病。乳酸乳球菌和白藜芦醇通过灭活丝裂原活化蛋白激酶(mitogen-activated protein kinase,MAPK)/NF-κB信号,恢复紧密连接,小鼠肝功能得以改善,包括血清转氨酶、胆红素水平,血清内毒素、甘油三酯、总胆固醇指标显著下降[48]。动物模型研究[49]表明,益生菌有助于抑制黄曲霉毒素B1诱导的HCC发生,恢复肠道生态平衡,降低LPS水平,减轻氧化应激。一项动物研究[50]表明,从中国传统发酵食品中分离的植物乳杆菌C88增加了小鼠粪便中黄曲霉毒素B1的排泄,可作为潜在的HCC治疗策略。
3.2 粪菌移植(fecal microbiota transfer,FMT)
FMT是一种将来自健康供体粪便中的功能菌群移植到患者的胃肠道,以重建新的肠道菌群治疗肠道和肠外疾病的新技术[51]。动物研究[52]显示,酒精敏感小鼠在FMT后肠道微生物群成分发生改变,阻止了酒精诱导的肝损伤。与益生菌相比,FMT对肠道黏膜屏障功能的改变更显著[53]。对慢加急性肝衰竭小鼠行FMT,可显著减轻肝脏炎症,恢复肠道微生物群的多样性[54]。一项纳入33例严重酒精性肝炎患者的临床试验[55]表明,FMT能够改善28、90天存活率以及出现肝性脑病患者的认知能力。FMT可能通过逆转肠道菌群失调、减少细胞毒性代谢物或炎症介质的产生,抑制HCC的发展。需要注意的是,FMT目前正被纳入非酒精性脂肪性肝炎和肝硬化的临床试验评估,但关于FMT在HCC中的治疗研究尚少,亟需开展更多动物试验以评估FMT的实用性和安全性。目前尚不清楚FMT是否能够永久恢复HCC患者肠道微生物群的改变,此类患者通常免疫受到抑制,由此造成的细菌易位是否有并发菌血症的风险难以评估。
3.3 抗生素
在HCC患者中,尽管使用利福昔明可降低自发性腹膜炎发生率,但与死亡风险无关[56],提示肿瘤本身可能比HCC并发症对生存率的影响更大。既往研究[57]报道,HCC是导致肝性脑病预后不良的独立危险因素,HCC患者的芳香族氨基酸水平高于非HCC患者,提示芳香族和支链氨基酸的失衡与HCC患者肝性脑病的发生有关,HCC的存在会影响肝性脑病的不良预后,并降低利福昔明在预防肝性脑病复发方面的作用。
3.4 TLR4阻滞剂
TLR4阻滞剂Eritoran tetrasodium是一种合成脂类球形红杆菌类似物,可通过减少HSC的激活以及降低α-平滑肌肌动蛋白、转化生长因子-β1的丰度,减轻肝纤维化,显著降低血清ALT水平及肝脏炎症细胞浸润[58]。在体外研究[59]中,Eritoran tetrasodium显著抑制了LPS诱导的原发性造血干细胞和Kupffer细胞的核易位,阻断NF-κB的激活,抑制促炎细胞因子、促纤维化介质以及活性氧的释放;在慢性肝损伤小鼠模型中,Eritoran tetrasodium阻断TLR4介导的炎症反应,减少肝脏炎症细胞浸润,减轻肝纤维化[60]。
3.5 FXR激活剂、TGR5激动剂
现有的FXR激活剂包括GW4064、奥贝胆酸(obeticholic acid,OCA)、熊去氧胆酸。经GW4064干预后,CYP7A1的表达水平下调,肝组织学和血清转氨酶活性均得到改善[61],表明GW4064可以纠正BA的代谢异常,使结合BA水平升高,从而减轻肝毒性。OCA是一种半合成BA类似物和有效的FXR激动剂,长期给药可改善肝纤维化、脂肪变性和小叶炎症,防止病情进展,抑制BA输入转运体,诱导BA输出泵[62]。在胆汁淤积小鼠模型中采用熊去氧胆酸治疗,可观察到血清和肝脏中BA显著降低,总BA含量恢复至正常水平,组织学观察到修复及肝细胞再生[63]。INT-767作为FXR与TGR5双激动剂,是一种新型的OCA硫酸盐衍生物,通过减少胆道BA输出,改善胆汁淤积表型[64]。研究[65]表明,INT-767可阻止饮食诱导的肝脂肪变性、炎症和纤维化进展,减少肠道微生物群失调,改善肝脏的组织学特征,并减少促炎细胞因子的产生及BA合成,但需要长期临床试验确定INT-767在HCC高危患者中的预防及治疗效果。
3.6 中草药
在萘硫氰酸酯诱导的胆汁淤积小鼠中,使用茉莉花多糖(G. jasminoides Ellis polysaccharide,GPS)能够使转氨酶水平降低3~7倍,改善肝组织损伤,上调FXR、PXR及其下游外排转运体的表达,从而降低BA水平。此外,GPS可改善肠道微生物群失调,改善肠道屏障功能,并使LPS水平降低1.5倍,GPS可抑制TLR4/NF-κB信号转导,降低炎症因子的表达,改善肝脏炎症[66],利用草药多糖可能对胆汁淤积性肝病具有独特的治疗前景。
4. 展望
HCC通常由慢性肝病引起,肠道微生物群能够介导HCC的发生,BA受体异常可能导致肝脏炎症、损伤、代谢稳态破坏,最终促进HCC的发展,通过纠正肠道生态失调以恢复BA正常水平可能是阻止HCC进展的有效方法。在治疗方面,TLR4、FXR、TGR5可能成为治疗HCC的新靶点,但在目前的研究中依然存在一些局限性:(1)小鼠产生的肿瘤不一定与人类相似,饲料是否对BA代谢产生影响、试验是否可代表人类HCC发展进程,上述问题值得进一步探讨;(2)来自不同种族或国家的不同个体,其肠道微生物组成可能存在差异,定植在人体的肠道固有菌群对植入菌种有一定抵抗性,补充同种、等量益生菌难以使所有人受益;(3)对于FMT供体的选择需慎重,如何控制病原体感染的风险需要进一步研究;(4)目前尚不清楚补充肠道益生菌对于早期或晚期HCC患者疗效的差异,仍需临床大样本队列研究观察微生物群和BA的动态变化是否与患者HCC分期相关。期待未来研究能够着眼于特定菌群在个体化治疗上的应用,以及更多BA受体的发掘,为HCC的早期诊断、预防及精准治疗提供新的依据。
-
[1] WANG GQ, JIANG Y, LU CD, et al. CircFOXM1 promotes proliferation and metastasis of hepatocellular carcinoma via regulating miR-1179/SPAG5 axis[J]. Sci Rep, 2021, 11( 1): 23890. DOI: 10.1038/s41598-021-03285-w. [2] COSTANTE F, AIROLA C, SANTOPAOLO F, et al. Immunotherapy for nonalcoholic fatty liver disease-related hepatocellular carcinoma: Lights and shadows[J]. World J Gastrointest Oncol, 2022, 14( 9): 1622- 1636. DOI: 10.4251/wjgo.v14.i9.1622. [3] WU LW, FENG J, LI JJ, et al. The gut microbiome-bile acid axis in hepatocarcinogenesis[J]. Biomed Pharmacother, 2021, 133: 111036. DOI: 10.1016/j.biopha.2020.111036. [4] CHIANG JYL, FERRELL JM. Bile acid metabolism in liver pathobiology[J]. Gene Expr, 2018, 18( 2): 71- 87. DOI: 10.3727/105221618X15156018385515. [5] GUPTA H, YOUN GS, SHIN MJ, et al. Role of gut microbiota in hepatocarcinogenesis[J]. Microorganisms, 2019, 7( 5): 121. DOI: 10.3390/microorganisms7050121. [6] SCHNEIDER KM, MOHS A, GUI WF, et al. Imbalanced gut microbiota fuels hepatocellular carcinoma development by shaping the hepatic inflammatory microenvironment[J]. Nat Commun, 2022, 13( 1): 3964. DOI: 10.1038/s41467-022-31312-5. [7] ZHANG R, MA WQ, FU MJ, et al. Overview of bile acid signaling in the cardiovascular system[J]. World J Clin Cases, 2021, 9( 2): 308- 320. DOI: 10.12998/wjcc.v9.i2.308. [8] CHEN MJ, LIU C, WAN Y, et al. Enterohepatic circulation of bile acids and their emerging roles on glucolipid metabolism[J]. Steroids, 2021, 165: 108757. DOI: 10.1016/j.steroids.2020.108757. [9] DONG SJ, ZHU M, WANG K, et al. Dihydromyricetin improves DSS-induced colitis in mice via modulation of fecal-bacteria-related bile acid metabolism[J]. Pharmacol Res, 2021, 171: 105767. DOI: 10.1016/j.phrs.2021.105767. [10] GRÜNER N, MATTNER J. Bile acids and microbiota: Multifaceted and versatile regulators of the liver-gut axis[J]. Int J Mol Sci, 2021, 22( 3): 1397. DOI: 10.3390/ijms22031397. [11] MOHAMADKHANI A. On the potential role of intestinal microbial community in hepatocarcinogenesis in chronic hepatitis B[J]. Cancer Med, 2018, 7( 7): 3095- 3100. DOI: 10.1002/cam4.1550. [12] SONG I, GOTOH Y, OGURA Y, et al. Comparative genomic and physiological analysis against Clostridium scindens reveals Eubacterium sp. c-25 as an atypical deoxycholic acid producer of the human gut microbiota[J]. Microorganisms, 2021, 9( 11): 2254. DOI: 10.3390/microorganisms9112254. [13] ZENG HW, UMAR S, RUST B, et al. Secondary bile acids and short chain fatty acids in the colon: A focus on colonic microbiome, cell proliferation, inflammation, and cancer[J]. Int J Mol Sci, 2019, 20( 5): 1214. DOI: 10.3390/ijms20051214. [14] LIU P, TANG QH, CHEN MM, et al. Hepatocellular senescence: Immunosurveillance and future senescence-induced therapy in hepatocellular carcinoma[J]. Front