肝硬化食管胃底静脉曲张破裂出血患者经颈静脉肝内门体分流术后发生显性肝性脑病的列线图预测模型建立及评价
DOI: 10.12449/JCH240816
Establishment and evaluation of a nomogram prediction model for overt hepatic encephalopathy after transjugular intrahepatic portosystemic shunt in patients with liver cirrhosis and esophagogastric variceal bleeding
-
摘要:
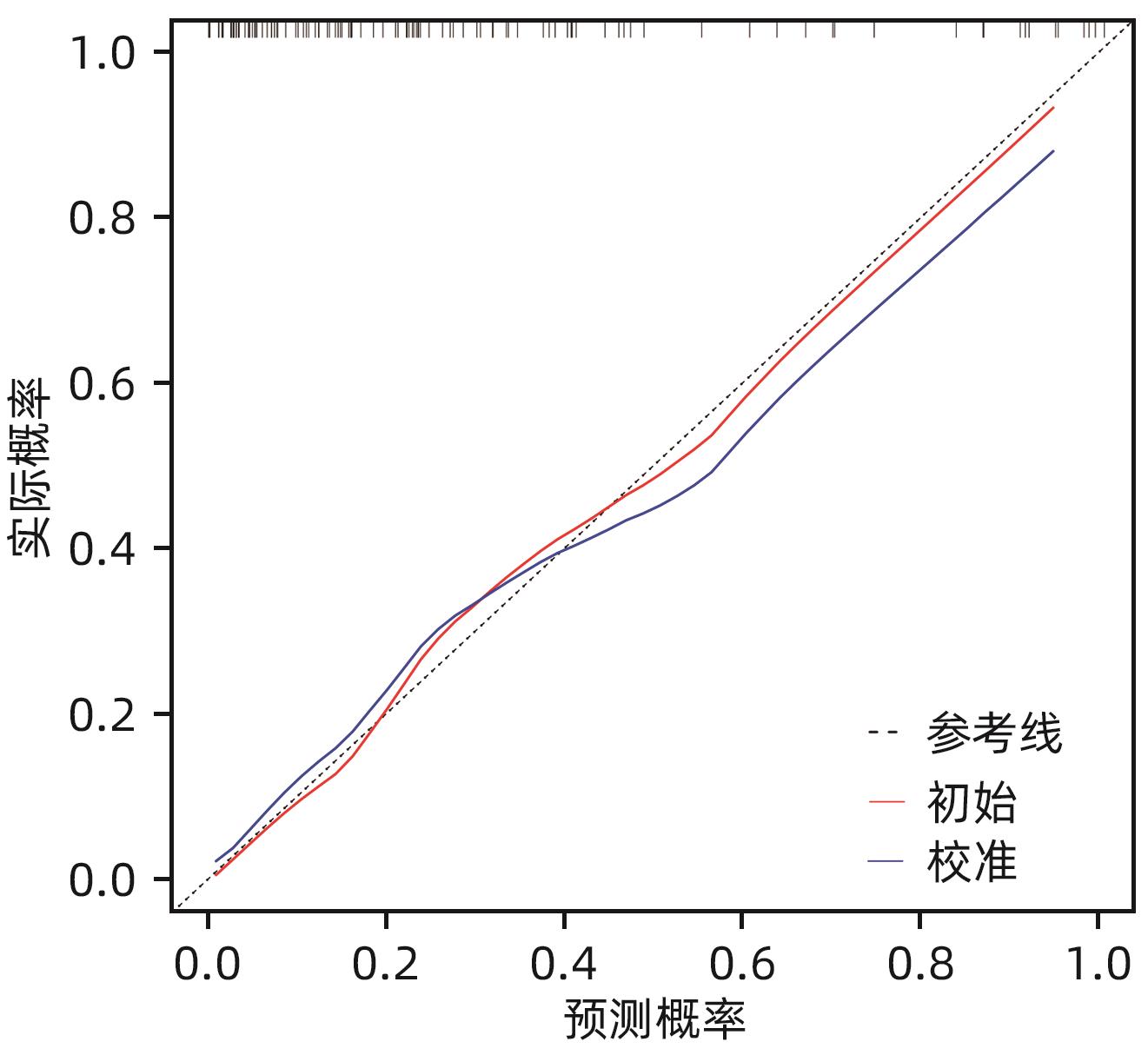
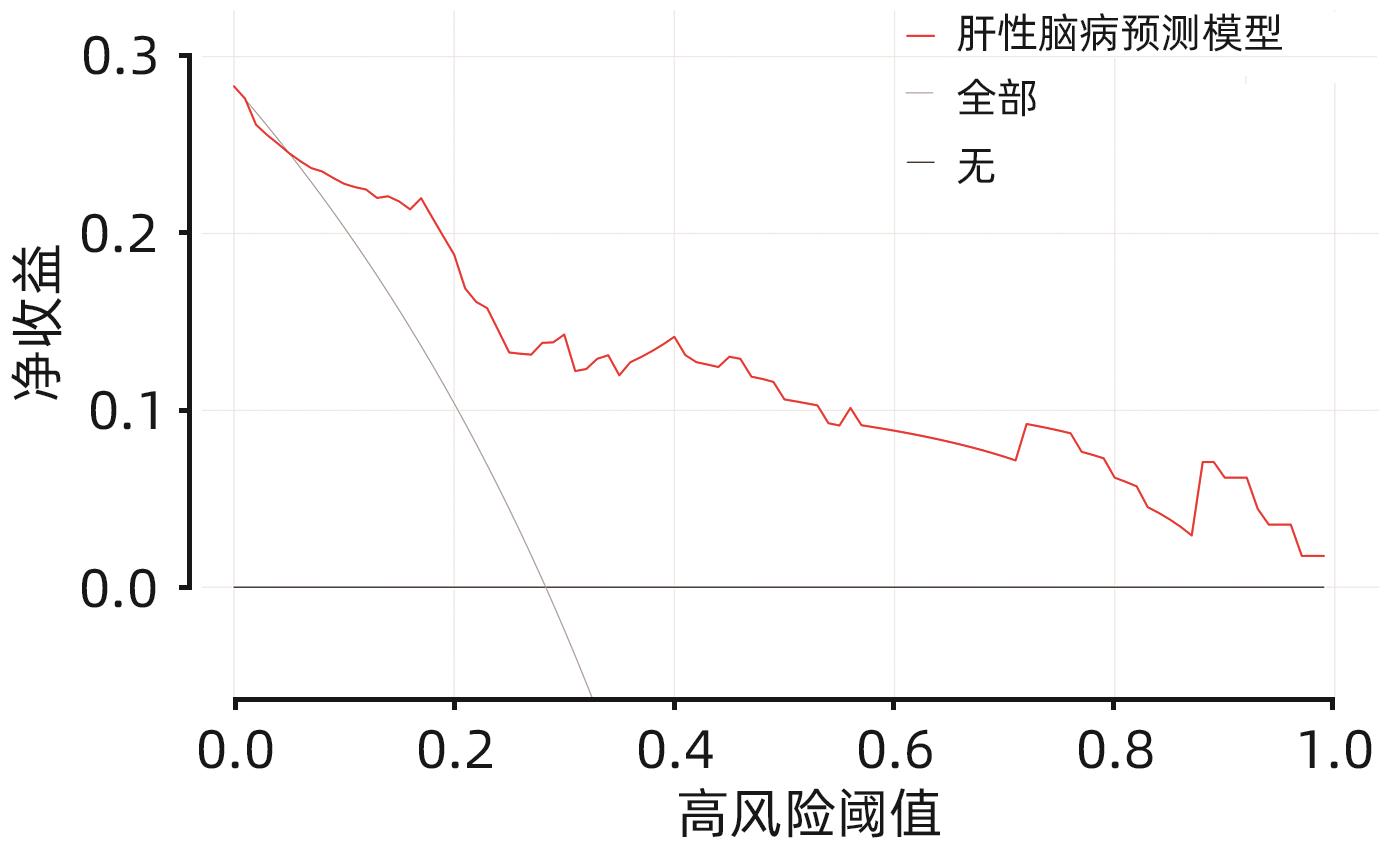
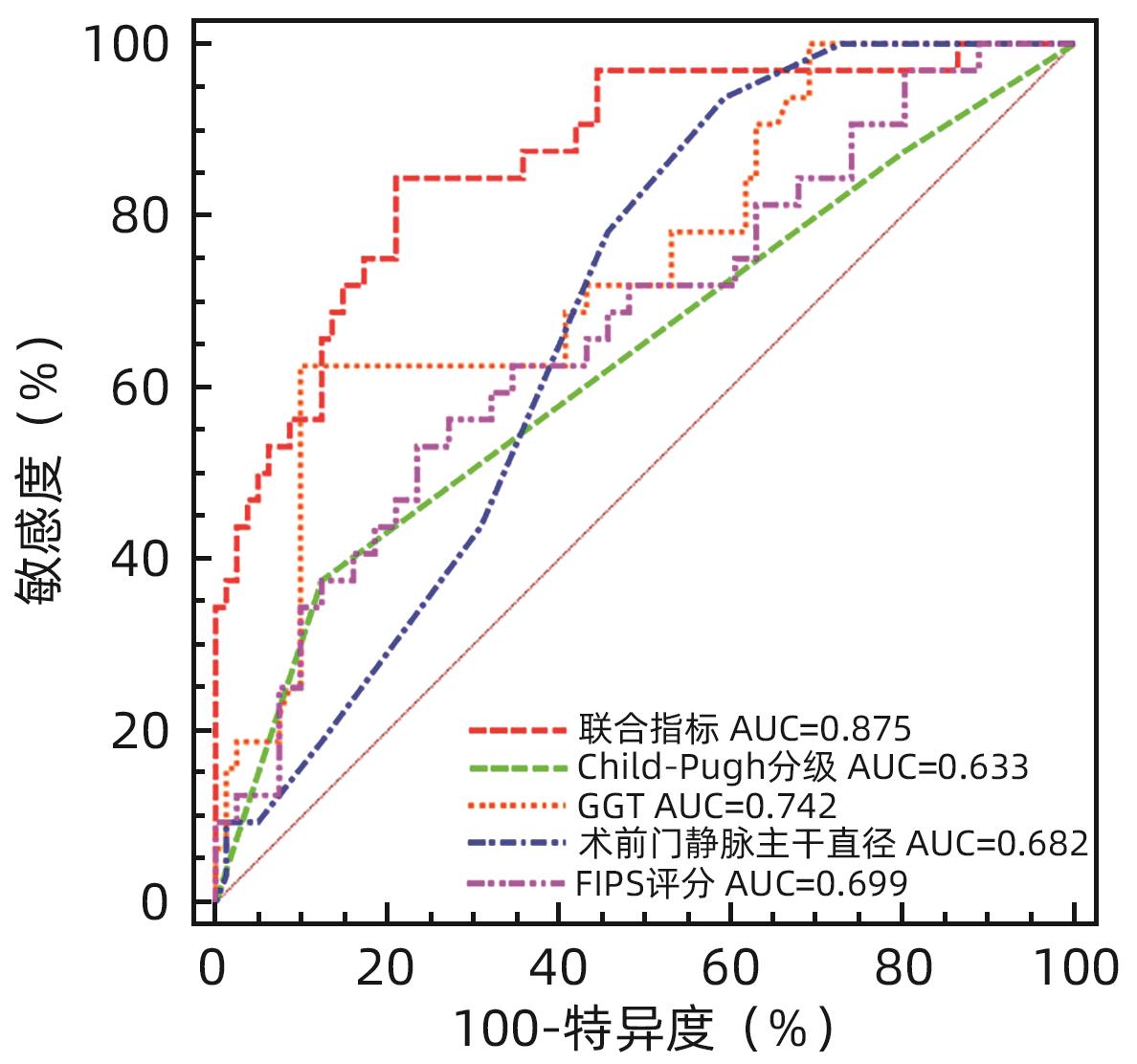
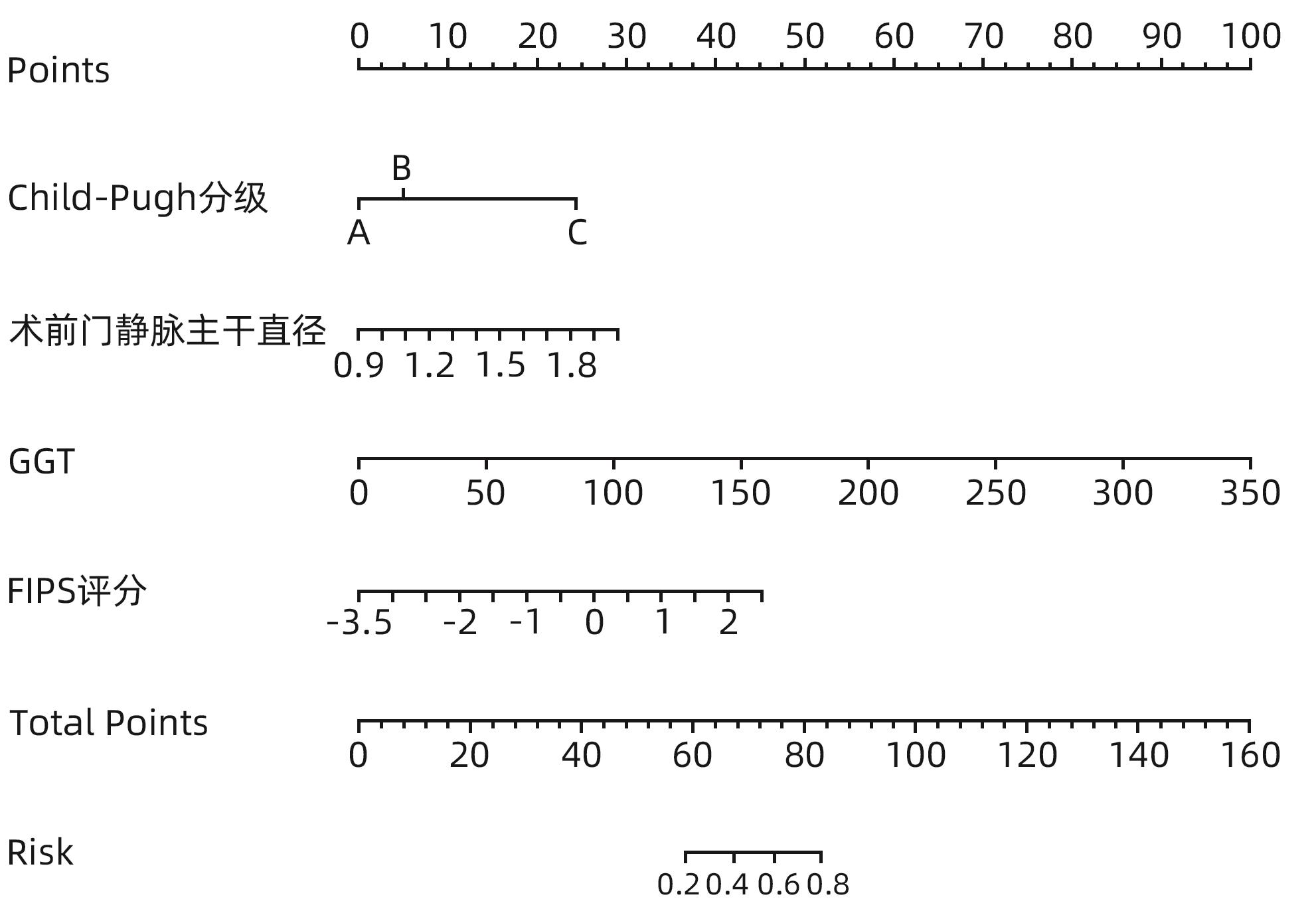
目的 构建肝硬化食管胃底静脉曲张破裂出血后行经颈静脉肝内门体分流术(TIPS)患者发生显性肝性脑病的预测模型列线图,并对模型预测能力进行评价。 方法 选取苏州大学附属第一医院2020年1月—2022年12月收治的因肝硬化食管胃底静脉曲张破裂出血经药物治疗或内镜下止血失败后行TIPS的患者113例,所有患者术后随访6个月,根据TIPS术后是否发生显性肝性脑病,分为显性肝性脑病组(n=32)和非显性肝性脑病组(n=81),收集患者的临床资料、血常规、血清生化学及血凝检查等结果。计量资料两组间比较采用成组t检验或Mann-Whitney U检验。计数资料组间比较采用χ2检验或Fisher精确概率法。利用多因素Logistic回归法分析TIPS术后肝性脑病发生的独立影响因素,并构建列线图预测模型,计算一致性指数(C-index)并绘制校准曲线,评价列线图预测能力。绘制临床决策曲线,分析模型临床净收益。通过受试者工作特征曲线(ROC曲线)对模型的预测能力进行验证。 结果 显性肝性脑病组与非显性肝性脑病组比较,年龄、糖尿病、Child-Pugh分级、腹水、术前门静脉主干直径、GGT水平、凝血酶原时间及弗赖堡术后生存指数(FIPS)评分差异均有统计学意义(P值均<0.05)。多因素Logistic回归分析结果显示,Child-Pugh分级(OR=17.498,95%CI:2.561~119.548,P=0.004)、术前门静脉主干直径(OR=1.361,95%CI:1.057~1.752,P=0.017)、GGT(OR=1.032,95%CI:1.013~1.052,P=0.001)和FIPS评分(OR=2.838,95%CI:1.277~6.311,P=0.010)是TIPS术后发生显性肝性脑病的独立影响因素。基于以上4项指标建立列线图模型,C-index为0.875,校准曲线拟合良好;模型的ROC曲线下面积为0.875(95%CI:0.799~0.929,P<0.001);决策曲线分析结果显示,0.3~0.9阈概率间模型具有较良好的净获益。 结论 Child-Pugh分级、术前门静脉主干直径、GGT及FIPS评分对肝硬化食管胃底静脉曲张破裂出血患者TIPS术后显性肝性脑病的发生具有一定的预测价值,基于上述指标构建的列线图模型可以个体化预测肝硬化食管胃底静脉曲张破裂出血患者TIPS术后显性肝性脑病的发生率。 -
关键词:
- 肝硬化 /
- 食管和胃静脉曲张 /
- 门体分流术, 经颈静脉肝内 /
- 肝性脑病 /
- 列线图
Abstract:Objective To establish a nomogram prediction model for the development of overt hepatic encephalopathy (OHE) in patients with liver cirrhosis undergoing transjugular intrahepatic portosystemic shunt (TIPS) after esophagogastric variceal bleeding, and to evaluate the predictive ability of the model. Methods This study was conducted among 113 patients with esophagogastric variceal bleeding due to liver cirrhosis who were admitted to The First Affiliated Hospital of Soochow University from January 2020 to December 2022 and underwent TIPS after failed medical or endoscopic therapy. All patients were followed up for 6 months after surgery, and according to the presence or absence of OHE after TIPS, they were divided into OHE group with 32 patients and non-OHE group with 81 patients. Related data were collected from all patients, including clinical data, routine blood test results, serum biochemistry, and coagulation test results. The independent-samples t test or the Mann-Whitney U test was used for comparison of continuous data between two groups, and the chi-square test or the Fisher’s exact test was used for comparison of categorical data between two groups. The multivariate Logistic regression analysis was used to investigate the independent risk factors for the onset of OHE after TIPS, and then a nomogram prediction model was established. The index of concordance (C-index) was calculated and the calibration curve was plotted to evaluate the predictive ability of the model, and the clinical decision curve was plotted to analyze the net clinical benefit of the model. The receiver operating characteristic (ROC) curve was used to validate the predictive ability of the model. Results There were significant differences between the OHE group and the non-OHE group in age, diabetes, Child-Pugh class, ascites, main portal vein diameter before surgery, gamma-glutamyl transpeptidase (GGT) level, prothrombin time, and Freiburg index of post-TIPS survival (FIPS) score (all P<0.05). The multivariate Logistic regression analysis showed that Child-Pugh class (odds ratio [OR]=17.498, 95% confidence interval [CI]: 2.561 — 119.548, P=0.004), main portal vein diameter before surgery (OR=1.361, 95%CI: 1.057 — 1.752, P=0.017), GGT (OR=1.032, 95%CI: 1.013 — 1.052, P=0.001), and FIPS score (OR=2.838, 95%CI: 1.277 — 6.311, P=0.010) were independent influencing factors for the development of OHE after TIPS. The nomogram model established based on the above four indicators had a C-index of 0.875, good fitting of the calibration curve, and an area under the ROC curve of 0.875 (95%CI: 0.799 — 0.929, P<0.001), and the decision curve analysis showed that the 0.3 — 0.9 threshold probability model had a good net benefit. Conclusion Child-Pugh class, main portal vein diameter before surgery, GGT, and FIPS score have a certain value in predicting the development of OHE after TIPS in patients with esophagogastric variceal bleeding due to liver cirrhosis, and the nomogram model established based on these indicators can be used to individually predict the onset of OHE after TIPS in patients with esophagogastric variceal bleeding due to liver cirrhosis. -
表 1 显性肝性脑病组和非显性肝性脑病组临床资料比较
Table 1. Figure 1 Comparison of clinical data between overt-hepatic encephalopathy group and covert-hepatic encephalopathy group
项目 显性肝性脑病组(n=32) 非显性肝性脑病组(n=81) 统计值 P值 性别[例(%)] χ2=1.732 0.188 男 15(46.88) 49(60.49) 女 17(53.13) 32(39.51) 年龄(岁) 63.53±10.73 57.01±12.63 t=2.575 0.011 病因[例(%)] χ2=3.510 0.476 病毒性 13(40.63) 37(45.68) 酒精性 3(9.38) 13(16.05) 血吸虫性 5(15.63) 14(17.28) 自身免疫性 9(28.13) 11(13.58) 其他 2(6.25) 6(7.41) 基础疾病[例(%)] 高血压 8(25.00) 12(14.81) χ2=1.634 0.201 糖尿病 14(43.75) 20(24.69) χ2=3.961 0.047 腹水[例(%)] χ2=11.803 0.007 无 6(18.75) 13(16.05) 少 5(15.63) 39(48.15) 中 6(18.75) 10(12.35) 大 15(46.88) 19(23.46) Child-Pugh分级[例(%)] χ2=8.461 0.015 A级 4(12.50) 16(19.75) B级 16(50.00) 55(67.90) C级 12(37.50) 10(12.35) 术前门静脉主干直径(cm) 1.47±0.19 1.31±0.23 t=3.807 <0.001 表 2 显性肝性脑病组和非显性肝性脑病组实验室指标比较
Table 2. Figure 2 Laboratory index comparison between overt-hepatic encephalopathy group and covert-hepatic encephalopathy group
实验室指标 显性肝性脑病组(n=32) 非显性肝性脑病组(n=81) 统计值 P值 WBC(×109/L) 4.10(3.17~4.95) 3.75(2.42~7.04) Z=-0.274 0.784 NEUT(×109/L) 2.94(1.99~3.56) 2.74(1.71~5.76) Z=-0.615 0.539 Hb(g/L) 78.09±13.36 77.10±15.67 t=0.316 0.753 PLT(×109/L) 61.00(39.25~70.75) 55.00(43.00~71.00) Z=-0.360 0.719 TBil(μmol/L) 30.77(20.55~31.50) 23.00(17.80~33.95) Z=-1.680 0.093 ALT(U/L) 31.05(22.38~39.48) 28.50(17.10~36.15) Z=-1.782 0.075 AST(U/L) 40.65(26.15~46.40) 33.00(23.75~46.15) Z=-1.440 0.150 GGT(U/L) 77.40(29.30~82.66) 31.70(19.10~38.50) Z=-4.004 0.001 Alb(g/L) 32.03±4.50 31.64±4.05 t=0.443 0.659 Glb(g/L) 23.46±5.46 23.04±5.99 t=0.346 0.730 肌酐(μmol/L) 63.20(52.60~72.51) 59.60(49.80~69.15) Z=-1.584 0.113 TC(mmol/L) 2.92(2.50~3.57) 2.84(2.46~3.35) Z=-0.096 0.924 LDL(mmol/L) 1.64(1.31~1.92) 1.47(1.17~1.85) Z=-1.039 0.299 HDL(mmol/L) 0.74(0.54~0.88) 0.72(0.52~0.87) Z=-0.182 0.856 Na(mmol/L) 140.03±2.96 139.39±2.39 t=1.203 0.232 Ca(mmol/L) 2.04±0.14 2.02±0.13 t=0.866 0.388 术前血氨(μmol/L) 49.79±15.04 43.17±18.10 t=1.766 0.080 PT(s) 16.81±1.44 15.93±1.66 t=2.626 0.010 APTT(s) 39.98±17.09 38.37±8.21 t=0.676 0.501 TT(s) 18.05(16.15~21.20) 17.90(16.70~19.85) Z=-0.599 0.549 FBG(g/L) 1.66±0.68 1.56±0.48 t=0.782 0.439 FIPS评分 -0.61±0.81 -1.18±0.94 t=2.984 0.004 注:NEUT,中性粒细胞计数;Glb,球蛋白;TC,总胆固醇;LDL,低密度脂蛋白;HDL,高密度脂蛋白;PT,凝血酶原时间;APTT,部分凝血活酶时间;TT,凝血酶时间;FBG,纤维蛋白原。
表 3 多因素Logistic回归分析显性肝性脑病发生的影响因素
Table 3. Figure 3 Multifactor Logistic regression analysis of factors affecting overt-hepatic encephalopathy
项目 B值 SE Wald P值 OR 95%CI 糖尿病(无 vs 有) 0.838 0.595 1.981 0.159 2.277 0.724~7.161 Child-Pugh分级 10.133 0.006 B级 vs A级 2.860 1.002 8.153 0.354 2.056 0.448~9.429 C级 vs A级 2.122 0.735 8.330 0.004 17.498 2.561~119.548 术前门静脉主干直径 0.308 0.129 5.717 0.017 1.361 1.057~1.752 GGT 0.032 0.010 10.823 0.001 1.032 1.013~1.052 FIPS评分 1.043 0.408 6.549 0.010 2.838 1.277~6.311 -
[1] LESMANA CRA, RAHARJO M, GANI RA. Managing liver cirrhotic complications: Overview of esophageal and gastric varices[J]. Clin Mol Hepatol, 2020, 26( 4): 444- 460. DOI: 10.3350/cmh.2020.0022. [2] LYU Y, FAN DM, HAN GH. Application status and future prospect of transjugular intrahepatic portosystemic shunt in gastroesophageal variceal bleeding in liver cirrhosis[J]. J Clin Hepatol, 2022, 38( 6): 1229- 1233. DOI: 10.3969/j.issn.1001-5256.2022.06.004.吕勇, 樊代明, 韩国宏. 经颈静脉肝内门体分流术在肝硬化食管胃底静脉曲张破裂出血中的应用现状与未来展望[J]. 临床肝胆病杂志, 2022, 38( 6): 1229- 1233. DOI: 10.3969/j.issn.1001-5256.2022.06.004. [3] ZHANG K, LIU JL, LIU YL, et al. The 3D model was constructed by computer based on CT thin-slice scanning data to guide the clinical observation of the clinical effect of intrahepatic portal shunt through jugular vein in the treatment of portal hypertension complicated with gastrointestinal bleeding in cirrhosis[J]. Clin J Med Offic, 2023, 51( 6): 655- 656, 660. DOI: 10.16680/j.1671-3826.2023.06.28.张凯, 刘晶磊, 刘燚隆, 等. 应用CT薄层扫描数据电脑构建3D模型指导经颈静脉肝内门体分流术治疗肝硬化门脉高压合并消化道出血临床效果观察[J]. 临床军医杂志, 2023, 51( 6): 655- 656, 660. DOI: 10.16680/j.1671-3826.2023.06.28. [4] STEPANOVA M, MISHRA A, VENKATESAN C, et al. In-hospital mortality and economic burden associated with hepatic encephalopathy in the United States from 2005 to 2009[J]. Clin Gastroenterol Hepatol, 2012, 10( 9): 1034- 1041. DOI: 10.1016/j.cgh.2012.05.016. [5] TONG H, GAN C, WEI B, et al. Risk factors for overt hepatic encephalopathy after transjugular intrahepatic portosystemic shunt creation in patients with liver cirrhosis[J]. J Dig Dis, 2021, 22( 1): 31- 40. DOI: 10.1111/1751-2980.12957. [6] BETTINGER D, STURM L, PFAFF L, et al. Refining prediction of survival after TIPS with the novel Freiburg index of post-TIPS survival[J]. J Hepatol, 2021, 74( 6): 1362- 1372. DOI: 10.1016/j.jhep.2021.01.023. [7] CAI WM, ZHENG BS, LIN XR, et al. Prediction of patient hepatic encephalopathy risk with Freiburg index of post-TIPS survival score following transjugular intrahepatic portosystemic shunts: A retrospective study[J]. Int J Gen Med, 2022, 15: 4007- 4016. DOI: 10.2147/IJGM.S359918. [8] Chinese Society of Hepatology, Chinese Medical Association. Guidelines on the management of hepatic encephalopathy in cirrhosis[J]. J Clin Hepatol, 2018, 34( 10): 2076- 2089. DOI: 10.3969/j.issn.1001-5256.2018.10.007.中华医学会肝病学分会. 肝硬化肝性脑病诊疗指南[J]. 临床肝胆病杂志, 2018, 34( 10): 2076- 2089. DOI: 10.3969/j.issn.1001-5256.2018.10.007. [9] BAJAJ JS, CORDOBA J, MULLEN KD, et al. Review article: The design of clinical trials in hepatic encephalopathy: An International Society for Hepatic Encephalopathy and Nitrogen Metabolism(ISHEN) consensus statement[J]. Aliment Pharmacol Ther, 2011, 33( 7): 739- 747. DOI: 10.1111/j.1365-2036.2011.04590.x. [10] MA JL, CHEN X, HE LL, et al. Value of Child-Pugh score, Model for End-Stage Liver Disease score, MELD combined with serum sodium concentration, APASAL score, and R-score in predicting rebleeding and death in cirrhotic patients with esophagogastric variceal bleeding[J]. J Clin Hepatol, 2020, 36( 6): 1278- 1283. DOI: 10.3969/j.issn.1001-5256.2020.06.018.马佳丽, 陈旭, 何玲玲, 等. Child-Pugh评分、MELD评分、MELD-Na评分、APASAL评分和R评分对肝硬化伴食管胃静脉曲张再出血及死亡的预测价值[J]. 临床肝胆病杂志, 2020, 36( 6): 1278- 1283. DOI: 10.3969/j.issn.1001-5256.2020.06.018. [11] Chinese Society of Hepatology, Chinese Society of Gastroenterology, Chinese Society of Digestive Endoscopology of Chinese Medical Association. Guidelines on the management of esophagogastric variceal bleeding in cirrhotic portal hypertension[J]. J Clin Hepatol, 2023, 39( 3): 527- 538. DOI: 10.3760/cmaj.cn501113-20220824-00436.中华医学会肝病学分会, 中华医学会消化病学分会, 中华医学会消化内镜学分会. 肝硬化门静脉高压食管胃静脉曲张出血的防治指南[J]. 临床肝胆病杂志, 2023, 39( 3): 527- 538. DOI: 10.3760/cmaj.cn501113-20220824-00436. [12] The Chinese College of Interventionalists. CCI clinical practice guidelines: Management of TIPS for portal hypertension(2019 edition)[J]. J Clin Hepatol, 2019, 35( 12): 2694- 2699. DOI: 10.3969/j.issn.1001-5256.2019.12.010.中国医师协会介入医师分会. 中国门静脉高压经颈静脉肝内门体分流术临床实践指南(2019年版)[J]. 临床肝胆病杂志, 2019, 35( 12): 2694- 2699. DOI: 10.3969/j.issn.1001-5256.2019.12.010. [13] GAO Y. Predictive value of Freiburg survival index(FIPS) for survival of patients with cirrhosis and portal hypertension treated by transjugular intrahepatic portosystemic shunt[D]. Changchun: Jilin University, 2022.高扬. 弗莱堡术后生存指数(FIPS)对经颈静脉肝内门体分流术治疗的肝硬化门脉高压患者生存期的预测价值[D]. 长春: 吉林大学, 2022. [14] LIU Y, ZHOU ZZ, LIU G, et al. The characteristics and risk factors of hepatic encephalopathy after emergent TIPS operation in patients with bleeding from esophageal and gastric varices[J]. Chin Hepatol, 2022, 27( 6): 658- 661. DOI: 10.3969/j.issn.1008-1704.2022.06.014.刘益, 周自忠, 刘刚, 等. 食管胃底静脉曲张破裂出血患者急诊TIPS术后肝性脑病发生特征及危险因素分析[J]. 肝脏, 2022, 27( 6): 658- 661. DOI: 10.3969/j.issn.1008-1704.2022.06.014. [15] ICHAI P, SAMUEL D. Etiology and prognosis of fulminant hepatitis in adults[J]. Liver Transpl, 2008, 14( Suppl 2): S67- S79. DOI: 10.1002/lt.21612. [16] KRISHNARAO A, GORDON FD. Prognosis of hepatic encephalopathy[J]. Clin Liver Dis, 2020, 24( 2): 219- 229. DOI: 10.1016/j.cld.2020.01.004. [17] FONIO P, DISCALZI A, CALANDRI M, et al. Incidence of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt(TIPS) according to its severity and temporal grading classification[J]. Radiol Med, 2017, 122( 9): 713- 721. DOI: 10.1007/s11547-017-0770-6. [18] YIN XC, ZHANG F, GUO HW, et al. A nomogram to predict the risk of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt in cirrhotic patients[J]. Sci Rep, 2020, 10( 1): 9381. DOI: 10.1038/s41598-020-65227-2. [19] LIU J, ZHOU C, WANG Y, et al. The combination of Child-Pugh score and quantitative CT-based spleen volume could predict the risk of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt creation[J]. Abdom Radiol(NY), 2021, 46( 7): 3464- 3470. DOI: 10.1007/s00261-021-02972-6. [20] WEISS N, BARBIER SAINT HILAIRE P, COLSCH B, et al. Cerebrospinal fluid metabolomics highlights dysregulation of energy metabolism in overt hepatic encephalopathy[J]. J Hepatol, 2016, 65( 6): 1120- 1130. DOI: 10.1016/j.jhep.2016.07.046. [21] MCMILLIN M, FRAMPTON G, QUINN M, et al. Bile acid signaling is involved in the neurological decline in a murine model of acute liver failure[J]. Am J Pathol, 2016, 186( 2): 312- 323. DOI: 10.1016/j.ajpath.2015.10.005. [22] AMPUERO J, RANCHAL I, DEL MAR DÍAZ-HERRERO M, et al. Role of diabetes mellitus on hepatic encephalopathy[J]. Metab Brain Dis, 2013, 28( 2): 277- 279. DOI: 10.1007/s11011-012-9354-2. [23] YIN XC, ZHANG F, XIAO JQ, et al. Diabetes mellitus increases the risk of hepatic encephalopathy after a transjugular intrahepatic portosystemic shunt in cirrhotic patients[J]. Eur J Gastroenterol Hepatol, 2019, 31( 10): 1264- 1269. DOI: 10.1097/MEG.0000000000001452. -



 PDF下载 ( 1030 KB)
PDF下载 ( 1030 KB)

 下载:
下载: