Resistance-associated variants in the non-structural protein 5B region in patients with hepatitis C virus genotype 1b infection in Guangxi, China
-
摘要: 目的分析我国广西地区HCV 1b型感染者NS5B区耐药基因相关变异(RAV)的发生率及不同性别间的差异。方法选取2016年4月-2018年9月广西医科大学第一附属医院收治的60例HCV 1b型感染初治患者,留取基线血清样本,采用巢式聚合酶链式反应扩增NS5B区片段并进行基因测序,测序结果与基因库标准株进行比对。不同性别患者间的年龄、HCV RNA、ALT和AST水平比较采用t检验,耐药位点变异率采用Fisher确切概率法检验。结果入选的60例患者中,55例获得完整的NS5B区序列信息,RAV发生率为96. 3%,其中C316 (94. 5%)、A338(70. 9%)、T19(74. 5%)为主要的变异位点,同时检测到C316+T19、C316+T19+A338等多位点联合突变。男性RAV发生率为95. 8%(23/24),女性为96. 8%(30/31),差异无统计学意义(P=1. 000)。结论广西地区HCV 1b型感染者NS5B区存在较高的RAV发生率,既有单位点突变,也存在多位点联合突变,且不同性别间RAV发生率无明显差异。Abstract: Objective To investigate the incidence rate of resistance-associated variants ( RAVs) in the non-structural protein 5B (NS5B) region in patients with hepatitis C virus ( HCV) genotype 1b ( GT1b) infection in Guangxi, China, as well as its difference between male and female patients. Methods A total of 60 previously untreated patients with HCV GT1b infection who were admitted to The First Affiliated Hospital of Guangxi Medical University from April 2016 to September 2018 were enrolled. Their baseline serum samples were collected. The NS5B region fragments were amplified by nested PCR and gene sequencing was performed, and then the sequencing results were compared with standard strains in GeneBank. The t-test was used for comparison of age, HCV RNA, alanine aminotransferase ( ALT) , and aspartate aminotransferase ( AST) between male and female patients, and the Fisher's exact test was used for comparison of mutation rate of drug-resistance sites. Results Of all 60 patients, 55 obtained the complete sequence information of the NS5B region, and the incidence rate of RAVs in the NS5B region was 96. 3%. C316 ( 94. 5%) , A338 ( 70. 9%) , and T19 ( 74. 5%) were the main mutation sites, and multisite mutations such as C316 + T19 and C316 + T19 + A338 were observed. There was no significant difference in the incidence rate of RAVs between male patients and female patients [95. 8% ( 23/24) vs 96. 8% ( 30/31) , P = 1. 000]. Conclusion Patients with HCV GT1b infection in Guangxi have a high incidence rate of RAVs in the NS5B region, with both single-site and multisite mutations.There is no significant difference in the incidence rate of RAVs between male and female patients.
-
Key words:
- hepacivirus /
- hepatitis C /
- genotype /
- drug resistance /
- cross-sectional studies
-
在我国,原发性肝癌是最常见的恶性肿瘤之一。外科手术是肝癌患者的首选治疗方式。然而,外科术后肿瘤复发是影响患者预后的重要因素[1]。近年来,微创介入治疗因其独特的优点,被肝癌患者尤其是复发性肝癌患者所接受。经肝动脉化疗栓塞(transcatheter arterial chemoembolization, TACE)联合局部消融被越来越多地应用于治疗外科术后复发性肝癌患者[2]。研究[2-3]显示,TACE联合局部消融治疗外科术后复发性肝癌具有较好的疗效。本研究对TACE联合局部消融治疗外科术后复发性肝癌患者的临床资料进行回顾性分析,现报道如下。
1. 资料与方法
1.1 研究对象
回顾性分析2017年1月—2020年12月于宁夏医科大学总医院接受TACE联合局部消融治疗外科术后复发性肝癌患者的资料。纳入标准:(1)既往因肝癌行外科手术治疗,术后影像学检查提示肝内出现新的病灶,具备典型的肝癌影像学特征;(2)复发性肝癌以TACE联合局部消融为首选治疗;(3)肝内单发病灶直径≤5 cm,或者多发结节病灶≤3个,且每个结节病灶直径≤3 cm;(4)肝功能Child-Pugh分级为A级或B级。排除标准:(1)BCLC分期C期或者D期;(2)合并有全身急性疾病或自身免疫系统疾病者;(3)消融治疗前肝功能Child-Pugh C级;(4)随访信息不全或者失访者。
1.2 TACE联合局部消融治疗
患者完善相关检查后行TACE术式。采用Seldinger技术行股动脉穿刺插管,行腹腔干、肝固有动脉或其分支造影。通过造影结果明确肿瘤局部情况后,超选择插管至肿瘤主要供血动脉,进行化疗药物灌注治疗,并用碘化油(用量10~20 mL)进行栓塞,根据肿瘤局部情况行个体化治疗。灌注药物有盐酸吡柔比星20 mg,洛铂30 mg。患者在TACE术后2~4周行射频消融或者微波消融。术前通过影像学检查(B超、CT、MRI)明确病灶部位及形态,确定穿刺位点,制订个体化治疗计划。术中在CT引导下行经皮穿刺肝内病灶,逐步进行病灶消融术,消融后立即行CT平扫了解消融范围是否基本覆盖病灶。若消融范围覆盖不完全,则可立即再次消融。于一次消融中完成所有病灶消融。
1.3 随访及疗效评价
局部消融治疗后开始随访。术后前3个月,患者每个月行肝脏增强CT或者MRI。之后间隔3个月1次,1年后间隔半年1次,行上述影像学检查。随访患者的总体生存时间(overall survival time, OS)。OS定义为:患者接受初次联合治疗至其死亡或随访截止时间(2020年10月)。随访过程中若局部病灶存活或者复发,可根据个体情况给予相应治疗(TACE、局部消融等)。疗效评价:依据美国肝病学会颁布的改良实体瘤疗效评价标准(mRECIST),评价患者的近期疗效(首次联合治疗术后3个月)。该标准内容包括:完全缓解(CR),影像学检查提示肝内所有病灶动脉期不增强,即肿瘤病灶完全坏死;部分缓解(PR),影像学检查提示肝内病灶部分坏死(病灶直径缩小≥30%),但仍有部分病灶存活;疾病进展(PD),影像学检查提示肝内病灶直径较前增大(病灶直径增加≥20%),或肝内出现新的病灶;疾病稳定(SD),经影像学检查评估后,肝内病灶变化情况介于部分缓解与疾病进展之间,即病灶缩小程度不及部分缓解,增加程度不及疾病进展。总体有效率(ORR)=(CR+PR)/总例数×100%;疾病控制率(DCR)=(CR+PR+SD)/总例数×100%。
1.4 统计学方法
采用SPSS 24.0软件进行分析。运用Kaplan-Meier法进行生存分析,采用log-rank检验分析组间差异。采用Cox比例风险回归模型评估影响外科术后复发性肝癌患者OS的可能危险因素。单因素分析结果有意义的变量被用于多因素分析检验。P<0.05为差异有统计学意义。
2. 结果
2.1 一般资料
共纳入47例患者,其中男33例,女14例。肝功能Child-Pugh A级29例,B级18例。单结节患者33例,多结节患者(肿瘤数目2~3个)14例。
2.2 近期疗效和不良反应
TACE联合局部消融治疗外科术后复发性肝癌47例,其中CR 33例,PR 9例,SD 3例,PD 2例,ORR为89.3%,DCR为95.7%。
47例复发性肝癌患者共行TACE 53次,局部消融67次。TACE及消融术后并发症均为轻度不良反应,其中TACE术后疼痛33例,恶心呕吐29例,发热19例;局部消融术后疼痛28例,发热23例。予以对症处理后均得以缓解。治疗中未发生与手术直接相关的死亡事件。
2.3 生存情况
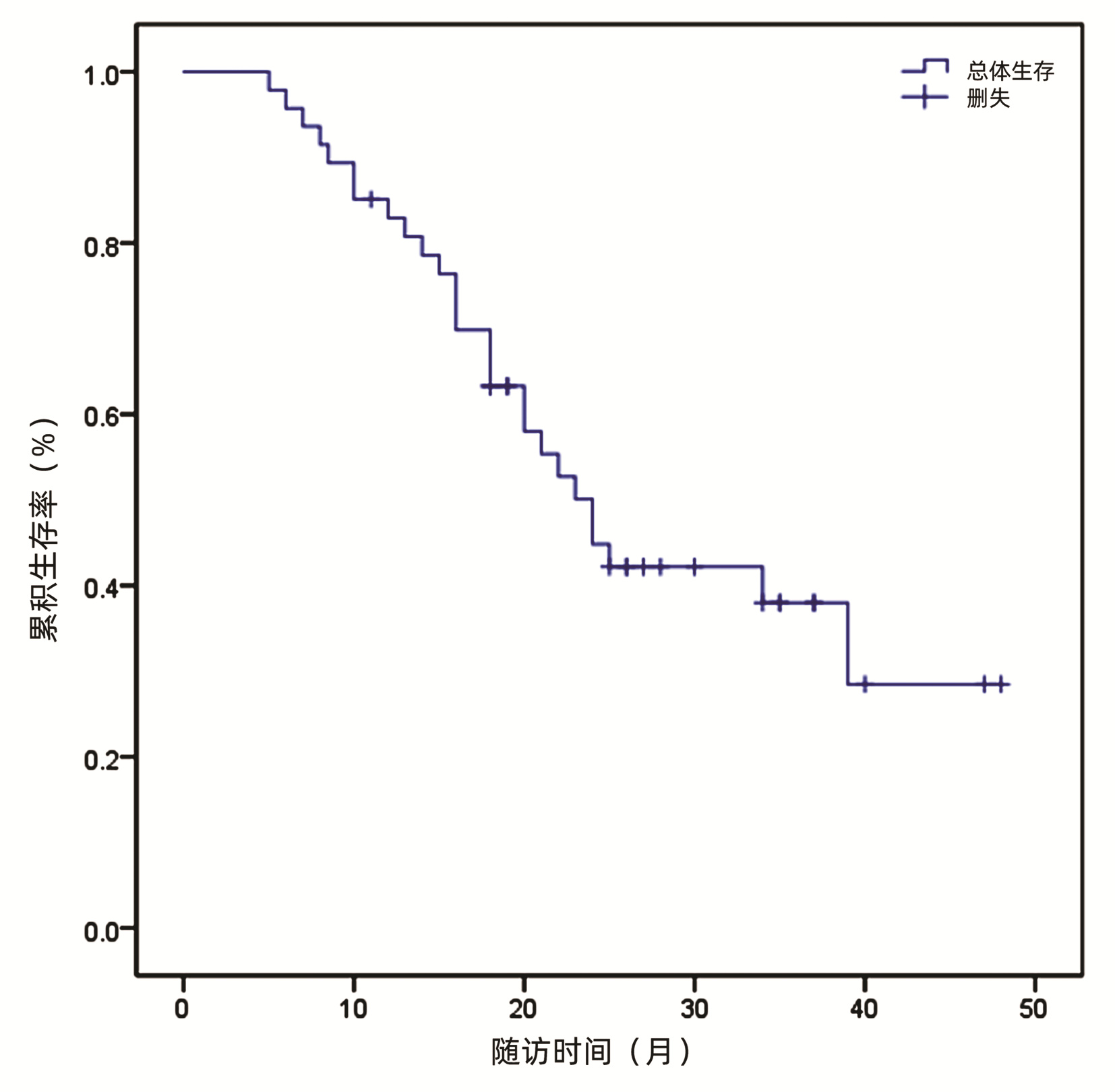
47例患者中位OS为24个月。6、12、18、24个月总生存率分别为95.70%、76.40%、63.30%、58.00%。截至研究终点(即随访截止时间),27例患者死亡,尚有20例患者存活(图 1)。
2.4 影响患者OS的单因素和多因素分析
Kaplan-Meier单因素分析显示外科术后复发性肝癌患者TACE联合局部消融治疗后OS与AFP≥40 ng/mL、肿瘤直径≥3 cm、肿瘤边界不规整和肿瘤位置临近危险区域(大血管、空腔脏器等)相关,差异均有统计学意义(P值均<0.05)。与性别、年龄、PLT、ALT、AST、肝功能分级和肿瘤数目无关(P值均>0.05)(表 1)。将单因素分析有意义的因素(AFP、肿瘤直径、肿瘤边界和肿瘤位置是否临近危险区域)导入Cox比例风险回归模型,结果显示:肿瘤边界不规整和肿瘤位置临近危险区域(大血管、空腔脏器等)是影响外科术后复发性肝癌患者TACE联合局部消融术后生存的危险因素(P值均<0.05)(表 2)。
表 1 影响患者OS的单因素分析Table 1. Univariate analysis of affecting patient OS因素 例数 中位OS(月) χ2值 P值 性别 0.754 0.385 男 33 22 女 14 25 年龄 0.003 0.953 ≥60岁 23 23 <60岁 24 24 PLT 0.066 0.798 ≥100×109/L 32 23 <100×109/L 15 22 ALT 0.294 0.588 ≥40 U/L 11 24 <40 U/L 36 22 AST 0.459 0.498 ≥45 U/L 30 24 <45 U/L 17 22 Child-Pugh分级 0.479 0.489 A级 29 25 B级 18 22 AFP 3.861 0.049 ≥40 ng/mL 20 16 <40 ng/mL 27 25 肿瘤直径 3.933 0.047 ≥3 cm 19 16 <3 cm 28 25 肿瘤数目 0.508 0.476 单个 32 23 2~3个 15 34 肿瘤边界 5.531 0.021 规整 28 25 不规整 19 18 肿瘤位置是否临近危险区域(大血管、空腔脏器等) 7.920 0.005 是 11 18 否 36 34 表 2 影响患者OS的多因素分析Table 2. Multivariate analysis of affecting patient OS因素 RR B值 SE Wald 95%CI P值 肿瘤边界(规整/不规整) 3.938 1.164 0.417 7.804 1.709~9.073 0.005 肿瘤位置是否临近危险区域(是/否) 3.202 1.371 0.426 10.361 1.415~7.245 0.001 3. 讨论
对于复发性肝癌,手术切除或者肝移植可能是最为理想的治疗方式。然而复发性肝癌患者常受剩余肝脏体积、远处转移、静脉侵犯、基础条件、个人意愿等情况的影响而难以耐受再次手术切除[4-5]。因而临床中多采用微创介入的治疗方式[6-7]。TACE被视为非手术治疗肝癌的首选方式[8]。然而多次行TACE对患者肝功能损害较大,且TACE受肿瘤乏血供、肿瘤直径等因素的影响[9],单一TACE治疗效果欠佳。
局部消融因具备微创、可重复等优势被广泛应用于原发性肝癌与复发性肝癌患者的治疗中。相较于外科手术,局部消融具备安全性能高、术后并发症少、住院时间短等优势[10-11]。但有研究[12]显示,两者的治疗效果与肿瘤直径存在一定的关系。就患者术后生存率而言,当肿瘤直径较小时(≤3 cm),两者在患者术后生存率方面无明显差异;当肿瘤直径较大时(>3 cm),局部消融组患者的生存率低于外科手术组。
近年来为改善肝癌患者微创化治疗的疗效,TACE联合局部消融被越来越广泛应用于临床[13-14]。本研究结果显示,复发性肝癌患者治疗后3个月ORR为89.3%,DCR为95.7%,且治疗后无严重手术直接相关并发症发生,提示对于外科术后复发性肝癌患者,TACE联合局部消融治疗可获得较好的的近期疗效及安全性。临床研究[12]显示,对于复发性肝癌患者远期生存率,TACE联合消融组与手术组治疗相比,其预后相近,两组在统计学上无明显差异。Peng等[15]亦称在患者1、3、5年生存率方面,TACE联合消融组与再次手术组无统计学差异。在本研究中,47例复发性肝癌患者TACE联合局部消融治疗后,中位OS可达到24个月,且12、24个月总生存率分别为76.40%、58.00%,与张辉等[16]的研究结果相近。提示对于接受TACE联合局部消融治疗的外科术后复发性肝癌患者,该治疗方式可获得较好的生存率。
本研究显示肿瘤边界不规整和肿瘤位置临近危险区域(大血管、空腔脏器等)是影响外科术后复发性肝癌患者TACE联合局部消融术后生存的危险因素(P值均<0.05),肿瘤边界不规整,肿瘤位置临近危险区域(大血管、空腔脏器等)的患者,治疗后生存率较低。当肿瘤位置临近大血管时,因管内血液对管壁的快速冲刷作用,即所谓的“热沉效应”,导致消融过程热量损减,使得消融效果降低,可能导致肿瘤消融不完全从而影响预后。当肿瘤位置临近空腔脏器时,消融过程可能因注意保护脏器而折损消融效果,且术前肿瘤可能已经对临近脏器产生微浸润现象[17],从而影响治疗效果及预后。
肿瘤边界不规整常由于癌组织的树突状生长或者癌细胞的不规则浸润引起[18],使得病灶更容易临近危险区域(大血管、空腔脏器等),且消融过程受到局部汽化的影响,可能导致消融范围不能完全覆盖病灶,从而引发肿瘤复发及转移,对患者的预后造成影响。因此对于复发性肝癌需要个体化治疗,在适宜情况下可联合其他治疗方式,以期延长患者的生存期。
付元等[19]探讨了TACE联合射频消融及索拉非尼在外科术后复发性肝癌患者中的治疗效果。结果显示,相较于对照组(TACE联合射频消融组),治疗组(TACE联合射频消融及口服索拉非尼)可明显延长患者的中位生存时间。然而在1、2、3年生存率方面,尽管治疗组均高于对照组,但其差异无统计学意义。笔者认为,如何选用适合的治疗方式以求最大程度延长复发性肝癌患者的生存,是临床研究需要突破的一个关键点。就本研究而言,在合并有危险因素的情形下(肿瘤边界不完整或肿瘤位置临近危险区域),局部消融过程可能存在“盲区”或“遗漏区”,对于此类患者是否可以联合一些其他的治疗手段,如靶向治疗等,进而降低局部复发率,目前尚未有这方面的研究。
综上所述,TACE联合局部消融治疗外科术后复发性肝癌安全可靠,可以成为此类患者的一种治疗方式。本研究还发现,肿瘤边界不完整和肿瘤位置临近危险区域(大血管、空腔脏器等)是影响患者联合治疗术后生存的危险因素。对于合并有此类危险因素的患者,术后应密切随访。本研究不足之处在于此研究为单一中心回顾性研究,纳入患者数量较少,随访时间较短,且分析的危险因素较少,结果可能存在一定偏倚。仍需多中心大样本的研究来进一步验证。
-
[1] XIE L, HAO P, WU RH, et al. Establishment and application of a database for hepatitis C virus NS3/4A protease inhibitors and their drug resistance data[J]. J Clin Hepatol, 2018, 34 (9) :1884-1890. (in Chinese) 谢磊, 郝沛, 吴瑞红, 等. HCV NS3/4A蛋白酶抑制剂药物及其耐药信息数据库的建立和应用[J].临床肝胆杂志, 2018, 34 (9) :1884-1890. [2] MESSINA JP, HUMPHREYS I, FLAXMAN A, et al. Global distribution and prevalence of hepatitis C virus genotypes[J].Hepatology, 2015, 61 (1) :77-87. [3] PAWLOTSKY JM, NEGRO F, AGHEMO A, et al. EASL Recommendations on treatment of hepatitis C 2018[J]. Hepatology, 2018, 69 (2) :461-511. [4] MOHD HANAFIA K, GROEGER J, FLAXMAN AD, et al. Global epidemiology of hepatitis C virus infection:New estimates of age-specific antibody to HCV seroprevalence[J]. Hepatology, 2013, 57 (4) :1333-1342. [5] TANG W, SU MH, JIANG JN, et al. Epidemiological characteristics and genotype distribution of hepatitis C virus in Guangxi[J]. World Chin J Dig, 2014, 22 (9) :1300-1306. (in Chinese) 唐维, 苏明华, 江建宁, 等.广西地区丙型肝炎病毒的基因型分布与流行病学特征[J].世界华人消化杂志, 2014, 22 (9) :1300-1306. [6] MANNS M, MARCELLIN P, POORDAD F, et al. Simeprevir with pegylated interferon alfa 2a or 2b plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1infection (QUEST-2) :A randomised, double-blind, placebo-controlled phase 3 trial[J]. Lancet, 2014, 384 (9941) :414-426. [7] DONALDSON EF, HARRINGTON PR, O'REAR JJ, et al. Clinical evidence and bioinformatics characterization of potential hepatitis C virus resistance pathways for sofosbuvir[J]. Hepatology, 2015, 61 (1) :56-65. [8] QUAN M, XING HC. Effects of hepatitis C virus resistance associated variants on the efficacy of direct-acting antiviral agents[J/CD]. Chin J Liver Dis:Electronic Edit, 2018, 10 (3) :32-36. (in Chinese) 全敏, 邢卉春.丙型肝炎病毒耐药相关变异对直接抗病毒药物疗效的影响[J/CD].中国肝脏病杂志:电子版, 2018, 10 (3) :32-36. [9] Chinese Society of Hepatology and Chinese Society of Infectious Diseases, Chinese Medical Association. The guide line of prevention and treatment for chronic hepatitis C:A 2015 update[J]. J Clin Hepatol, 2015, 31 (12) :1961-1979. (in Chinese) 中华医学会肝病学分会, 中华医学会感染病学分会.丙型肝炎防治指南 (2015年更新版) [J].临床肝胆病杂志, 2015, 31 (12) :1961-1979. [10] AN ZY, DING Y, DOU XG, et al. Selection and evaluation of treatment regimens with direct-acting antiviral agents for patients with chronic hepatitis C in the real world in China[J]. J Clin Hepatol, 2018, 34 (2) :233-237. (in Chinese) 安子英, 丁洋, 窦晓光, 等.我国慢性丙型肝炎患者真实世界中直接抗病毒药物治疗方案的选择与评价[J].临床肝胆杂志, 2018, 34 (2) :233-237. [11] LEMM JA, LIU M, GENTLES RG, et al. Preclinical characterization of BMS-791325, an allosteric inhibitor of hepatitis C Virus NS5B polymerase[J]. Antimicrob Agents Chemother, 2014, 58 (6) :3485-3495. [12] DVORY-SOBOL H, VOITENLEITNER C, MABERY E, et al.Clinical and in vitro resistance to GS-9669, a thumb site II nonnucleoside inhibitor of the hepatitis C virus NS5B polymerase[J]. Antimicrob Agents Chemother, 2014, 58 (11) :6599-6606. [13] SUN D, DAI M, SHEN S, et al. Analysis of naturally occurring resistance-associated variants to NS3/4A Protein inhibitors, NS5A protein inhibitors, and NS5B polymerase inhibitors in patients with chronic hepatitis C[J]. Gene Expression, 2018, 18 (1) :63-69. [14] CHARLTON M, GANE E, MANNS MP, et al. Sofosbuvir and ribavirin for treatment of compensated recurrent hepatitis C virus infection after liver transplantation[J]. Gastroenterology, 2015, 148 (1) :108-117. [15] ALVES R, QUEIROZ ATL, PESSOA MG, et al. The presence of resistance mutations to protease and polymerase inhibitors in hepatitis C virus sequences from the Los Alamosdatabank[J]. J Viral Hepat, 2013, 20 (6) :414-421. [16] WANG Y, RAO HY, XIE XW, et al. Direct-acting antiviral agents resistance-associated polymorphisms in chinese treatment-nave patients infected with genotype 1b hepatitis C virus[J]. Chin Med J (Engl) , 2015, 128 (19) :2625-2631. [17] JI H, KOZAK RA, BIONDI MJ, et al. Next generation sequencing of the hepatitis C virus NS5B gene reveals potential novel S282drug resistance mutations[J]. Virology, 2015, 477:1-9. [18] BARTELS DJ, SULLIVAN JC, ZHANG EZ, et al. Hepatitis C virus variants with decreased sensitivity to direct-acting antivirals (DAAs) were rarely observed in DAA-naive patients prior to treatment[J]. J Virol, 2013, 87 (3) :1544-1553. [19] HALFON P, SARRAZIN C. Future treatment of chronic hepatitis C with direct acting antivirals:Is resistance important?[J].Liver Int, 2012, 32 (Suppl 1) :79-87. [20] VOITENLEITNER C, CROSBY R, WALKER J, et al. In vitro characterization of GSK2485852, a novel hepatitis C virus polymerase inhibitor[J]. Antimicrob Agents Chemother, 2013, 57 (11) :5216-5224. 期刊类型引用(4)
1. 卢冰. 再次肝切除术和射频消融术在治疗肝切除术后早期复发性小肝癌中的疗效比较. 医药论坛杂志. 2024(06): 620-623+628 .  百度学术
百度学术2. 尚敏杰,张军港,顾宗庭,魏芳强,陶然,吴国清,文阳,沈坚,唐雨琪. 免疫联合靶向治疗预防肝细胞癌行ALPPS术后复发的临床疗效. 中华消化外科杂志. 2023(02): 281-285 .  百度学术
百度学术3. 姚爱武,廖和壁,张璟. 血清AFP、AFP-L3与肝细胞癌经肝动脉化疗栓塞术后疗效的关系分析. 分子诊断与治疗杂志. 2023(04): 690-693+698 .  百度学术
百度学术4. 蔡泽丰,祖庆泉,周春高,施海彬,王杰. TACE联合MWA治疗肝切除术后复发性肝癌的疗效及预后分析. 现代生物医学进展. 2023(23): 4578-4583 .  百度学术
百度学术其他类型引用(1)
-




 PDF下载 ( 2088 KB)
PDF下载 ( 2088 KB)


 下载:
下载:
 百度学术
百度学术
 下载:
下载: