基质金属蛋白酶7(MMP7)和硫酸乙酰肝素糖蛋白2(HSPG2)在胰腺癌中的表达及临床意义
DOI: 10.3969/j.issn.1001-5256.2023.09.020
Expression and clinical significance of matrix metalloproteinase 7 and heparan sulfate proteoglycan 2 in pancreatic cancer
-
摘要:
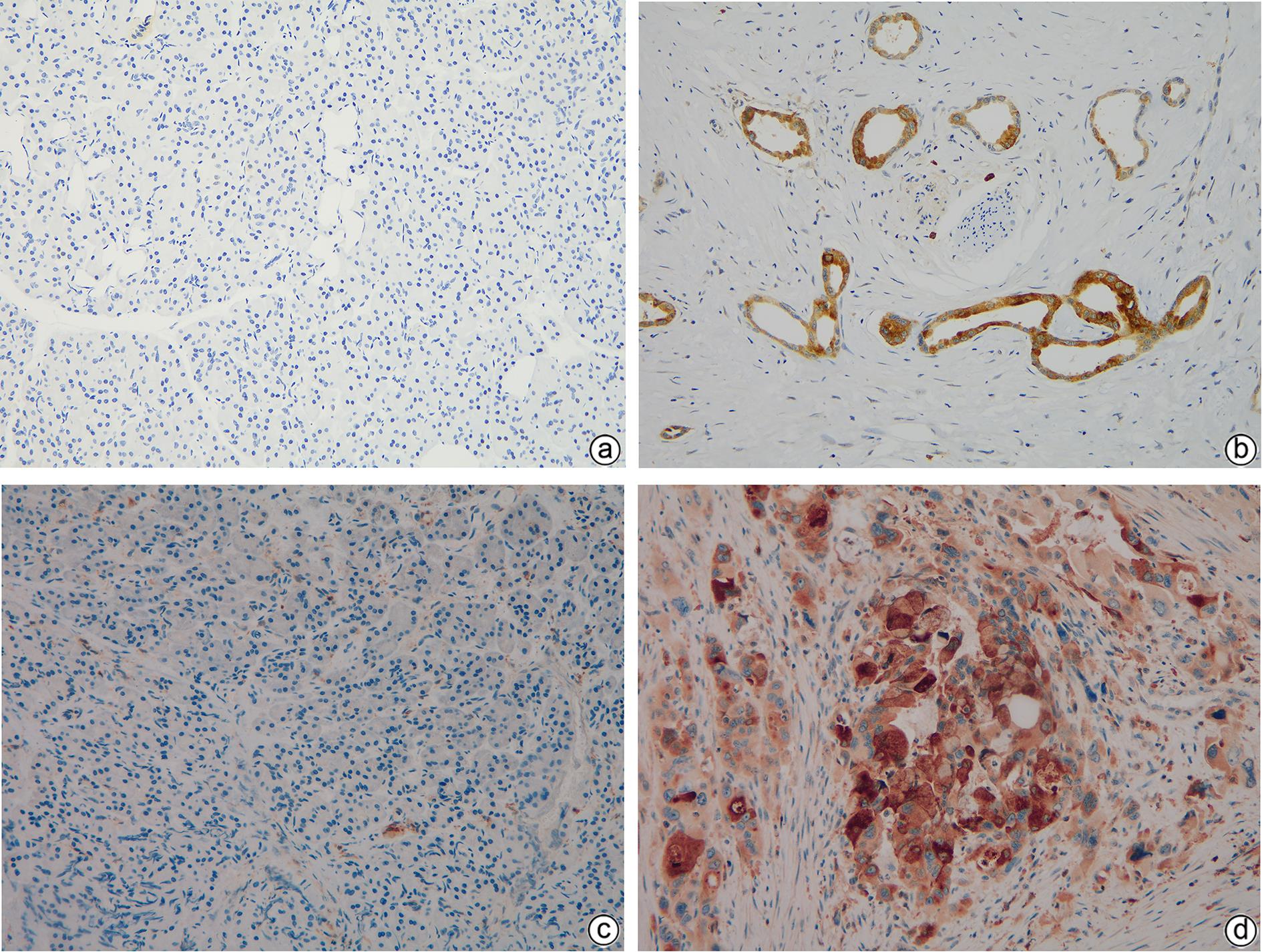
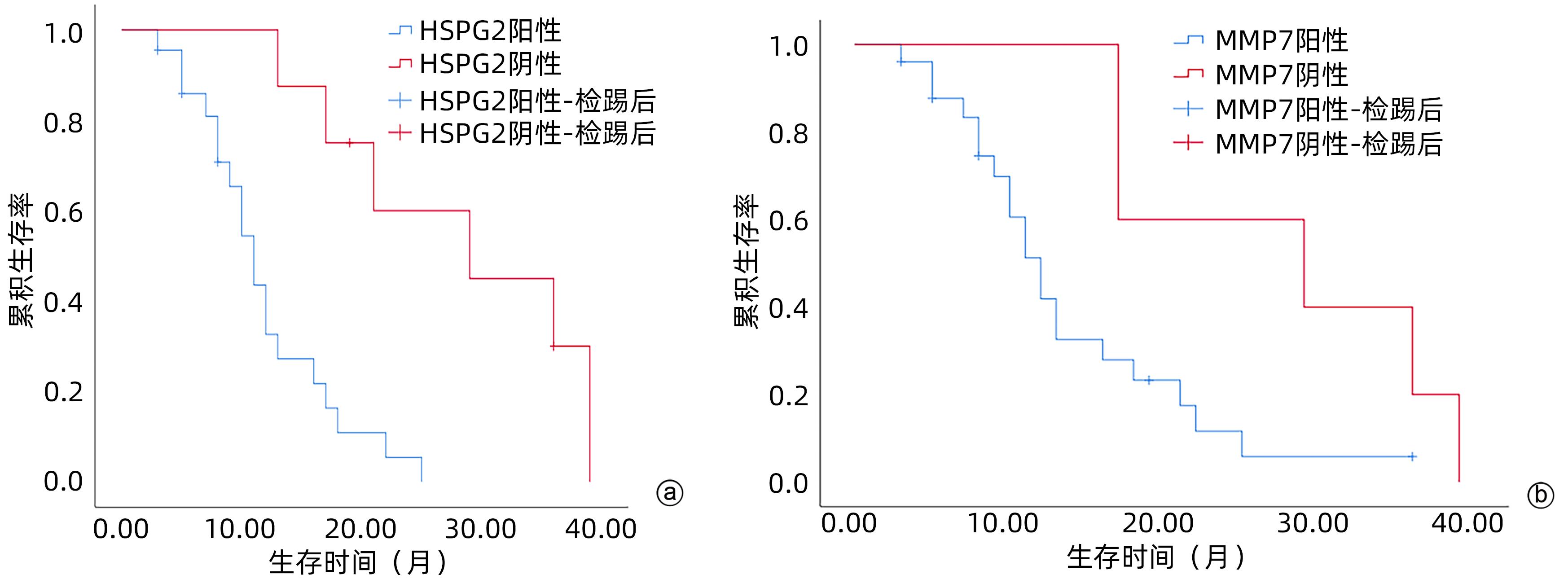
目的 探讨基质金属蛋白酶7(MMP7)和硫酸乙酰肝素糖蛋白2(HSPG2)在胰腺癌患者中的表达及临床意义。 方法 选取2018年1月—2020年12月广西医科大学附属肿瘤医院收治的胰腺癌患者30例,收集胰腺癌组织标本及其血清样本,同时收集癌旁正常组织以及30例健康志愿者血清样本作为对照。采用免疫组化方法检测胰腺癌组织及癌旁正常组织中MMP7和HSPG2蛋白表达;采用酶联免疫吸附法检测胰腺癌患者和健康对照者血清样本中MMP7和HSPG2的表达水平;收集患者临床资料并分析MMP7和HSPG2表达与临床病理特征、生存预后的关系。计量资料两组间比较采用成组t检验。计数资料组间比较采用χ2检验或Fisher确切概率法。MMP7与HSPG2表达的关系采用Spearman相关分析。采用Kaplan-Meier法绘制生存曲线,生存情况比较采用Log-rank检验。 结果 免疫组化结果显示,胰腺癌组织中MMP7和HSPG2蛋白阳性表达均高于癌旁组织,差异均有统计学意义(χ2值分别为31.093、35.623,P值<0.05)。胰腺癌患者血清中MMP7、HSPG2水平均显著高于健康对照组(t值分别为20.174、32.600,P值<0.05)。MMP7和HSPG2表达均与肿瘤直径、TNM分期有关(P值<0.05)。Spearman相关性分析显示,HSPG2与MMP7之间的表达呈正相关(r=0.539,P=0.002)。MMP7和HSPG2蛋白阳性表达患者总生存率明显低于阴性对照患者(χ2值分别为4.084、12.554,P值均<0.05)。 结论 MMP7和HSPG2在胰腺癌组织和血清中呈阳性表达,且与肿瘤的直径、TNM分期有关,两者可能在胰腺癌的发生发展中存在某种关联,有望成为胰腺癌诊断和判断预后的潜在标志物。 -
关键词:
- 胰腺肿瘤 /
- 基质金属蛋白酶7 /
- 类肝素硫酸蛋白聚糖类
Abstract:Objective To investigate the expression and clinical significance of matrix metalloproteinase 7 (MMP7) and heparan sulfate proteoglycan 2 (HSPG2) in patients with pancreatic cancer. Methods Pancreatic cancer tissue samples, normal adjacent tissue samples, and serum samples were collected from 30 patients who were admitted to Guangxi Medical University Cancer Hospital from January 2018 to December 2020, and serum samples were collected from 30 healthy volunteers as controls. Immunohistochemistry was used to measure the protein expression levels of MMP7 and HSPG2 in pancreatic cancer tissue samples and normal adjacent tissue samples; ELISA was used to measure the expression levels of MMP7 and HSPG2 in the serum samples of the pancreatic cancer patients and the healthy controls; related clinical data were collected to analyze the association of the expression of MMP7 and HSPG2 with clinicopathological features, survival, and prognosis. The independent-samples t test was used for comparison of continuous data between two groups, and the chi-square test or the Fisher’s exact test was used for comparison of categorical data between two groups; the Spearman correlation analysis was used to investigate the correlation between MMP7 and HSPG2; the Kaplan-Meier method was used to plot survival curves, and the Log-rank test was used for survival analysis. Results Immunohistochemistry showed that the protein expression levels of MMP7 and HSPG2 in pancreatic cancer tissue were significantly higher than those in adjacent tissue (χ2=31.093 and 35.623, both P<0.05). The patients with pancreatic cancer had significantly higher serum levels of MMP7 and HSPG2 than the healthy controls (t=20.174 and 32.600, both P<0.05). The expression of MMP7 and HSPG2 was associated with tumor diameter and TNM stage (both P<0.05). The Spearman correlation analysis showed a positive correlation between HSPG2 and MMP7 (r=0.539, P=0.002). The patients with positive protein expression of MMP7 and HSPG2 had a significantly lower overall survival rate than those with negative expression (χ2=4.084 and 12.554, both P<0.05). Conclusion MMP7 and HSPG2 are highly expressed in pancreatic cancer tissue and serum and are associated with tumor diameter and TNM stage. They may also be associated with the development and progression of pancreatic cancer, and therefore, they are expected to become potential markers for the diagnosis and prognostic evaluation of pancreatic cancer. -
表 1 MMP7和HSPG2在胰腺癌及癌旁正常组织中的阳性表达
Table 1. Positive expression of MMP7 and HSPG2 in pancreatic cancer and normal adjacent tissues
组别 例数 HSPG2阳性(例) MMP7阳性(例) 胰腺癌组织 30 22 25 癌旁正常组织 30 1 2 χ2值 31.093 35.623 P值 <0.001 <0.001 表 2 HSPG2和MMP7表达与胰腺癌临床病理特征的关系
Table 2. Relationship between HSPG2 and MMP7 expression and clinicopathological features of pancreatic cancer
项目 HSPG2 P值 MMP7 P值 阴性 (n=8) 阳性 (n=22) 阴性 (n=5) 阳性 (n=25) 性别(例) >0.05 >0.05 女 3 11 2 12 男 5 11 3 13 肿瘤直径(例) 0.003 0.019 >4 cm 6 3 4 5 ≤4 cm 2 19 1 20 肿瘤部位(例) >0.05 >0.05 头颈部 5 14 3 16 胰体尾部 3 8 2 9 分化程度(例) >0.05 >0.05 高 3 2 1 4 中+低 5 20 4 21 TNM分期(例) 0.039 0.031 Ⅰ~Ⅱ 1 13 0 14 Ⅲ~Ⅳ 7 9 5 11 有无转移(例) >0.05 >0.05 是 7 15 4 18 否 1 7 1 7 表 3 HSPG2和MMP7表达的相关性分析
Table 3. Correlation analysis of the expression of both HSPG2 and MMP7
HSPG2 MMP7 r值 P值 阳性 阴性 阳性 21 1 0.539 0.002 阴性 4 4 表 4 各组间血清中MMP7、HSPG2的表达水平比较
Table 4. Comparison of expression levels of MMP7 and HSPG2 in serum between groups
组别 例数 MMP7(ng/mL) HSPG2(ng/mL) 胰腺癌组 30 4.05±0.51 267.53±33.83 健康对照组 30 1.55±0.45 59.45±8.80 t值 20.174 32.600 P值 <0.001 <0.001 -
[1] SZYMOŃSKI K, MILIAN-CIESIELSKA K, LIPIEC E, et al. Current pathology model of pancreatic cancer[J]. Cancers(Basel), 2022, 14( 9): 2321. DOI: 10.3390/cancers14092321. [2] ANDERSSON R, HAGLUND C, SEPPÄNEN H, et al. Pancreatic cancer-the past, the present, and the future[J]. Scand J Gastroenterol, 2022, 57( 10): 1169- 1177. DOI: 10.1080/00365521.2022.2067786. [3] GRINDEL BJ, MARTINEZ JR, TELLMAN TV, et al. Matrilysin/MMP-7 cleavage of perlecan/HSPG2 complexed with semaphorin 3A supports FAK-mediated stromal invasion by prostate cancer cells[J]. Sci Rep, 2018, 8( 1): 7262. DOI: 10.1038/s41598-018-25435-3. [4] MENG N, LI Y, JIANG P, et al. A comprehensive pan-cancer analysis of the tumorigenic role of matrix metallopeptidase 7(MMP7) across human cancers[J]. Front Oncol, 2022, 12: 916907. DOI: 10.3389/fonc.2022.916907. [5] CRUZ LA, TELLMAN TV, FARACH-CARSON MC. Flipping the molecular switch: Influence of perlecan and its modifiers in the tumor microenvironment[J]. Adv Exp Med Biol, 2020, 1245: 133- 146. DOI: 10.1007/978-3-030-40146-7_6. [6] QIN W, YANG JY, CHEN TW, et al. Expression and significance of L1 cell adhesion molecule and transforming growth factor-β1 in pancreatic cancer tissue[J]. J Clin Hepatol, 2021, 37( 6): 1404- 1408. DOI: 10.3969/j.issn.1001-5256.2021.06.035.秦雯, 杨建宇, 陈泰文, 等. 胰腺癌组织中L1细胞黏附分子和转化生长因子β1的表达及意义[J]. 临床肝胆病杂志, 2021, 37( 6): 1404- 1408. DOI: 10.3969/j.issn.1001-5256.2021.06.035. [7] NIKŠIĆ M, MATZ M, VALKOV M, et al. World-wide trends in net survival from pancreatic cancer by morphological sub-type: An analysis of 1,258,329 adults diagnosed in 58 countries during 2000-2014(CONCORD-3)[J]. Cancer Epidemiol, 2022, 80: 102196. DOI: 10.1016/j.canep.2022.102196. [8] MANRAI M, TILAK T, DAWRA S, et al. Current and emerging therapeutic strategies in pancreatic cancer: Challenges and opportunities[J]. World J Gastroenterol, 2021, 27( 39): 6572- 6589. DOI: 10.3748/wjg.v27.i39.6572. [9] MAO N, HUANG ZJ, LIN ZT, et al. Current status of the application of translational medicine in the early diagnosis of pancreatic cancer[J]. Chin J Dig Surg, 2021, 20( 4): 466- 470. DOI: 10.3760/cma.j.cn115610-20210117-00029.毛宁, 黄子健, 林志涛, 等. 转化医学在胰腺癌早期诊断中的应用现状[J]. 中华消化外科杂志, 2021, 20( 4): 466- 470. DOI: 10.3760/cma.j.cn115610-20210117-00029. [10] PEZESHKIAN Z, NOBILI S, PEYRAVIAN N, et al. Insights into the role of matrix metalloproteinases in precancerous conditions and in colorectal cancer[J]. Cancers(Basel), 2021, 13( 24): 6226. DOI: 10.3390/cancers13246226. [11] WATTANAWONGDON W, BARTPHO TS, TONGTAWEE T. Expression of matrix metalloproteinase-7 predicts poor prognosis in gastric cancer[J]. Biomed Res Int, 2022, 2022: 2300979. DOI: 10.1155/2022/2300979. [12] YUAN JQ, ZHANG KJ, WANG SM, et al. YAP1/MMP7/CXCL16 axis affects efficacy of neoadjuvant chemotherapy via tumor environment immunosuppression in triple-negative breast cancer[J]. Gland Surg, 2021, 10( 9): 2799- 2814. DOI: 10.21037/gs-21-612. [13] TELLMAN TV, CRUZ LA, GRINDEL BJ, et al. Cleavage of the perlecan-semaphorin 3A-plexin A1-neuropilin-1(PSPN) complex by matrix metalloproteinase 7/matrilysin triggers prostate cancer cell dyscohesion and migration[J]. Int J Mol Sci, 2021, 22( 6): 3218. DOI: 10.3390/ijms22063218. [14] MARTINEZ JR, DHAWAN A, FARACH-CARSON MC. Modular proteoglycan perlecan/HSPG2: Mutations, phenotypes, and functions[J]. Genes(Basel), 2018, 9( 11): 556. DOI: 10.3390/genes9110556. [15] KAZANSKAYA GM, TSIDULKO AY, VOLKOV AM, et al. Heparan sulfate accumulation and perlecan/HSPG2 up-regulation in tumour tissue predict low relapse-free survival for patients with glioblastoma[J]. Histochem Cell Biol, 2018, 149( 3): 235- 244. DOI: 10.1007/s00418-018-1631-7. [16] LORD MS, JUNG M, CHENG B, et al. Transcriptional complexity of the HSPG2 gene in the human mast cell line, HMC-1[J]. Matrix Biol, 2014, 35: 123- 131. DOI: 10.1016/j.matbio.2013.12.005. [17] FARACH-CARSON MC, WARREN CR, HARRINGTON DA, et al. Border patrol: insights into the unique role of perlecan/heparan sulfate proteoglycan 2 at cell and tissue borders[J]. Matrix Biol, 2014, 34: 64- 79. DOI: 10.1016/j.matbio.2013.08.004. 期刊类型引用(12)
1. 郭龙鑫,高云娟,吴承钊,龙敏娟,祝胜凯,宋海波,赵旭,肖小河. 基于不良反应监测大数据的中药药源性肝损伤风险信号新发现及易感因素初探. 中国药物警戒. 2024(01): 15-19 .  百度学术
百度学术2. 李勇,张蕾,吴幸福. 老年药物性肝损伤患者临床特征分析. 实用肝脏病杂志. 2024(04): 547-550 .  百度学术
百度学术3. 徐媛,方忠宏,冉姗. 1例多因素所致药物性肝损伤病例的病因论证与药学监护. 药物流行病学杂志. 2023(03): 350-355 .  百度学术
百度学术4. 郭冬伟. 生物技术药物的免疫毒性和免疫原性的分析及探讨. 当代化工研究. 2021(03): 161-162 .  百度学术
百度学术5. 李任,苏庆全,黎运. 62例肺结核患者抗结核药物治疗致药物性肝损伤的危险因素分析及其防治对策. 抗感染药学. 2021(05): 724-726 .  百度学术
百度学术6. 杨焕芝,李兴德,陈学平,张仲安,钱彦华,蒋潇,徐艳琼,宋沧桑. 93例药物性肝损伤患者临床特征分析. 中国药业. 2021(15): 122-125 .  百度学术
百度学术7. 王巧玲,邹正升. 细胞色素P450基因多态性与药物性肝损伤的关系. 临床肝胆病杂志. 2020(05): 1150-1153 .  本站查看
本站查看8. 许维国. 中草药导致药物性肝损伤的现状及研究进展. 中国处方药. 2020(06): 14-16 .  百度学术
百度学术9. 刘毅,周淑娴,陈念辉. 1例药源性肝损伤合并2型糖尿病患者的病例分析. 中国药师. 2020(10): 1986-1989 .  百度学术
百度学术10. 陈龙,李钺. 肝切除术后并发症的危险因素及预测评分系统. 临床肝胆病杂志. 2019(01): 217-221 .  本站查看
本站查看11. 郭永涛. 抗肺结核药物所致肝损伤患者临床症状分布及危险因素分析. 山西医药杂志. 2019(05): 564-566 .  百度学术
百度学术12. 刘丽艳,唐晓雯,董春玲,陶茹,刘成海,张雅丽. 药物性肝损伤患者的护理研究进展. 护士进修杂志. 2019(24): 2253-2256 .  百度学术
百度学术其他类型引用(8)
-




 PDF下载 ( 962 KB)
PDF下载 ( 962 KB)

 下载:
下载:
 百度学术
百度学术

