金雀异黄素在去卵巢非酒精性脂肪性肝病小鼠模型中的保护作用及机制
DOI: 10.12449/JCH240411
伦理学声明:本研究方案于2022年6月20日经由安徽医科大学实验动物伦理委员会审批,批号:LLSC20221057,符合实验室动物管理与使用准则。
利益冲突声明:本文不存在任何利益冲突。
作者贡献声明:朱项羽负责实验操作,整理数据,撰写文章;金涌负责指导实验和写作,分析数据;朱项羽、金涌负责实验设计,数据分析,拟定写作思路,修改文章及定稿。
Protective effect of Genistein against nonalcoholic fatty liver disease in ovariectomized mice and its mechanism
-
摘要:
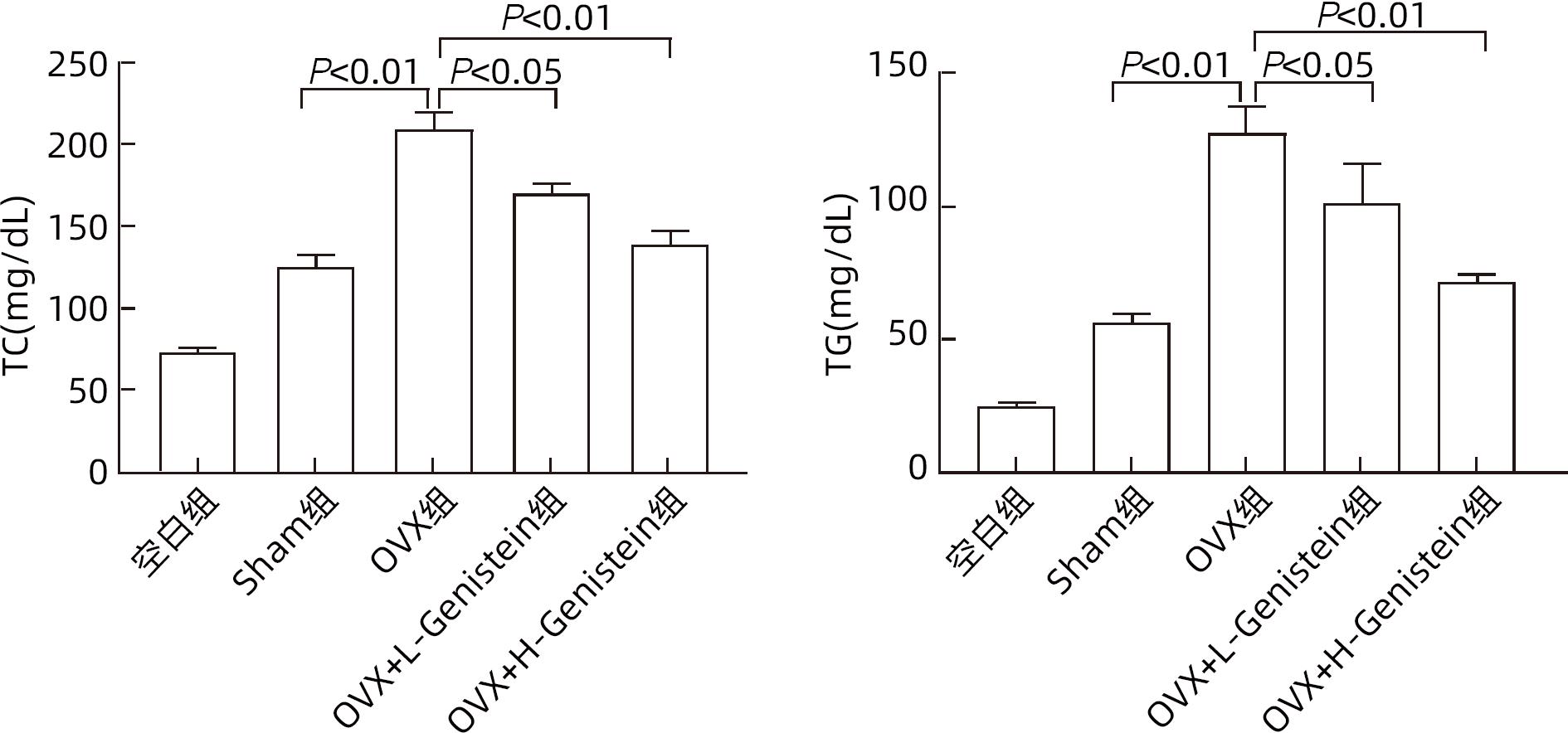
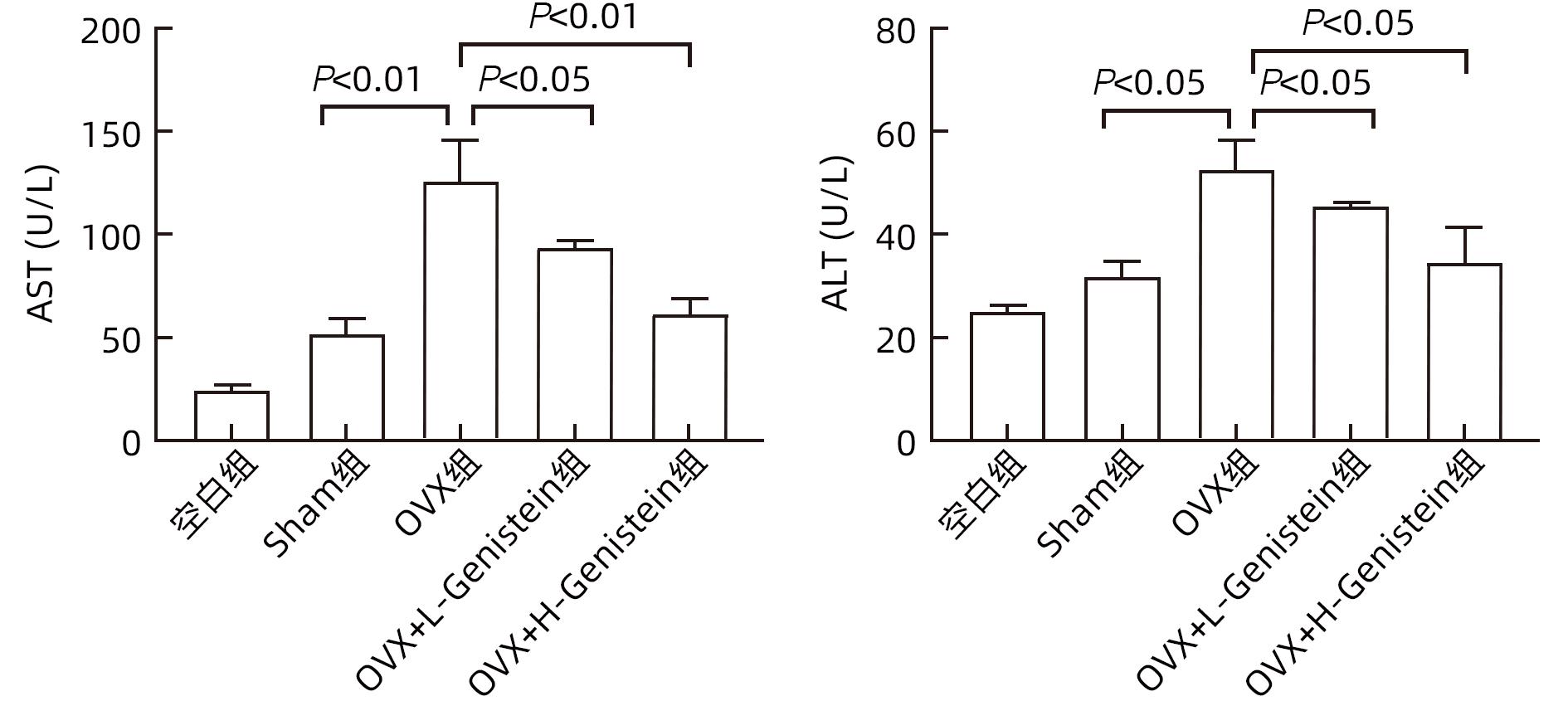
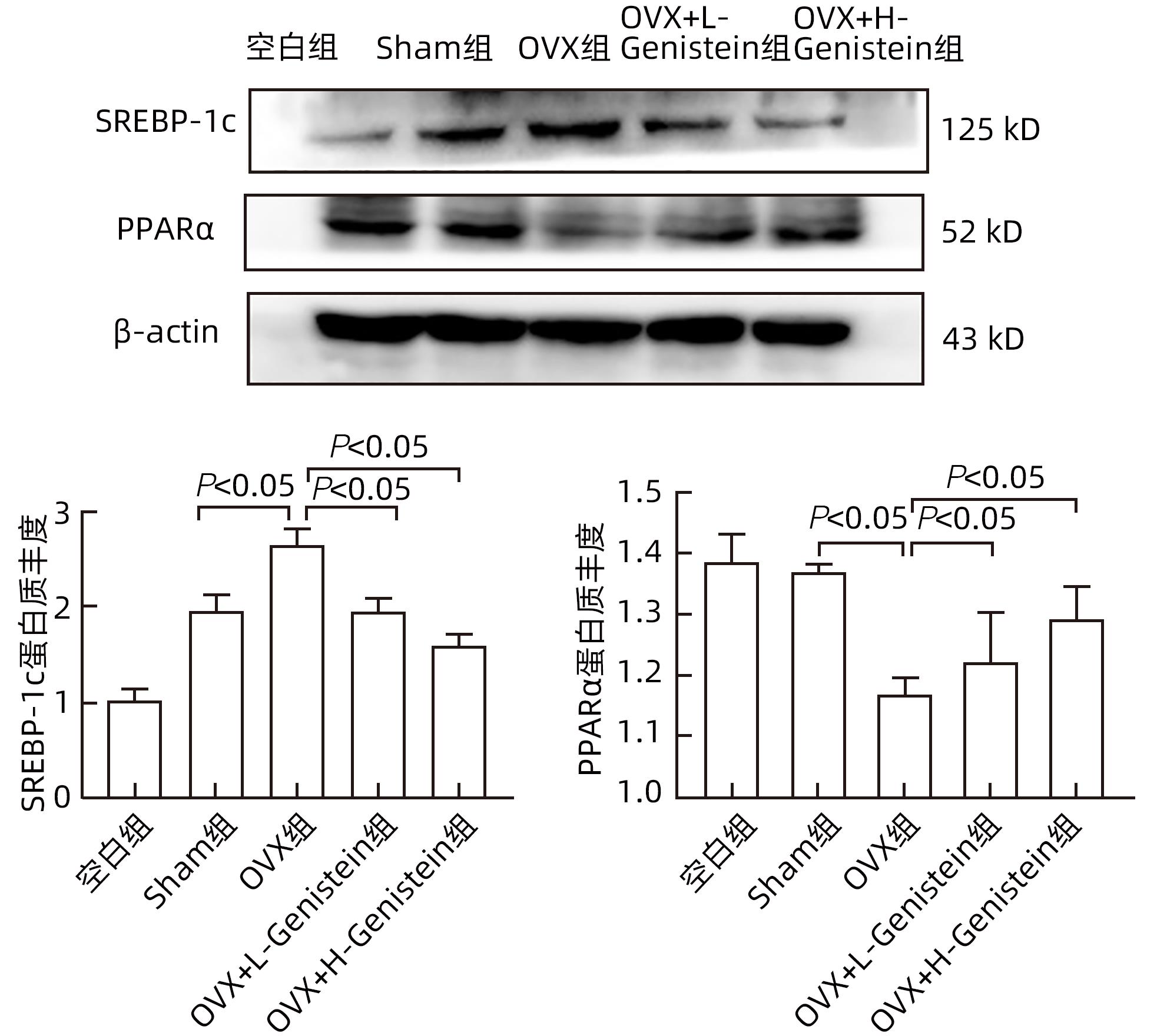
目的 探讨金雀异黄素(Genistein)在去卵巢(OVX)小鼠非酒精性脂肪性肝病(NAFLD)中的保护作用及机制。 方法 取40只6周龄雌性C57BL/6小鼠建立OVX小鼠模型,随机分为5组,每组8只,分别为对照组、模型4周组、模型6周组、模型8周组、模型10周组。相同环境下,采用高脂饮食对5组OVX小鼠进行饮食造模,病理学检查显示,经10周高脂饮食诱导NAFLD成功。另取40只6周龄雌性C57BL/6小鼠随机分为5组:空白组、假手术(Sham)组、OVX组、OVX+L-Genistein(4 mg/kg体质量)组、OVX+H-Genistein(8 mg/kg体质量)组。Sham组进行相同的OVX手术过程,但不结扎卵巢动脉和切除卵巢。空白组小鼠正常饮食喂养,其余各组供给高脂饮食。将Genistein溶解在DMSO中,Sham组和OVX组的动物仅用溶媒溶液处理,所有小鼠每天灌胃给药1次,持续10周。记录小鼠体质量及脏器指数,处死小鼠并收集血清及肝组织。试剂盒检测血清ALT、AST活性及TG、TC水平。HE和油红O染色观察肝组织病理。通过Western Blot分析肝组织中脂质代谢相关的固醇调节元件结合蛋白1(SREBP-1c)、过氧化物酶体增殖物激活受体α(PPARα)的蛋白表达。计量资料多组间比较采用单因素方差分析,进一步两组间比较采用Dunnett-t检验。 结果 高脂饮食10周后,Genistein低、高剂量组的体质量、肝指数和肝质量均低于OVX组(P值均<0.05)。此外,Genistein显著下调了血清TC、TG水平(P值均<0.05),降低了血清中AST和ALT活性(P值均<0.05)。HE和油红O染色结果表明,Genistein低、高剂量组较OVX组脂滴累积明显减少。Western Blot分析结果显示,给予Genistein干预后,SREBP-1c的蛋白表达量明显下降,PPARα的蛋白表达量明显升高(P值均<0.05)。 结论 Genistein对OVX小鼠NAFLD具有保护作用,其部分作用机制可能与调节SREBP-1c、PPARα的表达有关,从而促进脂肪酸氧化和抑制肝脏脂质合成。 -
关键词:
- 非酒精性脂肪性肝病 /
- 卵巢切除术 /
- 小鼠, 近交C57BL /
- 金雀异黄素
