半肝阻断法和全肝阻断法在原发性肝癌肝切除术中应用效果比较的Meta分析
DOI: 10.3969/j.issn.1001-5256.2021.01.015
Effect of hemihepatic vascular exclusion versus total hepatic vascular exclusion in hepatectomy for primary liver cancer: A Meta-analysis
-
摘要:
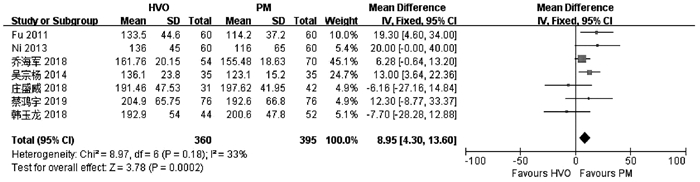
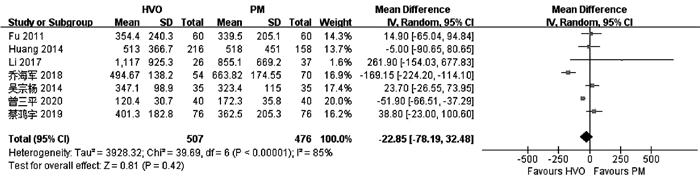
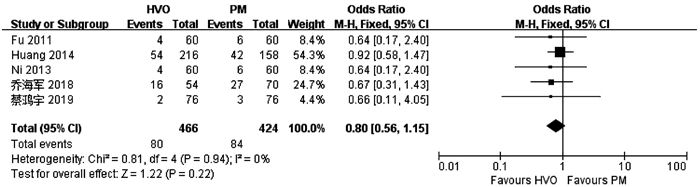
目的 比较普林格尔法(Pringle’s measure, PM)和半肝血管流入阻断法(HVO)在原发性肝癌肝切除术中的应用效果。 方法 计算机检索中、英文数据库中关于HVO和PM治疗原发性肝癌的对照研究,查找时间为数据库建立至2020年6月。对纳入的研究进行质量评价和数据提取后,采用RevMan5.3软件进行Meta分析。 结果 共纳入10项研究,合计1272例患者。HVO组术后第1、3天ALT水平低于PM组[均数差(MD)=-172.71,95%CI:-289.26~-56.16,P=0.004;MD=-130.35,95%CI:-221.25~-39.45,P=0.005],术后第3天AST水平低于PM组(MD=-84.56,95%CI:-166.47~-2.65,P=0.04),术后第1、3天Alb水平高于PM组(MD=1.31,95%CI:0.06~2.56,P=0.04;MD=1.81,95%CI:0.27~3.35,P=0.02),手术时间较PM组长(MD=8.95,95%CI:4.30~13.60;P<0.01)。 结论 HVO是一种安全有效的血流阻断方法,与PM相比能有效减轻肝损伤。但外科医生还应根据个人经验、患者病情以及术中具体情况选择适合患者的阻断方法。 -
关键词:
- 肝肿瘤 /
- 阻断治疗 /
- 肝切除术 /
- Meta分析(主题)
Abstract:Objective To systematically evaluate the effect of Pringle's measure (PM) versus hemihepatic vascular occlusion (HVO) in hepatectomy for primary liver cancer. Methods Related Chinese and English databases were searched for control studies on HVI versus PM in the treatment of primary liver cancer published up to June 2020. After quality evaluation and data extraction of the included studies, RevMan5.3 software was used for the meta-analysis. Results A total of 10 studies were included in the Meta-analysis, with 1272 patients in total. On days 1 and 3 after surgery, the HVO group had a significantly lower level of alanine aminotransferase than the PM group (day 1: mean difference [MD]=-172.71, 95% confidence interval [CI]: -289.26 to -56.16, P=0.004; day 3: MD=-130.35, 95%CI: -221.25 to -39.45, P=0.005). On day 3 after surgery, the HVO group had a significantly lower level of aspartate aminotransferase than the PM group (MD=-84.56, 95%CI: -166.47 to -2.65, P=0.04), and on days 1 and 3 after surgery, the HVO group had a significantly higher level of albumin than the PM group (day 1: MD=1.31, 95%CI: 0.06-2.56, P=0.04; day 3: MD=1.81, 95%CI: 0.27-3.35, P=0.02). The HVO group had a significantly longer time of operation than the PM group (MD=8.95, 95%CI: 4.30-13.60, P < 0.01). Conclusion HVO is a safe and effective method for vascular occlusion, and compared with PM, it can effectively alleviate liver injury. However, surgeons should select a suitable method for occlusion based on their own personal experience, patients' conditions, and specific situation during surgery. -
Key words:
- Liver Neoplasms /
- Therapeutic Occlusion /
- Hepatectomy /
- Meta-Analysis as Topic
-
肝细胞癌(HCC)是常见的消化系统恶性肿瘤之一, 其恶性程度高, 增殖能力强, 患者预后差。2018年, 全球新发肝癌84.1万例, 在恶性肿瘤发病率排名第6位, 死亡78.1万例[1]; 2015年我国的新发肝癌约37.0万例, 死亡人数约32.6万例[2]。因此, 寻找肝癌预后评估的生物标志物具有重要的临床意义。
葡萄糖-6-磷酸脱氢酶(glucose-6-phosphate dehydrogenase, G6PD是参与磷酸戊糖途径(pentose phosphate pathway, PPP)的第一种酶, 也是PPP的限速酶, 糖酵解产生的6-磷酸葡萄糖在G6PD的催化下进入PPP, 产生细胞活动所需要的能量[3]。研究发现, G6PD在黑色素瘤[4]、乳腺癌[5]、肺癌[6-7]、肝癌[8-9]、结直肠癌[10]等恶性肿瘤中表达升高, 在肿瘤的发生发展中起重要作用。
淋巴细胞/单核细胞比值(LMR)是反映机体炎症和免疫状态的一项敏感指标, 已被证实可用于预测卵巢癌[11]、肺癌[12]和肝癌[13]等实体肿瘤的预后。本文通过分析G6PD在肝癌组织中的表达与预后的关系, 以及不同G6PD表达水平患者的临床指标差异, 探讨G6PD表达在HCC预后评估中的临床价值。
1. 资料与方法
1.1 研究对象
收集2016年6月—2018年1月在本院就诊的44例首诊HCC患者的肝癌组织和相应的癌旁组织标本, 及其临床信息和实验室检查结果。纳入标准: (1)所有患者均符合《原发性肝癌诊疗规范(2019年版)》[14]; (2)有完整的基本资料和实验室检测结果(肝肾功能、血常规、凝血指标和肿瘤标志物)。排除标准: (1)合并其他恶性肿瘤或有全身感染症状; (2)术前接受过其他方式治疗。
1.2 仪器试剂
采用贝克曼奥林巴斯5800全自动生化分析仪检测肝肾功能, 贝克曼库尔特全自动血细胞分析仪H750检测血常规。贝克曼ACLTOP检测凝血指标。BeckmanDX1800全自动化学发光仪测定AFP。BIO-RAD荧光定量PCR系统CFX ConnectTM检测基因表达。
1.3 方法
使用Trizol(美国Thermo公司)提取组织RNA。逆转录试剂盒FSQ-301(日本TOYOBO公司)合成cDNA。β-actin为内参基因, 进行qPCR。G6PD: 上游5′-AACATCGCCTGCGTTATCCTC-3′, 下游5′-ACGTCCCGGATGATCCCAA-3′; β-actin: 上游5′-CCTCGCCTTTGCCGATCC-3′, 下游5′-GGATCTTCATGAGGTAGTC AGTC-3′。
用Gene Expression Profiling Interactive Analysis(GEPIA)数据库分析G6PD在HCC组织中的表达。Kaplan Meier Plotter(KM plotter)数据库评估HCC组织中G6PD表达水平与预后。
1.4 伦理学审查
本研究方案经由武汉大学中南医院伦理委员会审批, 批号: 2017058。
1.5 统计学方法
采用SPSS 21.0软件进行数据分析。正态分布的计量资料以x±s表示, 2组间比较采用t检验; 偏态分布的计量资料以M(P25~P75)表示, 2组间比较采用Mann-Whitney U检验。计数资料2组间比较采用Fisher确切概率法; Spearman相关用来分析G6PD与LMR的相关性; 采用Kaplan-Meier法绘制生存曲线。P<0.05为差异有统计学意义。
2. 结果
2.1 肝癌组织中G6PD mRNA表达
运用RT-qPCR检测44例HCC患者肝癌组织和癌旁肝脏组织中G6PD的mRNA表达水平。结果发现, G6PD mRNA在癌组织中表达明显高于癌旁组织(Z=-3.221, P=0.001), 癌组织G6PD表达水平是癌旁组织的2.09倍(图 1a)。
2.2 低G6PD组和高G6PD组的HCC患者基本资料
根据G6PD mRNA表达水平中位数, 将患者分为G6PD高表达组(n=22)及低表达组(n=22)。两组患者在年龄、性别、HBV DNA检测阳性率和TNM分期上差异均无统计学意义(P值均>0.05)(表 1)。
表 1 HCC患者低G6PD组和高G6PD组的基本资料指标 低G6PD组(n=22) 高G6PD组(n=22) P值 年龄(岁) 57.04±9.88 52.00±8.74 0.080 男/女(例) 17/5 17/5 1.000 HBV DNA(例) 0.537 <500 IU/ml 7 10 ≥500 IU/ml 15 12 TNM分期(例) 0.736 Ⅰ~Ⅱ 15 17 Ⅲ~Ⅳ 7 5 2.3 肝癌患者预后与G6PD表达的关系分析
运用GEPIA数据库分析肝癌组织(n=369)和癌旁组织(n=160)中的G6PD表达。结果发现, G6PD在癌组织中表达明显高于癌旁组织(P<0.01)(图 1b)。运用KM plotter数据库, 分析两组患者预后差异。结果发现, G6PD高表达是不良预后的危险因素: 总生存期(HR=1.84, 95%CI: 1.30~2.61; P=0.000 52)和无进展生存期(HR=1.75, 95%CI: 1.27~2.42;P=0.000 54), 即G6PD高表达患者预后不良(图 1c、d)。
2.4 肝癌组织中G6PD表达与临床指标的关系
为了进一步比较肝癌患者中G6PD mRNA表达与临床指标的关系, 比较HCC患者低G6PD组(n=22)和高G6PD组(n=22)的肝功能、血常规、凝血功能和AFP差异。
结果显示, LMR在高G6PD组患者中明显低于低G6PD组(P=0.011), 其他指标两组间差异均无统计学意义(P值均>0.05)(表 2)。
表 2 肝癌患者G6PD表达水平与术前临床指标关系指标 低G6PD组(n=22) 高G6PD组(n=22) 统计值 P值 ALT(U/L) 41.09±28.89 43.64±39.56 t=-0.244 0.809 AST(U/L) 49.00±33.74 47.23±30.33 t=0.183 0.855 总胆红素(μmol/L) 16.60±6.83 20.44±8.93 t=-1.604 0.116 直接胆红素(μmol/L) 4.49±2.82 4.94±2.20 t=-0.579 0.566 间接胆红素(μmol/L) 12.35±4.96 15.05±7.87 t=-1.335 0.187 TP(g/L) 66.80±5.96 67.58±6.27 t=-0.422 0.675 Alb(g/L) 39.66±3.75 39.65±5.63 t=0.006 0.995 Glb(g/L) 27.14±4.17 27.92±4.82 t=-0.575 0.568 GGT(U/L) 61.00±46.68 66.05±43.69 t=-0.366 0.716 ALP(U/L) 103.82±52.41 108.05±56.92 t=-0.256 0.799 WBC(×109/L) 5.35±1.66 5.61±2.34 t=-0.413 0.682 RBC(×1012/L) 4.24±0.85 4.41±0.70 t=-0.691 0.494 HGB(g/L) 129.59±25.11 135.34±18.16 t=-0.844 0.404 PLT(×109/L) 150.10±54.03 173.19±82.75 t=-1.052 0.229 中性粒细胞计数(×109/L) 3.41±1.64 3.80±1.80 t=-0.717 0.478 淋巴细胞计数(×109/L) 1.39±0.55 1.19±0.59 t=1.116 0.271 单核细胞计数(×109/L) 0.42±0.14 0.50±0.21 t=-1.531 0.134 LMR 3.49±1.44 2.45±0.96 t=2.681 0.011 PT(s) 11.59±1.06 11.70±0.88 t=-0.348 0.730 INR 1.06±0.10 1.07±0.08 t=-0.295 0.770 PTTA(%) 97.81±14.29 97.15±15.26 t=0.143 0.887 APTT(s) 32.20±2.78 32.63±3.65 t=-0.433 0.668 TT(s) 14.97±1.22 14.30±1.77 t=1.438 0.158 FIB(mg/dl) 286.61±56.74 308.19±81.70 t=-0.994 0.326 AFP(μg/L) 143.80(6.22~2 386.96) 1 481.35(56.20~4 453.63) Z=-1.723 0.085 2.5 G6PD表达与LMR相关性分析
进一步分析G6PD mRNA表达量与LMR相关性, 结果显示G6PD表达水平与LMR呈负相关(r=-0.439, P=0.005)(图 2), 提示G6PD表达与免疫功能、炎症状态有关。
3. 讨论
HCC是一种进展迅速, 恶性程度高, 预后极差的肿瘤[15]。随着各种分子生物学层面的研究深入, 越来越多的研究表明肝癌的发生发展常伴随着糖代谢紊乱[16]、多种基因表达改变和信号通路异常[17]。
肿瘤细胞由于瓦伯格效应(Warburg effect)主要通过糖酵解的方式分解葡萄糖获能, 所产生的6-磷酸葡萄糖可以通过PPP为核苷酸、脂质等生物分子合成提供前体以及NADPH[16, 18]。本研究发现G6PD基因在HCC中表达明显高于配对癌旁组织。相关研究指出, PI3K/Akt、Ras和Src等促癌信号通路的过度激活, 可通过翻译后调控机制促进G6PD激活[3], 从而引起肿瘤组织中G6PD表达升高, 产生的高水平NADPH可以减少细胞活性氧(ROS)的生成[19]。ROS在多种癌症中被检测到, 一方面被认为可以激活肿瘤信号, 但同时ROS累积也能启动氧化应激诱导肿瘤细胞的死亡, 肿瘤细胞G6PD表达升高, 可以减少升高的ROS, 建立氧化还原平衡[20]。同时细胞内ROS累积会引起NF-κB的激活, 可引起大量细胞因子如IL-6、IL-24、IL-32等的释放, 细胞因子招募单核细胞、巨噬细胞到肿瘤部位, 引起肿瘤微环境炎症反应, 促进肿瘤发展[21]。
已经有研究证实HBV感染会引起G6PD的升高[22], 同时HBV感染可能会引起肿瘤微环境炎症反应, 引起免疫细胞数量和状态改变[23]。而高LMR是多种癌症预后的保护因素[9, 24], 在肝癌的相关研究[25]中, 肝癌术前LMR可分为高值组(≥3.03)和低值组(<3.03), 高LMR组患者肝癌切除术后5年生存率较好。也有学者[26]指出肝移植术后LMR<2.75的患者的5年生存率明显低于LMR≥2.75的患者。而本研究发现LMR在G6PD高表达组中明显低于G6PD低表达组, 且肝癌组织中G6PD表达水平与LMR呈负相关, 提示G6PD高表达的HCC患者不良预后可能与肿瘤微环境炎症有关。
综上, 在肝癌组织中高G6PD的表达与不良预后有关, G6PD高表达和低表达组的LMR有明显差异, 且G6PD的表达量与LMR呈负相关, 提示G6PD表达可能与肿瘤微环境炎症反应有关。本研究所用样本量较小, 具体的分子机制尚需进一步研究。
-
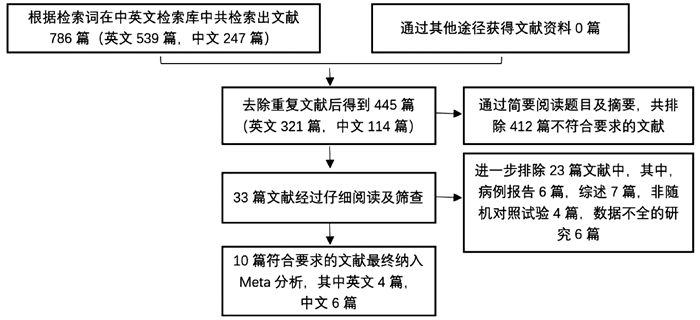
表 1 纳入文献基本特征及质量评价
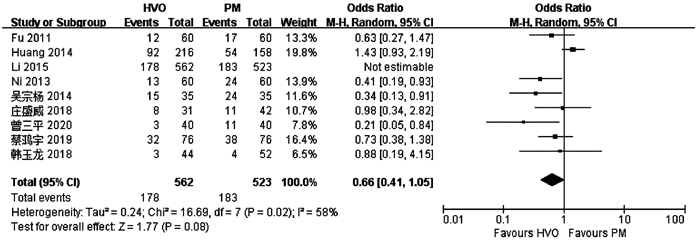
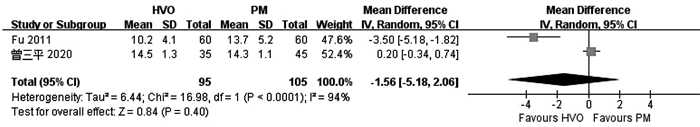
作者 年份 国家 研究类型 手术分组 样本量(例) 年龄(岁) 男/女(例) 肿瘤大小(cm) 质量评分(NOS/Jadad量表) Huang等[13] 2014 中国 RCS HVO 126 49.3±12.8 97/29 - 8 PM 158 48.5±12.0 129/29 - Li等[14] 2017 中国 RCS HVO 26 53(34~72)1) 20/6 - 8 PM 37 47.5(25~70)1) 33/4 - Ni等[15] 2013 中国 RCT HVO 60 56.1±9.3 47/13 4.7±2.2 3 PM 60 55.2±8.7 45/15 5.4±3.1 Fu等[16] 2011 中国 RCT HVO 60 49.3±9.2 41/19 6±2.8 3 PM 60 48.6±8.7 46/14 6.8±2.8 蔡鸿宇等[17] 2019 中国 RCT HVO 76 54.11±7.75 59/17 5.03±2.86 3 PM 76 53.24±7.83 56/20 5.77±3.74 曾三平[18] 2020 中国 RCT HVO 40 48.6±3.1 22/18 - 3 PM 40 48.8±3.0 23/17 - 韩玉龙等[19] 2018 中国 RCS HVO 44 - - 10.1±3.1 7 PM 52 - - 9.6±3.0 乔海军等[20] 2018 中国 RCS HVO 54 57.35±9.06 35/19 6.47±1.42 8 PM 70 56.94±8.72 46/24 6.51±1.38 吴宗杨等[21] 2014 中国 RCT HVO 35 - - - 3 PM 35 - - - 庄盛威等[22] 2018 中国 RCS HVO 31 49.62±4.91 24/7 9.07±0.99 8 PM 42 49.09±4.51 32/10 9.31±0.75 注:1)M(P最小值~P最大值);“-”表示未提及。 -
[1] BRUIX J, REIG M, SHERMAN M. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma[J]. Gastroenterology, 2016, 150(4): 835-853. DOI: 10.1053/j.gastro.2015.12.041 [2] TAN YH, SHANG YY, ZHANG T, et al. Current status and perspectives of multimodality therapy for hepatocellular carcinoma[J]. J Clin Hepatol, 2019, 35(8): 1858-1860. (in Chinese) DOI: 10.3969/j.issn.1001-5256.2019.08.047谭运华, 商阳阳, 张涛, 等. 肝细胞癌综合治疗的现状和前景[J]. 临床肝胆病杂志, 2019, 35(8): 1858-1860. DOI: 10.3969/j.issn.1001-5256.2019.08.047 [3] KOOBY DA, STOCKMAN J, BEN-PORAT L, et al. Influence of transfusions on perioperative and long-term outcome in patients following hepatic resection for colorectal metastases[J]. Ann Surg, 2003, 237(6): 860-869; discussion 869-870. [4] TORZILLI G, MAKUUCHI M, INOUE K, et al. No-mortality liver resection for hepatocellular carcinoma in cirrhotic and noncirrhotic patients: Is there a way? A prospective analysis of our approach[J]. Arch Surg, 1999, 134(9): 984-992. DOI: 10.1001/archsurg.134.9.984 [5] PRINGLE JH. V. Notes on the arrest of hepatic hemorrhage due to trauma[J]. Ann Surg, 1908, 48(4): 541-549. DOI: 10.1097/00000658-190810000-00005 [6] TORZILLI G, PROCOPIO F, DONADON M, et al. Safety of intermittent Pringle maneuver cumulative time exceeding 120 minutes in liver resection: A further step in favor of the "radical but conservative" policy[J]. Ann Surg, 2012, 255(2): 270-280. DOI: 10.1097/SLA.0b013e318232b375 [7] GLANEMANN M, VOLLMAR B, NUSSLER AK, et al. Ischemic preconditioning protects from hepatic ischemia/reperfusion-injury by preservation of microcirculation and mitochondrial redox-state[J]. J Hepatol, 2003, 38(1): 59-66. DOI: 10.1016/S0168-8278(02)00327-6 [8] KUPIEC-WEGLINSKI JW, BUSUTTIL RW. Ischemia and reperfusion injury in liver transplantation[J]. Transpl Proc, 2005, 37(4): 1653-1656. DOI: 10.1016/j.transproceed.2005.03.134 [9] MAN K, FAN ST, NG IO, et al. Tolerance of the liver to intermittent pringle maneuver in hepatectomy for liver tumors[J]. Arch Surg, 1999, 134(5): 533-539. DOI: 10.1001/archsurg.134.5.533 [10] WU CC, HWANG CR, LIU TJ, et al. Effects and limitations of prolonged intermittent ischaemia for hepatic resection of the cirrhotic liver[J]. Br J Surg, 1996, 83(1): 121-124. DOI: 10.1002/bjs.1800830139 [11] BISMUTH H. Surgical anatomy and anatomical surgery of the liver[J]. World J Surg, 1982, 6(1): 3-9. DOI: 10.1007/BF01656368 [12] CLAUDIO L, BRENDON S, MARCO S, et al. Assessing the quality of studies in meta-analyses: Advantage and limitations of the Newcastie Ottawa Scale[J]. WJMA, 2017, 5(4): 80. DOI: 10.13105/wjma.v5.i4.80 [13] HUANG Z, ZHANG P, WANG H, et al. Comparing outcomes of two vascular inflow occlusion techniques and treatment without vascular occlusion during major hepatectomy in patients with hepatitis B-related hepatocellular carcinoma[J]. PLoS One, 2014, 9(9): e107303. DOI: 10.1371/journal.pone.0107303 [14] LI M, ZHANG T, WANG L, et al. Selective hemihepatic vascular occlusion versus pringle maneuver in hepatectomy for primary liver cancer[J]. Med Sci Monit, 2017, 23: 2203-2210. DOI: 10.12659/MSM.900859 [15] NI JS, LAU WY, YANG Y, et al. A prospective randomized controlled trial to compare pringle manoeuvre with hemi-hepatic vascular inflow occlusion in liver resection for hepatocellular carcinoma with cirrhosis[J]. J Gastrointest Surg, 2013, 17(8): 1414-1421. DOI: 10.1007/s11605-013-2236-z [16] FU SY, LAU WY, LI GG, et al. A prospective randomized controlled trial to compare Pringle maneuver, hemihepatic vascular inflow occlusion, and main portal vein inflow occlusion in partial hepatectomy[J]. Am J Surg, 2011, 201(1): 62-69. DOI: 10.1016/j.amjsurg.2009.09.029 [17] CAI HY, SHAO BF, ZHOU Y. Comparative observation of selective hemihepatic blood flow occlusion and Pringle blood flow occlusion in the surgical treatment of HBV-related liver cancer[J]. Shandong Med J, 2019, 59(6): 53-55. (in Chinese) https://www.cnki.com.cn/Article/CJFDTOTAL-SDYY201906014.htm蔡鸿宇, 邵冰峰, 周元, 等. HBV相关性肝癌手术治疗中选择性半肝血流阻断与Pringle法血流阻断的应用对比观察[J]. 山东医药, 2019, 59(6): 53-55. https://www.cnki.com.cn/Article/CJFDTOTAL-SDYY201906014.htm [18] ZENG SP. Application effect comparison the first hepatic portal blood flow occlusion method and hemi-hepatic blood flow occlusion method in the treatment of primary hepatocellular carcinoma patients with hemi-hepatectomy[J]. Chin Mod Med, 2020, 27(3): 122-128. (in Chinese) DOI: 10.3969/j.issn.1674-4721.2020.03.036曾三平. 第一肝门血流阻断法与半肝血流阻断法在行半肝切除术治疗原发性肝癌患者中的应用效果比较[J]. 中国当代医药, 2020, 27(3): 122-128. DOI: 10.3969/j.issn.1674-4721.2020.03.036 [19] HAN YL, MIAO J, YIN JJ. Comparison of different hepatic inflow occlusion in hepatectomy in treatm ent of patients with primary large liver cancer[J]. J Prac Hepatol, 2018, 21(1): 104-107. (in Chinese) DOI: 10.3969/j.issn.1672-5069.2018.01.025韩玉龙, 苗健, 尹家俊. 不同肝血流阻断方案对手术切除原发性大肝癌患者治疗效果比较[J]. 实用肝脏病杂志, 2018, 21(1): 104-107. DOI: 10.3969/j.issn.1672-5069.2018.01.025 [20] QIAO HJ, SONG YC, ZHAO XY. Application of different blood flow occlusion methods of hepatectomy in the treatment of primary liver cancer[J]. J Hepatobiliary Surg, 2018, 26(4): 277-280. (in Chinese) https://www.cnki.com.cn/Article/CJFDTOTAL-GDWZ201804012.htm乔海军, 宋玉成, 赵小勇. 肝癌切除术中不同血流阻断方式在原发性肝癌治疗中的应用[J]. 肝胆外科杂志, 2018, 26(4): 277-280. https://www.cnki.com.cn/Article/CJFDTOTAL-GDWZ201804012.htm [21] WU ZY, FENG JY, WANG JB, et al. Clinical study of Glisson pedicle transverse hepatectomy in the treatment of liver cancer[J]. J Hepatopancreatobiliary Surg, 2014, 26(4): 342-344. (in Chinese) https://www.cnki.com.cn/Article/CJFDTOTAL-GDYW201404026.htm吴宗杨, 冯济业, 王金波, 等. Glisson蒂横断式肝切除术治疗肝癌的临床研究[J]. 肝胆胰外科杂志, 2014, 26(4): 342-344. https://www.cnki.com.cn/Article/CJFDTOTAL-GDYW201404026.htm [22] ZHUANG SW, LIN YY. Influence of different hepatic blood flow blocking methods on the curative effect of surgical resection of patients with primary massive liver cancer[J]. Chin Mod Doc, 2018, 56(29): 38-40. (in Chinese) https://www.cnki.com.cn/Article/CJFDTOTAL-ZDYS201829011.htm庄盛威, 林燕燕. 不同肝血流阻断方法对外科手术切除原发性大肝癌患者疗效的影响[J]. 中国现代医生, 2018, 56(29): 38-40. https://www.cnki.com.cn/Article/CJFDTOTAL-ZDYS201829011.htm [23] ZHANG NP, ZHANG XJ. Evaluation of different hepatic blood flow blocking methods in laparoscopic hepatectomy of patients with liver cancer[J/CD]. Chin J Liver Dis (Electronic Version), 2019, 11(3): 58-63. (in Chinese)张能平, 张雄杰. 不同肝血流阻断方式在肝癌患者腹腔镜肝切除术中的应用[J/CD]. 中国肝脏病杂志(电子版), 2019, 11(3): 58-63. [24] ZHU P, LAU WY, CHEN YF, et al. Randomized clinical trial comparing infrahepatic inferior vena cava clamping with low central venous pressure in complex liver resections involving the Pringle manoeuvre[J]. Br J Surg, 2012, 99(6): 781-788. DOI: 10.1002/bjs.8714 [25] SELZNER N, RUDIGER H, GRAF R, et al. Protective strategies against ischemic injury of the liver[J]. Gastroenterology, 2003, 125(3): 917-936. DOI: 10.1016/S0016-5085(03)01048-5 [26] GRACE PA. Ischaemia-reperfusion injury[J]. Br J Surg, 1994, 81(5): 637-647. DOI: 10.1002/bjs.1800810504 [27] XIAOBIN F, ZIPEI L, SHUGUO Z, et al. The Pringle manoeuvre should be avoided in hepatectomy for cancer patients due to its side effects on tumor recurrence and worse prognosis[J]. Med Hypotheses, 2009, 72(4): 398-401. [28] BELGHITI J, NOUN R, MALAFOSSE R, et al. Continuous versus intermittent portal triad clamping for liver resection: A controlled study[J]. Ann Surg, 1999, 229(3): 369-375. [29] GUO T, XIAO Y, LIU Z, et al. The impact of intraoperative vascular occlusion during liver surgery on postoperative peak ALT levels: A systematic review and meta-analysis[J]. Int J Surg, 2016, 27: 99-104. [30] WANG HQ, YANG JY, YAN LN. Hemihepatic versus total hepatic inflow occlusion during hepatectomy: A systematic review and meta-analysis[J]. World J Gastroenterol, 2011, 17(26): 3158-3164. [31] DING HY, TAI QW, ZHOU CM, et al. Hemihepatic inflow occlusion versus total hepatic inflow occlusion in liver resection: A meta-analysis[J]. Chin J Evid-based Med, 2014, 14(4): 469-477. (in Chinese) https://www.cnki.com.cn/Article/CJFDTOTAL-ZZXZ201404019.htm丁贺义, 邰沁文, 周成明, 等. 肝切除术应用半肝与全肝入肝血流阻断法效果的Meta分析[J]. 中国循证医学杂志, 2014, 14(4): 469-477 https://www.cnki.com.cn/Article/CJFDTOTAL-ZZXZ201404019.htm [32] RAHBARI NN, KOCH M, MEHRABI A, et al. Portal triad clamping versus vascular exclusion for vascular control during hepatic resection: A systematic review and meta-analysis[J]. J Gastrointest Surg, 2009, 13(3): 558-568. 期刊类型引用(1)
1. 姚元谦,吕建林,柳琳琳,王光耀. 铁死亡基因及相关长链非编码RNA在肝细胞癌发生与预后中的作用. 中国医药导报. 2022(26): 9-14 .  百度学术
百度学术其他类型引用(0)
-




 PDF下载 ( 3827 KB)
PDF下载 ( 3827 KB)


 下载:
下载:

 下载:
下载:




 百度学术
百度学术