Oncol, 2020, 10: 589908. DOI: 10.3389/fonc.2020.589908. [15] GENG SJ, CHENG SS, LI Y, et al. Faecal microbiota transplantation reduces susceptibility to epithelial injury and modulates tryptophan metabolism of the microbial community in a piglet model[J]. J Crohns Colitis, 2018, 12( 11): 1359- 1374. DOI: 10.1093/ecco-jcc/jjy103. [16] SEBŐK C, TRÁJ P, VÖRÖSHÁZI J, et al. Two sides to every question: Attempts to activate chicken innate immunity in 2D and 3D hepatic cell cultures[J]. Cells, 2021, 10( 8): 1910. DOI: 10.3390/cells10081910. [17] ZHANG WJ, LI KY, HUANG BH, et al. The hepatocyte in the innate immunity[J]. Virology, 2022, 576: 111- 116. DOI: 10.1016/j.virol.2022.09.011. [18] CHEN SN, TAN Y, XIAO XC, et al. Deletion of TLR4 attenuates lipopolysaccharide-induced acute liver injury by inhibiting inflammation and apoptosis[J]. Acta Pharmacol Sin, 2021, 42( 10): 1610- 1619. DOI: 10.1038/s41401-020-00597-x. [19] GAO B, AHMAD MF, NAGY LE, et al. Inflammatory pathways in alcoholic steatohepatitis[J]. J Hepatol, 2019, 70( 2): 249- 259. DOI: 10.1016/j.jhep.2018.10.023. [20] WANG SC, CHEN YC, CHEN SJ, et al. Alcohol addiction, gut microbiota, and alcoholism treatment: A review[J]. Int J Mol Sci, 2020, 21( 17): 6413. DOI: 10.3390/ijms21176413. [21] ROHR MW, NARASIMHULU CA, RUDESKI-ROHR TA, et al. Negative effects of a high-fat diet on intestinal permeability: A review[J]. Adv Nutr, 2020, 11( 1): 77- 91. DOI: 10.1093/advances/nmz061. [22] MALESZA IJ, MALESZA M, WALKOWIAK J, et al. High-fat, western-style diet, systemic inflammation, and gut microbiota: A narrative review[J]. Cells, 2021, 10( 11): 3164. DOI: 10.3390/cells10113164. [23] GILLARD J, CLERBAUX LA, NACHIT M, et al. Bile acids contribute to the development of non-alcoholic steatohepatitis in mice[J]. JHEP Rep, 2021, 4( 1): 100387. DOI: 10.1016/j.jhepr.2021.100387. [24] BEYOĞLU D, IDLE JR. The gut microbiota- A vehicle for the prevention and treatment of hepatocellular carcinoma[J]. Biochem Pharmacol, 2022, 204: 115225. DOI: 10.1016/j.bcp.2022.115225. [25] XUE R, SU LY, LAI SY, et al. Bile acid receptors and the gut-liver axis in nonalcoholic fatty liver disease[J]. Cells, 2021, 10( 11): 2806. DOI: 10.3390/cells10112806. [26] WANG TY, WEI W, ZHONG H, et al. Progress on the treatment of metabolic dysfunction-associated fatty liver disease by farnesol X receptor agonist[J/CD]. Chin J Liver Dis Electron Version, 2023, 15( 1): 6- 11. DOI: 10.3969/j.issn.1674-7380.2023.01.002.王霆宇, 魏尉, 钟黄, 等. 法尼醇X受体激动剂对非酒精性脂肪性肝炎治疗作用研究进展[J/CD]. 中国肝脏病杂志(电子版), 2023, 15( 1): 6- 11. DOI: 10.3969/j.issn.1674-7380.2023.01.002. [27] LI YQ, TIAN YY, CAI WZ, et al. Novel ι-carrageenan tetrasaccharide alleviates liver lipid accumulation via the bile acid-FXR-SHP/PXR pathway to regulate cholesterol conversion and fatty acid metabolism in insulin-resistant mice[J]. J Agric Food Chem, 2021, 69( 34): 9813- 9821. DOI: 10.1021/acs.jafc.1c04035. [28] HARTMANN P, HOCHRATH K, HORVATH A, et al. Modulation of the intestinal bile acid/farnesoid X receptor/fibroblast growth factor 15 axis improves alcoholic liver disease in mice[J]. Hepatology, 2018, 67( 6): 2150- 2166. DOI: 10.1002/hep.29676. [29] ZHONG XC, LIU YM, GAO XX, et al. Caffeic acid phenethyl ester suppresses intestinal FXR signaling and ameliorates nonalcoholic fatty liver disease by inhibiting bacterial bile salt hydrolase activity[J]. Acta Pharmacol Sin, 2023, 44( 1): 145- 156. DOI: 10.1038/s41401-022-00921-7. [30] IRACHETA-VELLVE A, CALENDA CD, PETRASEK J, et al. FXR and TGR5 agonists ameliorate liver injury, steatosis, and inflammation after binge or prolonged alcohol feeding in mice[J]. Hepatol Commun, 2018, 2( 11): 1379- 1391. DOI: 10.1002/hep4.1256. [31] SHAPIRO H, KOLODZIEJCZYK AA, HALSTUCH D, et al. Bile acids in glucose metabolism in health and disease[J]. J Exp Med, 2018, 215( 2): 383- 396. DOI: 10.1084/jem.20171965. [32] JIA W, XIE GX, JIA WP. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis[J]. Nat Rev Gastroenterol Hepatol, 2018, 15( 2): 111- 128. DOI: 10.1038/nrgastro.2017.119. [33] LI CL, LIN YK, CHEN HA, et al. Smoking as an independent risk factor for hepatocellular carcinoma due to the α7-nachr modulating the JAK2/STAT3 signaling axis[J]. J Clin Med, 2019, 8( 9): 1391. DOI: 10.3390/jcm8091391. [34] SPATZ M, CIOCAN D, MERLEN G, et al. Bile acid-receptor TGR5 deficiency worsens liver injury in alcohol-fed mice by inducing intestinal microbiota dysbiosis[J]. JHEP Rep, 2021, 3( 2): 100230. DOI: 10.1016/j.jhepr.2021.100230. [35] ZHANG GH, LIU MJ, SONG M, et al. Patchouli alcohol activates PXR and suppresses the NF-κB-mediated intestinal inflammatory[J]. J Ethnopharmacol, 2020, 248: 112302. DOI: 10.1016/j.jep.2019.112302. [36] SHIN DJ, WANG L. Bile acid-activated receptors: A review on FXR and other nuclear receptors[J]. Handb Exp Pharmacol, 2019, 256: 51- 72. DOI: 10.1007/164_2019_236. [37] LIU X, WANG Y. An overview of bile acid synthesis and its physiological and pathological functions[J]. Hereditas, 2019, 41( 5): 365- 374. DOI: 10.16288/j.yczz.19-011.刘笑, 王琰. 胆汁酸的合成调控及其在生理与病理中的功能机制[J]. 遗传, 2019, 41( 5): 365- 374. DOI: 10.16288/j.yczz.19-011. [38] LIU L, PANZITT K, RACEDO S, et al. Bile acids increase steroidogenesis in cholemic mice and induce cortisol secretion in adrenocortical H295R cells via S1PR2, ERK and SF-1[J]. Liver Int, 2019, 39( 11): 2112- 2123. DOI: 10.1111/liv.14052. [39] KWONG EK, ZHOU HP. Sphingosine-1-phosphate signaling and the gut-liver axis in liver diseases[J]. Liver Res, 2019, 3( 1): 19- 24. DOI: 10.1016/j.livres.2019.02.003. [40] GOETTEL M, FEGERT I, HONARVAR N, et al. Comparative studies on the effects of sodium phenobarbital and two other constitutive androstane receptor(CAR) activators on induction of cytochrome P450 enzymes and replicative DNA synthesis in cultured hepatocytes from wild type and CAR knockout rats[J]. Toxicology, 2020, 433-434: 152394. DOI: 10.1016/j.tox.2020.152394. [41] NOH K, CHOW ECY, QUACH HP, et al. Significance of the vitamin D receptor on crosstalk with nuclear receptors and regulation of enzymes and transporters[J]. AAPS J, 2022, 24( 4): 71. DOI: 10.1208/s12248-022-00719-9. [42] QIN X, WANG X. Role of vitamin D receptor in the regulation of CYP3A gene expression[J]. Acta Pharm Sin B, 2019, 9( 6): 1087- 1098. DOI: 10.1016/j.apsb.2019.03.005. [43] DONG BN, ZHOU Y, WANG W, et al. Vitamin D receptor activation in liver macrophages ameliorates hepatic inflammation, steatosis, and insulin resistance in mice[J]. Hepatology, 2020, 71( 5): 1559- 1574. DOI: 10.1002/hep.30937. [44] ŠARENAC TM, MIKOV M. Bile acid synthesis: From nature to the chemical modification and synthesis and their applications as drugs and nutrients[J]. Front Pharmacol, 2018, 9: 939. DOI: 10.3389/fphar.2018.00939. [45] FUKUI H. Role of gut dysbiosis in liver diseases: What have we learned so far?[J]. Diseases, 2019, 7( 4): 58. DOI: 10.3390/diseases7040058. [46] LIU YH, CHEN KF, LI FY, et al. Probiotic Lactobacillus rhamnosus GG prevents liver fibrosis through inhibiting hepatic bile acid synthesis and enhancing bile acid excretion in mice[J]. Hepatology, 2020, 71( 6): 2050- 2066. DOI: 10.1002/hep.30975. [47] CHEN QW, LI QR, CAO MW, et al. Hierarchy-assembled dual probiotics system ameliorates cholestatic drug-induced liver injury via gut-liver axis modulation[J]. Adv Sci, 2022, 9( 17): e2200986. DOI: 10.1002/advs.202200986. [48] MENDES KL, DE FARIAS LELIS D, DE FREITAS DF, et al. Acute oral treatment with resveratrol and Lactococcus Lactis Subsp. Lactis decrease body weight and improve liver proinflammatory markers in C57BL/6 mice[J]. Mol Biol Rep, 2021, 48( 2): 1725- 1734. DOI: 10.1007/s11033-021-06190-7. [49] GUERRERO-ENCINAS I, GONZÁLEZ-GONZÁLEZ JN, SANTIAGO-LÓPEZ L, et al. Protective effect of Lacticaseibacillus casei CRL 431 postbiotics on mitochondrial function and oxidative status in rats with aflatoxin B1-induced oxidative stress[J]. Probiotics Antimicrob Proteins, 2021, 13( 4): 1033- 1043. DOI: 10.1007/s12602-021-09747-x. [50] HUANG L, ZHAO ZJ, DUAN CC, et al. Lactobacillus plantarum C88 protects against aflatoxin B1-induced liver injury in mice via inhibition of NF-κB-mediated inflammatory responses and excessive apoptosis[J]. BMC Microbiol, 2019, 19( 1): 170. DOI: 10.1186/s12866-019-1525-4. [51] DUAN RX, ZHAO YF, YE YJ, et al. Mechanism of action of bile acid metabolism in regulating inflamm atory bowel disease and the research and development of drugs[J]. Chin J Chin Pharmacol Ther, 2022, 27( 10): 1171- 1181. DOI: 10.12092/j.issn.1009-2501.2022.10.012.段睿潇, 赵一帆, 叶永娟, 等. 胆汁酸代谢调节炎症性肠病的作用机制及药物研发[J]. 中国临床药理学与治疗学, 2022, 27( 10): 1171- 1181. DOI: 10.12092/j.issn.1009-2501.2022.10.012. [52] LIU HX, KANG X, YANG XD, et al. Compound probiotic ameliorates acute alcoholic liver disease in mice by modulating gut microbiota and maintaining intestinal barrier[J]. Probiotics Antimicrob Proteins, 2023, 15( 1): 185- 201. DOI: 10.1007/s12602-022-10005-x. [53] KIM ER, PARK JS, KIM JH, et al. A GLP-1/GLP-2 receptor dual agonist to treat NASH: Targeting the gut-liver axis and microbiome[J]. Hepatology, 2022, 75( 6): 1523- 1538. DOI: 10.1002/hep.32235. [54] GAO A, XU YJ, LU SW, et al. Effect of fecal microbiota transplantation on intestinal flora in mice with acute-on-chronic liver failure[J]. J Clin Hepatol, 2021, 37( 6): 1379- 1385. DOI: 10.3969/j.issn.1001-5256.2021.06.031.高安, 徐玉静, 陆圣威, 等. 粪菌移植对慢加急性肝衰竭小鼠模型肠道菌群的影响[J]. 临床肝胆病杂志, 2021, 37( 6): 1379- 1385. DOI: 10.3969/j.issn.1001-5256.2021.06.031. [55] SHARMA A, ROY A, PREMKUMAR M, et al. Fecal microbiota transplantation in alcohol-associated acute-on-chronic liver failure: An open-label clinical trial[J]. Hepatol Int, 2022, 16( 2): 433- 446. DOI: 10.1007/s12072-022-10312-z. [56] KANG SH, LEE YB, LEE JH, et al. Rifaximin treatment is associated with reduced risk of cirrhotic complications and prolonged overall survival in patients experiencing hepatic encephalopathy[J]. Aliment Pharmacol Ther, 2017, 46( 9): 845- 855. DOI: 10.1111/apt.14275. [57] TAJIRI K, SHIMIZU Y. Branched-chain amino acids in liver diseases[J]. Transl Gastroenterol Hepatol, 2018, 3: 47. DOI: 10.21037/tgh.2018.07.06. [58] SIVAK KV, STOSMAN KI, RASSOKHA TA, et al. The effect of TLR4 blockade on some indicators of systemic inflammatory response to Proteus mirabilis LPS in rats[J]. Bull Exp Biol Med, 2020, 169( 6): 795- 797. DOI: 10.1007/s10517-020-04981-9. [59] PARADA E, CASAS AI, PALOMINO-ANTOLIN A, et al. Early toll-like receptor 4 blockade reduces ROS and inflammation triggered by microglial pro-inflammatory phenotype in rodent and human brain ischaemia models[J]. Br J Pharmacol, 2019, 176( 15): 2764- 2779. DOI: 10.1111/bph.14703. [60] HSIEH YC, LEE KC, WU PS, et al. Eritoran attenuates hepatic inflammation and fibrosis in mice with chronic liver injury[J]. Cells, 2021, 10( 6): 1562. DOI: 10.3390/cells10061562. [61] CAO Y, XIAO YT, ZHOU KJ, et al. FXR agonist GW4064 improves liver and intestinal pathology and alters bile acid metabolism in rats undergoing small intestinal resection[J]. Am J Physiol Gastrointest Liver Physiol, 2019, 317( 2): G108- G115. DOI: 10.1152/ajpgi.00356.2017. [62] DENG H, ZHANG B, ZHU B, et al. Research advances in the role of gut microbiota in chronic hepatitis B, chronic hepatitis C, and related liver diseases[J]. J Clin Hepatol, 2022, 38( 5): 1143- 1147. DOI: 10.3969/j.issn.1001-5256.2022.05.035.邓辉, 张斌, 朱彬, 等. 肠道菌群影响慢性乙型肝炎、慢性丙型肝炎及相关肝病的研究进展[J]. 临床肝胆病杂志, 2022, 38( 5): 1143- 1147. DOI: 10.3969/j.issn.1001-5256.2022.05.035. [63] de BOER JF, de VRIES HD, PALMIOTTI A, et al. Cholangiopathy and biliary fibrosis in Cyp2c70-deficient mice are fully reversed by ursodeoxycholic acid[J]. Cell Mol Gastroenterol Hepatol, 2021, 11( 4): 1045- 1069. DOI: 10.1016/j.jcmgh.2020.12.004. [64] ITO K, OKUMURA A, TAKEUCHI JS, et al. Dual agonist of farnesoid X receptor and takeda G protein-coupled receptor 5 inhibits hepatitis B virus infection in vitro and in vivo[J]. Hepatology, 2021, 74( 1): 83- 98. DOI: 10.1002/hep.31712. [65] WANG XX, XIE C, LIBBY AE, et al. The role of FXR and TGR5 in reversing and preventing progression of Western diet-induced hepatic steatosis, inflammation, and fibrosis in mice[J]. J Biol Chem, 2022, 298( 11): 102530. DOI: 10.1016/j.jbc.2022.102530. [66] FANG S, WANG TM, LI YY, et al. Gardenia jasminoides Ellis polysaccharide ameliorates cholestatic liver injury by alleviating gut microbiota dysbiosis and inhibiting the TLR4/NF-κB signaling pathway[J]. Int J Biol Macromol, 2022, 205: 23- 36. DOI: 10.1016/j.ijbiomac.2022.02.056. 期刊类型引用(5)
1. 蓝婧,陈丽芬,江秋维,程读创,韦月辽. 肥胖人群肝脂肪变性相关因素及与IGN代谢指标的关系. 吉林医学. 2025(03): 527-529 .  百度学术
百度学术2. 卢美希,姜润秋. 肝癌相关成纤维细胞中FXR和TGR5表达与T细胞浸润及预后的相关性分析. 江苏大学学报(医学版). 2025(02): 139-144+153 .  百度学术
百度学术3. 储云茜,薛雅,蒋华,戚春建,戴菡珏,仙晴颖,朱文宇. 肠菌移植联合免疫检查点抑制剂在终末期恶性肿瘤患者治疗中的探索性研究. 中国临床药理学与治疗学. 2025(04): 509-516 .  百度学术
百度学术4. 江林,张玉涵,张凌宵,黄旲,刘颖斌. 肿瘤内菌群在消化系统肿瘤中的研究进展. 中华消化外科杂志. 2024(06): 868-875 .  百度学术
百度学术5. 唐郑,林伟刚,余仁欢,蔡俊普,梁文欣,安路,张亚迪. 基于中焦理论的肠道-器官轴中医内涵综述. 时珍国医国药. 2024(10): 2421-2426 .  百度学术
百度学术其他类型引用(3)
-




 PDF下载 ( 811 KB)
PDF下载 ( 811 KB)


 下载:
下载:
 下载:
下载:
 百度学术
百度学术