Abstract:Objective To investigate the protective effect of Genistein against nonalcoholic fatty liver disease (NAFLD) in ovariectomized (OVX) mice and its mechanism. Methods A total of 40 female C57BL/6 mice, aged 6 weeks, were used to establish an OVX mouse model, and then they were randomly divided into blank group, 4-week model group, 6-week model group, 8-week model group, and 10-week model group, with 8 mice in each group. Under the same environmental conditions, the mice were given high-fat diet for modeling, and pathological examination showed that NAFLD was successfully induced by 10-week high-fat diet. Another 40 female C57BL/6 mice, aged 6 weeks, were randomly divided into blank group, sham operation group (Sham group), OVX group, OVX+L-Genistein (4 mg/kg body weight) group, and OVX+H-Genistein (8 mg/kg body weight) group. The mice in the Sham group were given the same procedure of OVX, without the ligation of the ovarian artery and the resection of the ovary. The mice in the blank group were given normal diet, and those in the other groups were given high-fat diet. Genistein was dissolved in DMSO, and the mice in the Sham group and the OVX group were treated with solvent solution alone by gavage, once a day for 10 consecutive weeks. Body weight and visceral index were recorded, and the mice were sacrificed to collect serum and liver tissue. Kits were used to measure the activity of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) and the serum levels of triglyceride (TG) and total cholesterol (TC), and HE staining and oil red O staining were used to observe liver histopathology; Western blot was used to measure the protein expression levels of sterol regulatory element-binding protein 1c (SREBP-1c) and peroxisome proliferator-activated receptor alpha (PPARα) associated with lipid metabolism in liver tissue. A one-way analysis of variance was used for comparison of continuous data between multiple groups, and the Dunnett-t test was used for further comparison between two groups. Results After 10 weeks of high-fat diet, the OVX+L-Genistein group and the OVX+H-Genistein group had significantly lower body weight, liver index, and liver tissue weight (all P<0.05). In addition, Genistein significantly downregulated the serum levels of TC and TG (P<0.05) and reduced the activities of serum AST and ALT (P<0.05). HE and oil red O staining showed that compared with the OVX group, the OVX+L-Genistein group and the OVX+H-Genistein group had a significant reduction in the accumulation of lipid droplets. Western blot showed that after Genistein intervention, there was a significant reduction in the protein expression level of SREBP-1c and a significant increase in the protein expression level of PPARα (P<0.05). Conclusion Genistein exerts a protective effect against NAFLD in OVX mice possibly by regulating the expression of SREBP-1c and PPARα, thereby promoting fatty acid oxidation and inhibiting liver lipid synthesis. -
Key words:
- Non-alcoholic Fatty Liver Disease /
- Ovariectomy /
- Mice, Inbred C57BL /
-
Genistein
-
表 1 各组小鼠的肝脏系数
Table 1. Liver coefficients of mice in each group
组别 动物数(只) 体质量(g) 肝质量(g) 肝指数(%) 空白组 6 24.15±0.45 1.03±0.05 4.26±0.15 Sham组 6 26.91±0.361) 1.31±0.041) 4.86±0.141) OVX组 6 30.92±0.892) 1.63±0.042) 5.28±0.142) OVX+L-Genistein组 6 28.91±0.353) 1.46±0.043) 5.05±0.133) OVX+H-Genistein组 6 27.36±0.523) 1.35±0.033) 4.92±0.103) F值 18.225 15.919 8.529 P值 <0.05 <0.05 <0.05 注:与空白组比较,1)P<0.05;与Sham组比较,2)P<0.05;与OVX组比较,3)P<0.05。 -
[1] PAFILI K, RODEN M. Nonalcoholic fatty liver disease(NAFLD) from pathogenesis to treatment concepts in humans[J]. Mol Metab, 2021, 50: 101122. DOI: 10.1016/j.molmet.2020.101122. [2] YU Y, CAI JJ, SHE ZG, et al. Insights into the epidemiology, pathogenesis, and therapeutics of nonalcoholic fatty liver diseases[J]. Adv Sci(Weinh), 2018, 6( 4): 1801585. DOI: 10.1002/advs.201801585. [3] MANNE V, HANDA P, KOWDLEY KV. Current treatment of non-alcoholic fatty liver disease[J]. Clin Liver Dis, 2018, 22( 1): 23- 37. DOI: 10.1016/j.cld.2017.08.007. [4] CHOUDHURI G, SHAH S, KULKARNI A, et al. Non-alcoholic steatohepatitis in asians: Current perspectives and future directions[J]. Cureus, 2023, 15( 8): e42852. DOI: 10.7759/cureus.42852. [5] PAWLOWSKI JW, MARTIN BR, MCCABE GP, et al. Impact of equol-producing capacity and soy-isoflavone profiles of supplements on bone calcium retention in postmenopausal women: A randomized crossover trial[J]. Am J Clin Nutr, 2015, 102( 3): 695- 703. DOI: 10.3945/ajcn.114.093906. [6] ZHAO L, WANG Y, LIU J, et al. Protective effects of genistein and puerarin against chronic alcohol-induced liver injury in mice via antioxidant, anti-inflammatory, and anti-apoptotic mechanisms[J]. J Agric Food Chem, 2016, 64( 38): 7291- 7297. DOI: 10.1021/acs.jafc.6b02907. [7] AMANAT S, EFTEKHARI MH, FARAROUEI M, et al. Genistein supplementation improves insulin resistance and inflammatory state in non-alcoholic fatty liver patients: A randomized, controlled trial[J]. Clin Nutr, 2018, 37( 4): 1210- 1215. DOI: 10.1016/j.clnu.2017.05.028. [8] XIA HG, ZHU XY, ZHANG XY, et al. Alpha-naphthoflavone attenuates non-alcoholic fatty liver disease in oleic acid-treated HepG2 hepatocytes and in high fat diet-fed mice[J]. Biomed Pharmacother, 2019, 118: 109287. DOI: 10.1016/j.biopha.2019.109287. [9] MOLINA-MOLINA E, FURTADO GE, JONES JG, et al. The advantages of physical exercise as a preventive strategy against NAFLD in postmenopausal women[J]. Eur J Clin Invest, 2022, 52( 3): e13731. DOI: 10.1111/eci.13731. [10] ZHU XY, XIA HG, WANG ZH, et al. In vitro and in vivo approaches for identifying the role of aryl hydrocarbon receptor in the development of nonalcoholic fatty liver disease[J]. Toxicol Lett, 2020, 319: 85- 94. DOI: 10.1016/j.toxlet.2019.10.010. [11] CALIGIONI CS. Assessing reproductive status/stages in mice[J]. Curr Protoc Neurosci, 2009, Appendix 4: Appendix 4 I. DOI: 10.1002/0471142301.nsa04is48. [12] DEMIR M, BORNSTEIN SR, MANTZOROS CS, et al. Liver fat as risk factor of hepatic and cardiometabolic diseases[J]. Obes Rev, 2023, 24( 10): e13612. DOI: 10.1111/obr.13612. [13] POWELL EE, WONG VWS, RINELLA M. Non-alcoholic fatty liver disease[J]. Lancet, 2021, 397( 10290): 2212- 2224. DOI: 10.1016/S0140-6736(20)32511-3. [14] WANG CE, XU WT, GONG J, et al. Research progress in the treatment of non-alcoholic fatty liver disease[J]. Clin J Med Offic, 2022, 50( 9): 897- 899, 903. DOI: 10.16680/j.1671-3826.2022.09.06.王彩娥, 许文涛, 宫建, 等. 非酒精性脂肪性肝病治疗研究进展[J]. 临床军医杂志, 2022, 50( 9): 897- 899, 903. DOI: 10.16680/j.1671-3826.2022.09.06. [15] FRIEDMAN SL, NEUSCHWANDER-TETRI BA, RINELLA M, et al. Mechanisms of NAFLD development and therapeutic strategies[J]. Nat Med, 2018, 24( 7): 908- 922. DOI: 10.1038/s41591-018-0104-9. [16] ALEXANDER KS, ZAKAI NA, LIDOFSKY SD, et al. Non-alcoholic fatty liver disease, liver biomarkers and stroke risk: The reasons for geographic and racial differences in stroke cohort[J]. PLoS One, 2018, 13( 3): e0194153. DOI: 10.1371/journal.pone.0194153. [17] KAWAMURA S, MATSUSHITA Y, KUROSAKI S, et al. Inhibiting SCAP/SREBP exacerbates liver injury and carcinogenesis in murine nonalcoholic steatohepatitis[J]. J Clin Invest, 2022, 132( 11): e151895. DOI: 10.1172/JCI151895. -



 PDF下载 ( 1690 KB)
PDF下载 ( 1690 KB)


 下载:
下载: