基于生物信息学分析构建肝细胞癌患者新型双硫死亡相关预后模型
DOI: 10.12449/JCH240917
Construction of a novel disulfidptosis-related prognostic model for patients with hepatocellular carcinoma based on bioinformatics analysis
-
摘要:
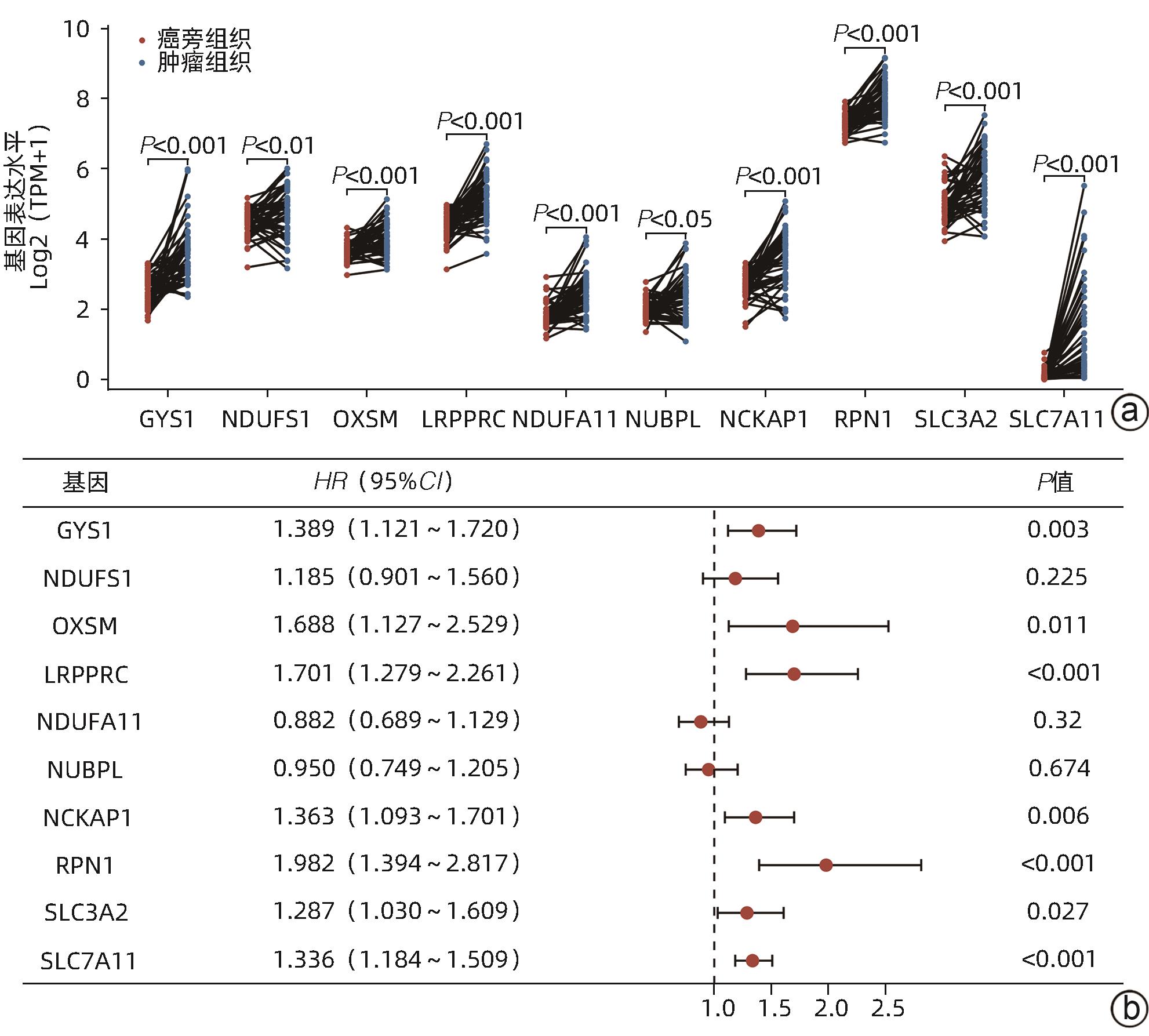
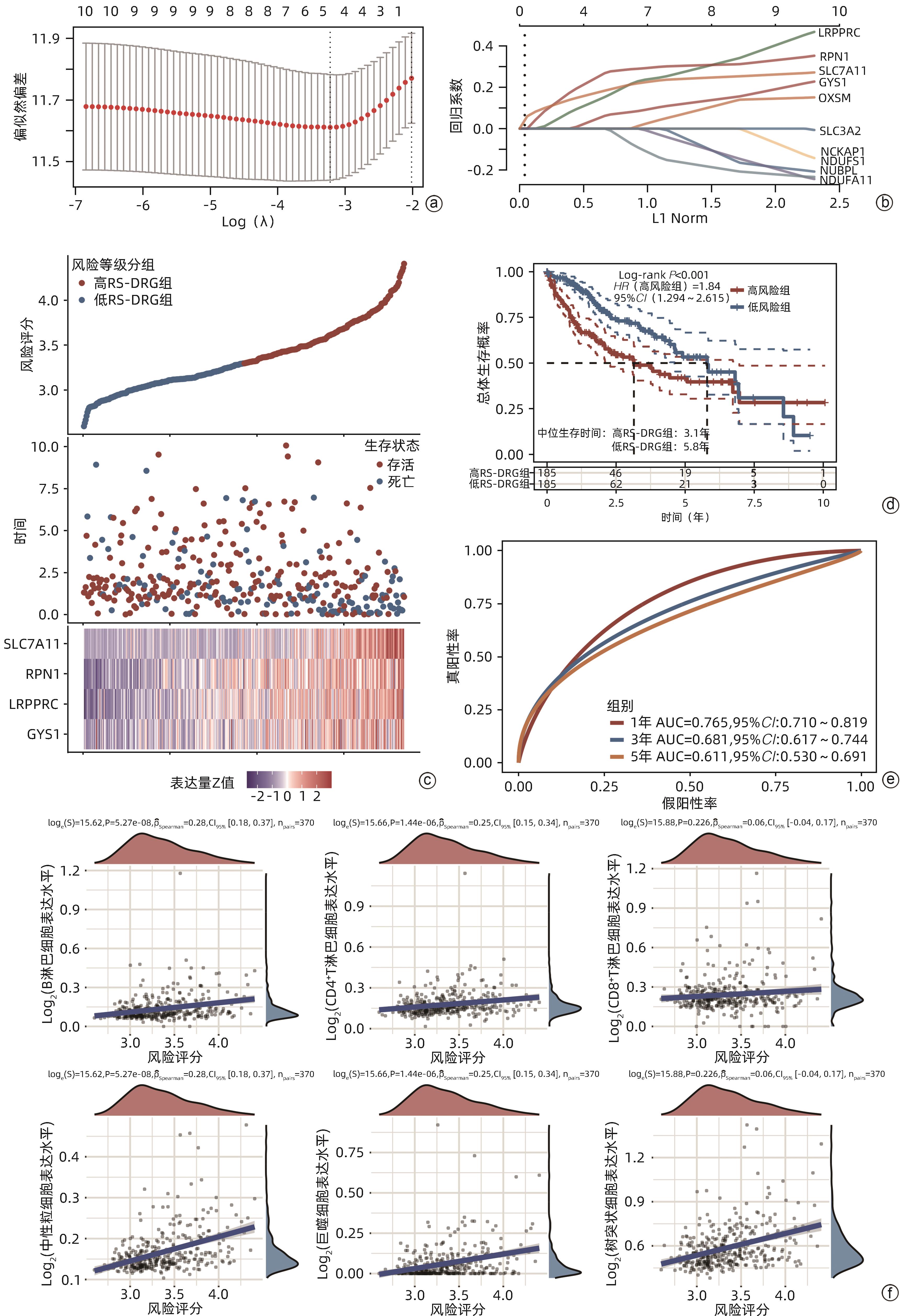
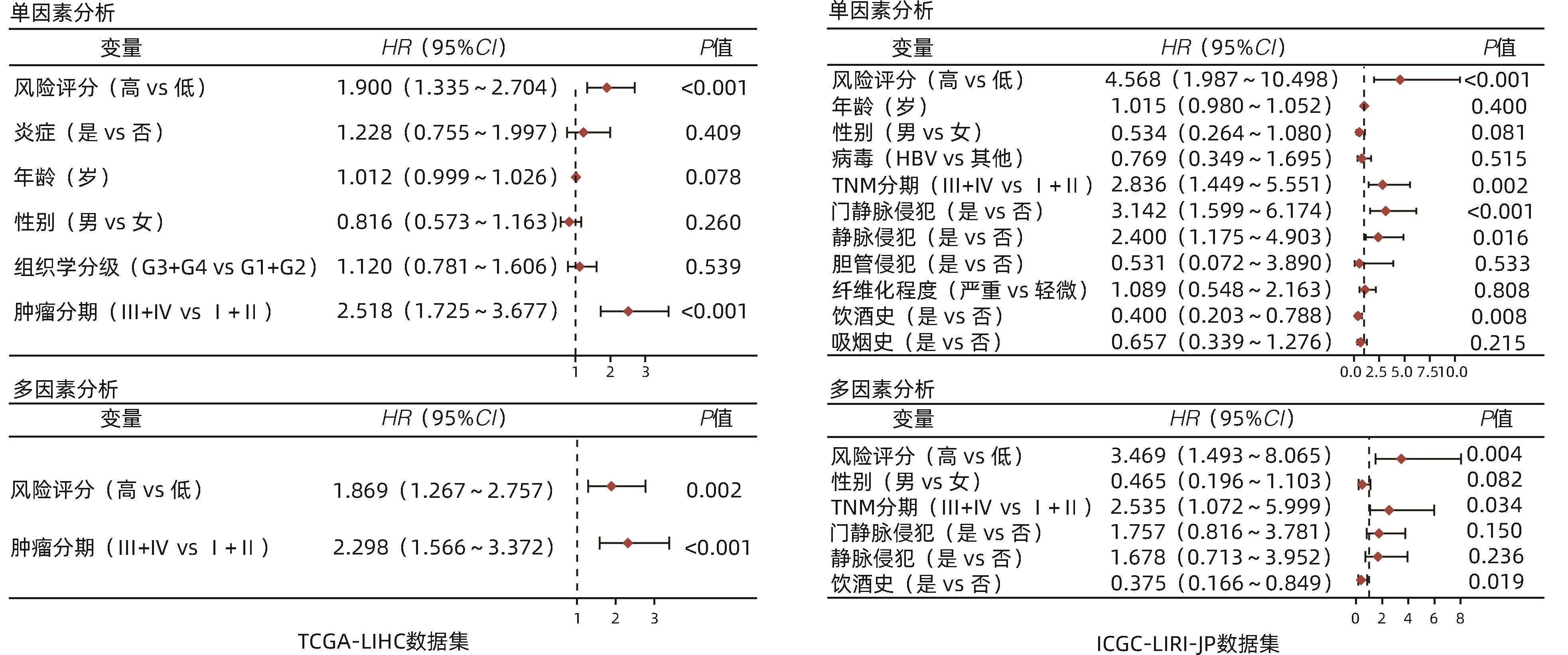
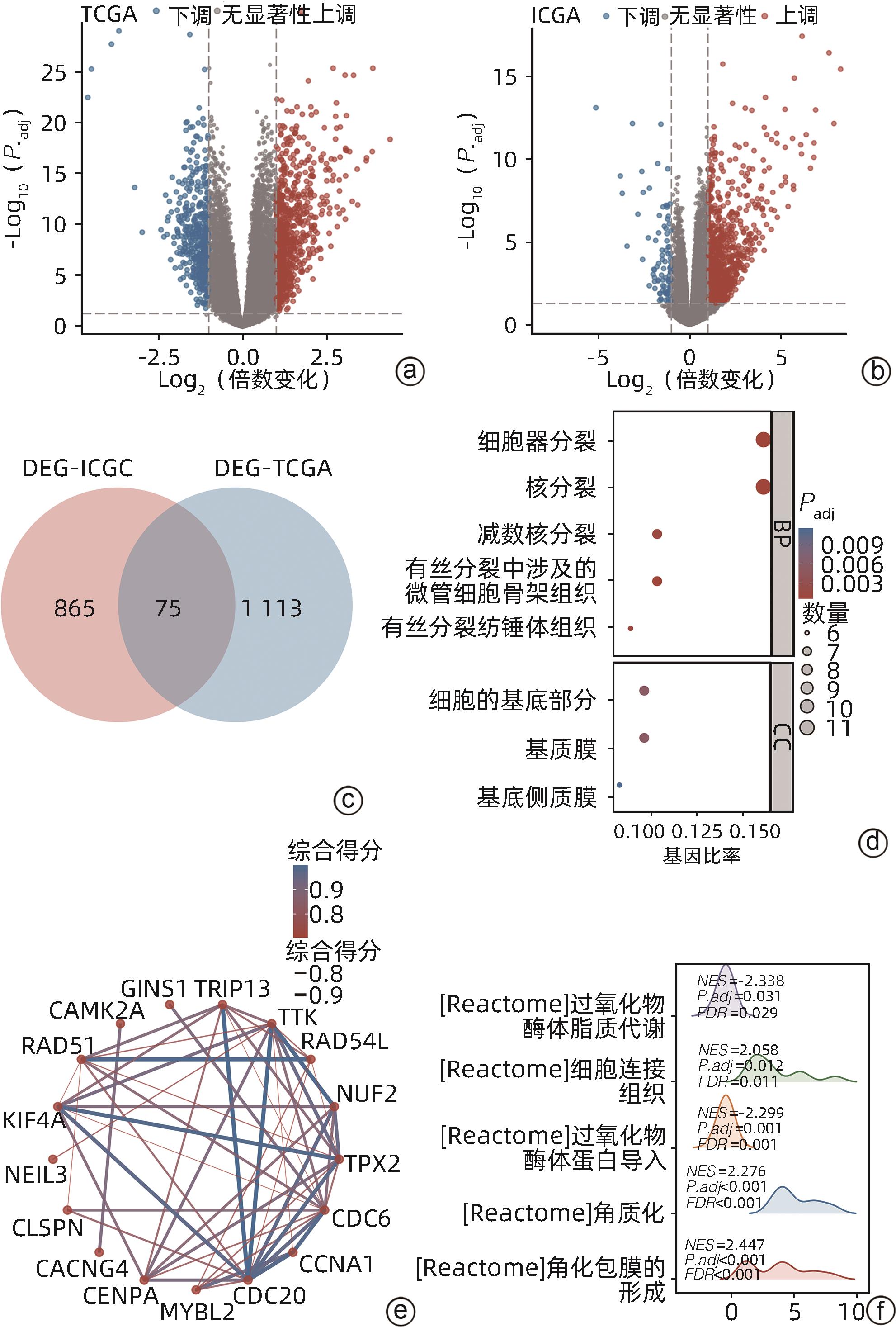
目的 探讨双硫死亡基因在肝细胞癌(HCC)中的表达情况,研究双硫死亡对HCC的预后价值并构建预后模型,分析其影响HCC的生物学过程和对索拉非尼耐药的影响。 方法 从TCGA-LIHC数据库中收集HCC患者的mRNA表达谱和相应临床数据。利用LASSO-Cox回归算法构建TCGA队列中的4基因预后预测模型。在外部数据集ICGC和GSE14520队列中验证模型的预后效能。基于癌症药物敏感性基因组学数据分析双硫死亡模型对索拉非尼治疗反应的预测作用。此外,进行GO和KEGG功能分析,以深入了解双硫死亡相关基因的生物学功能。计量资料两组间比较采用成组t检验;计数资料两组间比较采用χ2检验。采用Kaplan-Meier曲线和Log-rank检验评估预后差异;采用单因素和多因素Cox回归分析研究风险评分是否独立影响患者预后。 结果 在TCGA队列中行单因素Cox回归分析,7个已知的双硫死亡相关基因与HCC总生存期(OS)显著相关(P值均<0.05);进一步通过LASSO-Cox回归分析,构建双硫死亡相关的基因(DRG)预后模型,并计算风险评分(RS-DRG),RS-DRG=0.061 6×GYS1表达水平+0.152 8×LRPPRC表达水平+0.268 3×RPN1表达水平+0.183 5×SLC7A11表达水平。Log-rank检验表明双硫死亡模型中高风险评分患者的OS明显低于低风险评分患者(P<0.001)。根据多因素Cox回归结果,在TCGA和ICGC队列中,风险评分都是OS的独立预测因子(TCGA:HR=1.869,P=0.002;ICGC:HR=3.469,P=0.004)。Spearman相关分析表明RS-DRG与肿瘤微环境中的多种免疫细胞类型(包括B淋巴细胞、CD4+ T淋巴细胞、中性粒细胞、巨噬细胞和树突状细胞)的浸润水平呈显著正相关(P值均<0.05)。高风险评分组患者索拉非尼IC50值更低,对索拉非尼更敏感(P<0.001)。KEGG/GO富集分析显示双硫死亡风险差异基因显著富集于多种有丝分裂相关的分子功能。 结论 本研究构建了一种新的双硫死亡相关基因预后模型,在预测HCC预后方面具有潜在的临床应用价值;以双硫死亡基因为靶点可能是治疗HCC的一种很有前景的方法。 Abstract:Objective To investigate the expression of disulfidptosis-related genes in hepatocellular carcinoma (HCC) and the prognostic value of disulfidptosis in HCC, to construct a prognostic model, and to analyze its impact on the biological processes of HCC and sorafenib resistance. Methods The TCGA-LIHC database was used to collect the mRNA expression profiles and corresponding clinical data of HCC patients, and the LASSO-Cox regression algorithm was used to construct a four-gene predictive model for prognosis in the TCGA cohort. The external datasets ICGC and GSE14520 were used to validate the prognostic efficacy of the model, and the Cancer Drug Sensitivity Genomics (GDSC) data were used to investigate the value of the disulfidptosis model in predicting sorafenib treatment response, and gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses were used to investigate the biological functions of disulfidptosis-related genes. The independent-samples t test was used for comparison of continuous data between two groups, and the chi-square test was used for comparison of categorical data between two groups. The Kaplan-Meier curve and the log-rank test were used to evaluate the difference in prognosis, and univariate and multivariate Cox regression analyses were used to investigate whether risk score was an independent influencing factor for patient prognosis. Results The univariate Cox regression analysis in the TCGA cohort showed that seven known disulfidptosis-related genes were significantly associated with overall survival (OS) in HCC (all P<0.05). The LASSO-Cox regression analysis was used to construct a prognostic model based on disulfidptosis-related genes (DRG), and the risk score RS-DRG was calculated as RS-DRG=(0.061 6)×GYS1 expression level+(0.152 8)×LRPPRC expression level+(0.268 3)×RPN1 expression level+(0.183 5)×SLC7A11 expression level. The log-rank test showed that the patients with a high risk score based on the disulfidptosis model had a significantly lower OS than those with a low risk score (P<0.001). Based on the results of the multivariate Cox regression analysis, risk score was an independent predictive factor for OS in both TCGA and ICGC cohorts (TCGA: hazard ratio [HR]=1.869, P=0.002; ICGC: HR=3.469, P=0.004). The Spearman correlation analysis showed that RS-DRG was significantly positively correlated with the infiltration level of various immune cells (including B lymphocytes, CD4+ T lymphocytes, neutrophils, macrophages, and dendritic cells) in tumor microenvironment (all P<0.05). The patients in the high-risk score group had a significantly lower IC50 value of sorafenib and were more sensitive to sorafenib (P<0.001). The KEGG/GO enrichment analysis showed that the differentially expressed disulfidptosis-related genes were significantly enriched in various mitosis-related molecular functions. Conclusion This study constructed a novel prognostic model based on disulfidptosis-related genes, which has a potential clinical value in predicting the prognosis of HCC, and targeting disulfidptosis-related genes may provide a promising approach for HCC treatment. -
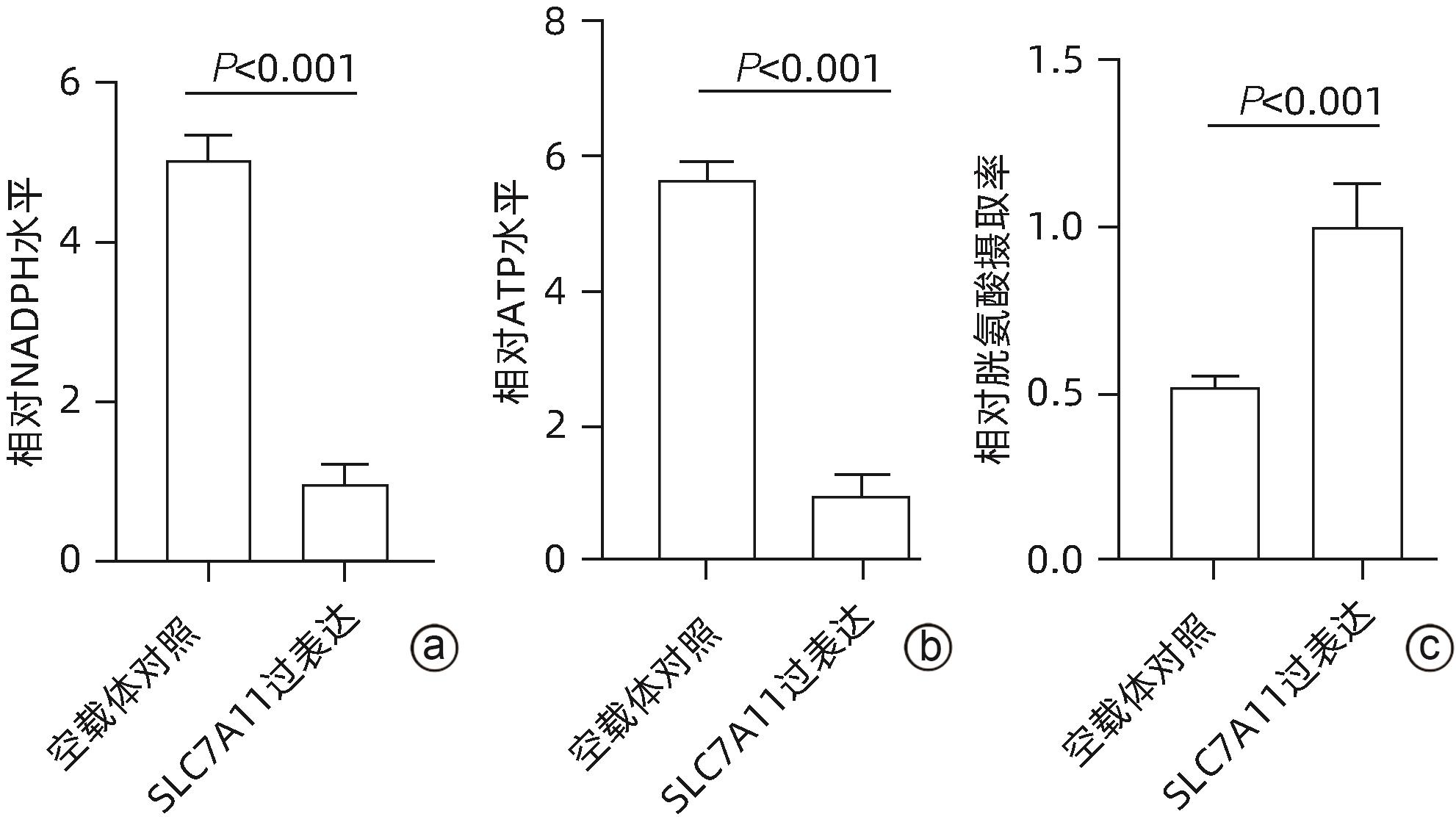
注: a,SLC7A11在HCC和癌旁组织中的表达;b,GSE146609中细胞簇划分;c,SLC7A11在HCC组织不同细胞簇中的表达;d,SLC7A11高、低表达的HCC患者免疫细胞评分;e,SLC7A11高、低表达的HCC患者免疫检查点水平;f,qRT-PCR检测SLC7A11在肝癌细胞和肝细胞中表达水平;g、h,qRT-PCR检测HepG2细胞、Huh7细胞中SLC7A11过表达;i、j,Western Blot检测HepG2细胞、Huh7细胞中SLC7A11过表达;k、l,CCK8实验检测过表达SLC7A11的HepG2细胞、Huh7细胞在葡萄糖缺乏的环境下24 h后的细胞活力。
图 1 SLC7A11在HCC中的差异表达和潜在生物学功能
Figure 1. Differential expression and potential biological function of SLC7A11 in HCC
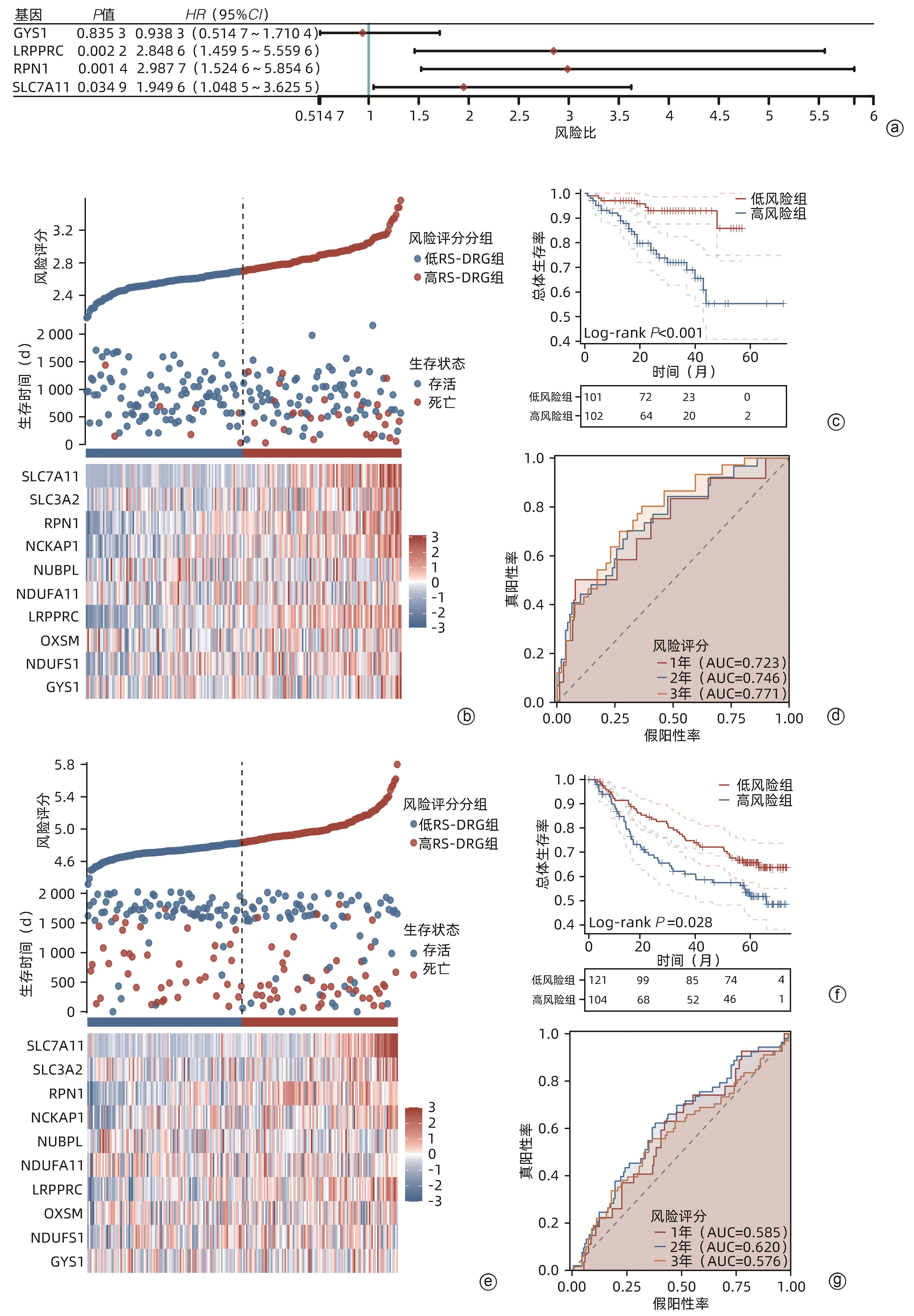
注: a,ICGC队列中单因素Cox回归分析与OS有关的基因;b,ICGC队列中不同风险组HCC患者的生存分布;c,ICGC队列中高风险组和低风险组HCC患者OS的Kaplan-Meier曲线;d,ICGC队列中时间依赖ROC曲线验;e,GSE14520队列中不同风险组HCC患者的生存分布;f,GSE14520队列中高风险组和低风险组HCC患者OS的Kaplan-Meier曲线;g,GSE14520队列中时间依赖ROC曲线。
图 5 在ICGC队列和GSE14520队列中验证预后模型
Figure 5. Validation of the prognostic model in the ICGC and GSE14520 cohort
-
[1] CAO PB, YANG AQ, LI PY, et al. Genomic gain of RRS1 promotes hepatocellular carcinoma through reducing the RPL11-MDM2-p53 signaling[J]. Sci Adv, 2021, 7( 35): eabf4304. DOI: 10.1126/sciadv.abf4304. [2] FENG M, SURESH K, SCHIPPER MJ, et al. Individualized adaptive stereotactic body radiotherapy for liver tumors in patients at high risk for liver damage: A phase 2 clinical trial[J]. JAMA Oncol, 2018, 4( 1): 40- 47. DOI: 10.1001/jamaoncol.2017.2303. [3] WEI L, LEE D, LAW CT, et al. Genome-wide CRISPR/Cas9 library screening identified PHGDH as a critical driver for Sorafenib resistance in HCC[J]. Nat Commun, 2019, 10( 1): 4681. DOI: 10.1038/s41467-019-12606-7. [4] FENG MX, MA MZ, FU Y, et al. Elevated autocrine EDIL3 protects hepatocellular carcinoma from anoikis through RGD-mediated integrin activation[J]. Mol Cancer, 2014, 13: 226. DOI: 10.1186/1476-4598-13-226. [5] LIU XG, NIE LT, ZHANG YL, et al. Actin cytoskeleton vulnerability to disulfide stress mediates disulfidptosis[J]. Nat Cell Biol, 2023, 25( 3): 404- 414. DOI: 10.1038/s41556-023-01091-2. [6] ZHENG PJ, ZHOU CT, DING YM, et al. Disulfidptosis: A new target for metabolic cancer therapy[J]. J Exp Clin Cancer Res, 2023, 42( 1): 103. DOI: 10.1186/s13046-023-02675-4. [7] ZHAO SY, WANG LY, DING W, et al. Crosstalk of disulfidptosis-related subtypes, establishment of a prognostic signature and immune infiltration characteristics in bladder cancer based on a machine learning survival framework[J]. Front Endocrinol(Lausanne), 2023, 14: 1180404. DOI: 10.3389/fendo.2023.1180404. [8] KUDO Y, SUGIMOTO M, ARIAS E, et al. PKCλ/ι loss induces autophagy, oxidative phosphorylation, and NRF2 to promote liver cancer progression[J]. Cancer Cell, 2020, 38( 2): 247- 262.e11. DOI: 10.1016/j.ccell.2020.05.018. [9] WU QC, ZHOU WH, YIN SY, et al. Blocking triggering receptor expressed on myeloid cells-1-positive tumor-associated macrophages induced by hypoxia reverses immunosuppression and anti-programmed cell death ligand 1 resistance in liver cancer[J]. Hepatology, 2019, 70( 1): 198- 214. DOI: 10.1002/hep.30593. [10] CALDERARO J, ZIOL M, PARADIS V, et al. Molecular and histological correlations in liver cancer[J]. J Hepatol, 2019, 71( 3): 616- 630. DOI: 10.1016/j.jhep.2019.06.001. [11] ZHONG ZY, ZHANG CJ, NI S, et al. NFATc1-mediated expression of SLC7A11 drives sensitivity to TXNRD1 inhibitors in osteoclast precursors[J]. Redox Biol, 2023, 63: 102711. DOI: 10.1016/j.redox.2023.102711. [12] FAVARO E, BENSAAD K, CHONG MG, et al. Glucose utilization via glycogen phosphorylase sustains proliferation and prevents premature senescence in cancer cells[J]. Cell Metab, 2012, 16( 6): 751- 764. DOI: 10.1016/j.cmet.2012.10.017. [13] XIE H, SONG J, GODFREY J, et al. Glycogen metabolism is dispensable for tumour progression in clear cell renal cell carcinoma[J]. Nat Metab, 2021, 3( 3): 327- 336. DOI: 10.1038/s42255-021-00367-x. [14] LIU JY, CHEN YJ, FENG HH, et al. LncRNA SNHG17 interacts with LRPPRC to stabilize c-Myc protein and promote G1/S transition and cell proliferation[J]. Cell Death Dis, 2021, 12( 11): 970. DOI: 10.1038/s41419-021-04238-x. [15] WANG HH, TANG AM, CUI YY, et al. LRPPRC facilitates tumor progression and immune evasion through upregulation of m6A modification of PD-L1 mRNA in hepatocellular carcinoma[J]. Front Immunol, 2023, 14: 1144774. DOI: 10.3389/fimmu.2023.1144774. [16] LIU XY, XIAO WD, ZHANG YN, et al. Reversible phosphorylation of Rpn1 regulates 26S proteasome assembly and function[J]. Proc Natl Acad Sci USA, 2020, 117( 1): 328- 336. DOI: 10.1073/pnas.1912531117. [17] CHEN X, KANG R, KROEMER G, et al. Broadening horizons: The role of ferroptosis in cancer[J]. Nat Rev Clin Oncol, 2021, 18( 5): 280- 296. DOI: 10.1038/s41571-020-00462-0. -
 附录A 和B 见二维码.pdf
附录A 和B 见二维码.pdf
-



 PDF下载 ( 6149 KB)
PDF下载 ( 6149 KB)


 下载:
下载: