Should patients in the immune tolerance stage of chronic hepatitis B virus infection be treated?
-
摘要: 国际主要指南对慢性HBV感染免疫耐受期的定义不完全一致,但几乎所有指南均不推荐对免疫耐受期慢性乙型肝炎患者启动治疗。本文讨论了关于对HBV感染免疫耐受期患者进行抗病毒治疗的最新证据,以预防其肝硬化和肝细胞癌的发生。Abstract: Major international guidelines have not yet reached a consensus on the definition of the immune tolerance stage of chronic hepatitis B virus infection; however, almost all guidelines do not recommend starting treatment for patients in the immune tolerance stage of chronic hepatitis B. This article discusses the latest evidence for the treatment of such patients to prevent liver cirrhosis and hepatocellular carcinoma.
-
Key words:
- Hepatitis B Virus /
- Hepatitis B, Chronic /
- Immune Tolerance /
- Therapeutics
-
图 1 我国1990年—2014年161个疾病监测点城乡男女HCC年龄死亡专率[36]
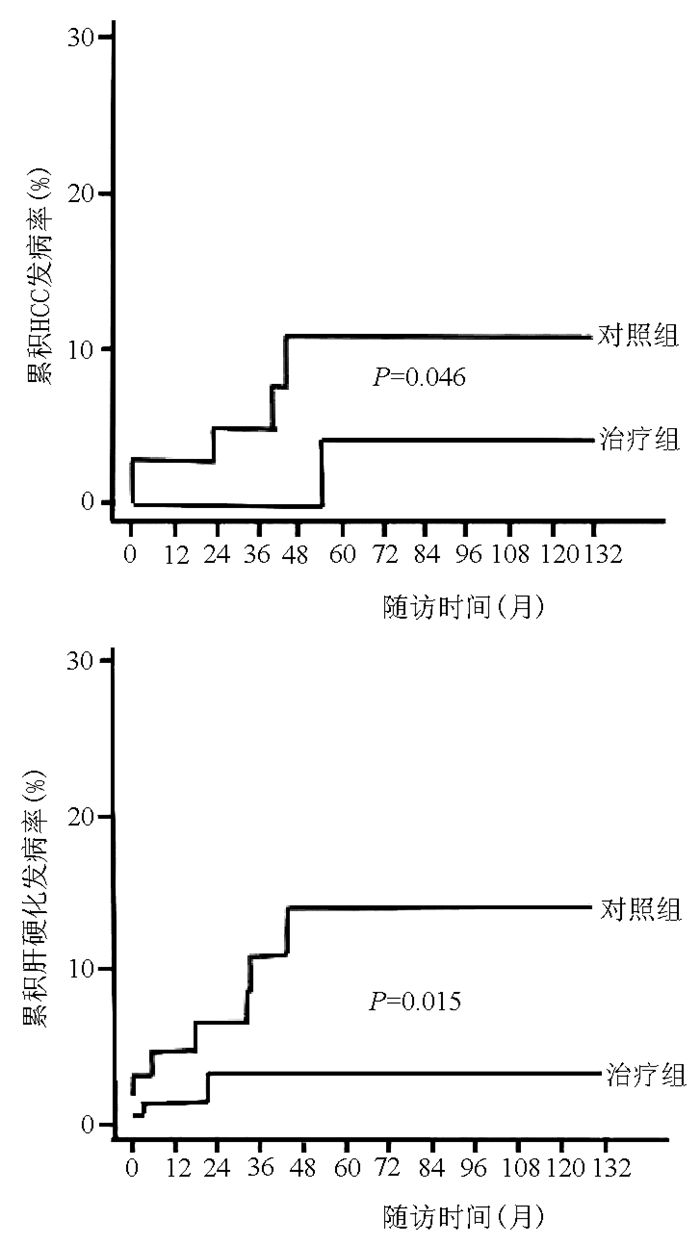
图 2 韩国多中心IT-CHB患者抗病毒治疗回顾性分析HCC及肝硬化累积发生率[48]
表 1 ALT持续正常的IT-CHB患者有明显肝细胞炎症坏死和肝纤维化病理学改变
-
[1] Polaris Observatory Collaborators. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: A modelling study[J]. Lancet Gastroenterol Hepatol, 2018, 3(6): 383-403. DOI: 10.1016/S2468-1253(18)30056-6 [2] TERRAULT NA, LOK A, MCMAHON BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance[J]. Hepatology, 2018, 67(4): 1560-1599. DOI: 10.1002/hep.29800 [3] European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection[J]. J Hepatol, 2017, 67(2): 370-398. DOI: 10.1016/j.jhep.2017.03.021 [4] SARIN SK, KUMAR M, LAU GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: A 2015 update[J]. Hepatol Int, 2016, 10(1): 1-98. DOI: 10.1007/s12072-015-9675-4 [5] JENG WJ, LOK AS. Should Treatment indications for chronic hepatitis B be expanded?[J]. Clin Gastroenterol Hepatol, 2020. [Epub ahead of print] [6] KAO JH, HU TH, JIA J, et al. East Asia expert opinion on treatment initiation for chronic hepatitis B[J]. Aliment Pharmacol Ther, 2020, 52(10): 1540-1550. [7] CORNBERG M, LOK AS, TERRAULT NA, et al. Guidance for design and endpoints of clinical trials in chronic hepatitis B-Report from the 2019 EASL-AASLD HBV Treatment Endpoints Conference[J]. J Hepatol, 2020, 72(3): 539-557. DOI: 10.1016/j.jhep.2019.11.003 [8] CHU CM, HUNG SJ, LIN J, et al. Natural history of hepatitis B e antigen to antibody seroconversion in patients with normal serum aminotransferase levels[J]. Am J Med, 2004, 116(12): 829-834. DOI: 10.1016/j.amjmed.2003.12.040 [9] ANDREANI T, SERFATY L, MOHAND D, et al. Chronic hepatitis B virus carriers in the immunotolerant phase of infection: Histologic findings and outcome[J]. Clin Gastroenterol Hepatol, 2007, 5(5): 636-641. DOI: 10.1016/j.cgh.2007.01.005 [10] LEE HW, KIM SU, BAATARKHUU O, et al. Comparison between chronic hepatitis B patients with untreated immune-tolerant phase vs. those with virological response by antivirals[J]. Sci Rep, 2019, 9(1): 2508. DOI: 10.1038/s41598-019-39043-2 [11] LEE HA, LEE HW, KIM IH, et al. Extremely low risk of hepatocellular carcinoma development in patients with chronic hepatitis B in immune-tolerant phase[J]. Aliment Pharmacol Ther, 2020, 52(1): 196-204. DOI: 10.1111/apt.15741 [12] LEE HW, KIM EH, LEE J, et al. Correction to: Natural history of untreated HBeAg-positive chronic HBV infection with persistently elevated HBV DNA but normal alanine aminotransferase[J]. Clin Transl Gastroenterol, 2020, 11(5): e00183. DOI: 10.14309/ctg.0000000000000183 [13] HUI CK, LEUNG N, YUEN ST, et al. Natural history and disease progression in Chinese chronic hepatitis B patients in immune-tolerant phase[J]. Hepatology, 2007, 46(2): 395-401. DOI: 10.1002/hep.21724 [14] MCMAHON BJ. Epidemiology and natural history of hepatitis B[J]. Semin Liver Dis, 2005, 25(Suppl 1): 3-8. [15] WU IC, LAI CL, HAN SH, et al. Efficacy of entecavir in chronic hepatitis B patients with mildly elevated alanine aminotransferase and biopsy-proven histological damage[J]. Hepatology, 2010, 51(4): 1185-1189. DOI: 10.1002/hep.23424 [16] WONG VW, HUI AJ, WONG GL, et al. Four-year outcomes after cessation of tenofovir in immune-tolerant chronic hepatitis B patients[J]. J Clin Gastroenterol, 2018, 52(4): 347-352. DOI: 10.1097/MCG.0000000000000852 [17] ROSENTHAL P, LING SC, BELLE SH, et al. Combination of entecavir/peginterferon alfa-2a in children with hepatitis B e antigen-positive immune tolerant chronic hepatitis B virus infection[J]. Hepatology, 2019, 69(6): 2326-2337. DOI: 10.1002/hep.30312 [18] FELD JJ, TERRAULT NA, LIN HS, et al. Entecavir and peginterferon alfa-2a in adults with hepatitis B e antigen-positive immune-tolerant chronic hepatitis B virus infection[J]. Hepatology, 2019, 69(6): 2338-2348. [19] LEE HW, CHAN HL. Unresolved issues of immune tolerance in chronic hepatitis B[J]. J Gastroenterol, 2020, 55(4): 383-389. DOI: 10.1007/s00535-020-01665-z [20] WU JF, SU YR, CHEN CH, et al. Predictive effect of serial serum alanine aminotransferase levels on spontaneous HBeAg seroconversion in chronic genotype B and C HBV-infected children[J]. J Pediatr Gastroenterol Nutr, 2012, 54(1): 97-100. DOI: 10.1097/MPG.0b013e31822a033e [21] CHEN YC, CHU CM, LIAW YF. Age-specific prognosis following spontaneous hepatitis B e antigen seroconversion in chronic hepatitis B[J]. Hepatology, 2010, 51(2): 435-444. DOI: 10.1002/hep.23348 [22] DOLMAN GE, KOFFAS A, MASON WS, et al. Why, who and when to start treatment for chronic hepatitis B infection[J]. Curr Opin Virol, 2018, 30: 39-47. DOI: 10.1016/j.coviro.2018.03.006 [23] BERTOLETTI A, KENNEDY PT. The immune tolerant phase of chronic HBV infection: New perspectives on an old concept[J]. Cell Mol Immunol, 2015, 12(3): 258-263. DOI: 10.1038/cmi.2014.79 [24] MASON WS, GILL US, LITWIN S, et al. HBV DNA integration and clonal hepatocyte expansion in chronic hepatitis B patients considered immune tolerant[J]. Gastroenterology, 2016, 151(5): 986-998. DOI: 10.1053/j.gastro.2016.07.012 [25] PROTZER U, KNOLLE P. "To Be or Not to Be": Immune tolerance in chronic hepatitis B[J]. Gastroenterology, 2016, 151(5): 805-806. DOI: 10.1053/j.gastro.2016.09.038 [26] MILICH DR. The concept of immune tolerance in chronic hepatitis B virus infection is alive and well[J]. Gastroenterology, 2016, 151(5): 801-804. DOI: 10.1053/j.gastro.2016.09.037 [27] LIAW YF, CHU CM. Immune tolerance phase of chronic hepatitis B[J]. Gastroenterology, 2017, 152(5): 1245-1246. DOI: 10.1053/j.gastro.2016.11.057 [28] KENNEDY PTF, BERTOLETTI A, MASON WS. Reply to immune tolerance phase of chronic hepatitis B[J]. Gastroenterology, 2017, 152(5): 1246-1247. DOI: 10.1053/j.gastro.2017.03.002 [29] WONG GL. Management of chronic hepatitis B patients in immunetolerant phase: What latest guidelines recommend[J]. Clin Mol Hepatol, 2018, 24(2): 108-113. DOI: 10.3350/cmh.2017.0068 [30] KLAIR JS, VANCURA J, MURALI AR. PRO: Patients with chronic hepatitis B in immune-tolerant phase should be treated[J]. Clin Liver Dis (Hoboken), 2020, 15(1): 21-24. DOI: 10.1002/cld.892 [31] HOWELL J, CHAN H, FELD JJ, et al. Closing the stable door after the horse has bolted: Should we be treating people with immune-tolerant chronic hepatitis B to prevent hepatocellular carcinoma?[J]. Gastroenterology, 2020, 158(8): 2028-2032. DOI: 10.1053/j.gastro.2020.02.027 [32] KOFFAS A, PETERSEN J, KENNEDY PT. Reasons to consider early treatment in chronic hepatitis B patients[J]. Antiviral Res, 2020, 177: 104783. DOI: 10.1016/j.antiviral.2020.104783 [33] KIM HL, KIM GA, PARK JA, et al. Cost-effectiveness of antiviral treatment in adult patients with immune-tolerant phase chronic hepatitis B[J]. Gut, 2020. [Epub ahead of print] [34] CHEN CF, LEE WC, YANG HI, et al. Changes in serum levels of HBV DNA and alanine aminotransferase determine risk for hepatocellular carcinoma[J]. Gastroenterology, 2011, 141(4): 1240-1248. DOI: 10.1053/j.gastro.2011.06.036 [35] BEASLEY RP, HWANG LY, LIN CC, et al. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22 707 men in Taiwan[J]. Lancet, 1981, 2(8256): 1129-1133. [36] SUN Y, WANG Y, LI M, et al. Long-term trends of liver cancer mortality by gender in urban and rural areas in China: An age-period-cohort analysis[J]. BMJ Open, 2018, 8(2): e020490. DOI: 10.1136/bmjopen-2017-020490 [37] KIM GA, LIM YS, HAN S, et al. High risk of hepatocellular carcinoma and death in patients with immune-tolerant-phase chronic hepatitis B[J]. Gut, 2018, 67(5): 945-952. DOI: 10.1136/gutjnl-2017-314904 [38] WANG C, DEUBNER H, SHUHART M, et al. High prevalence of significant fibrosis in patients with immunotolerance to chronic hepatitis B infection[J]. Hepatology, 2005, 42(Suppl 1): a573. [39] LAI M, HYATT BJ, NASSER I, et al. The clinical significance of persistently normal ALT in chronic hepatitis B infection[J]. J Hepatol, 2007, 47(6): 760-767. DOI: 10.1016/j.jhep.2007.07.022 [40] PARK JY, PARK YN, KIM DY, et al. High prevalence of significant histology in asymptomatic chronic hepatitis B patients with genotype C and high serum HBV DNA levels[J]. J Viral Hepat, 2008, 15(8): 615-621. DOI: 10.1111/j.1365-2893.2008.00989.x [41] KUMAR M, SARIN SK, HISSAR S, et al. Virologic and histologic features of chronic hepatitis B virus-infected asymptomatic patients with persistently normal ALT[J]. Gastroenterology, 2008, 134(5): 1376-1384. DOI: 10.1053/j.gastro.2008.02.075 [42] NGUYEN MH, GARCIA RT, TRINH HN, et al. Histological disease in Asian-Americans with chronic hepatitis B, high hepatitis B virus DNA, and normal alanine aminotransferase levels[J]. Am J Gastroenterol, 2009, 104(9): 2206-2213. DOI: 10.1038/ajg.2009.248 [43] SETO WK, LAI CL, IP PP, et al. A large population histology study showing the lack of association between ALT elevation and significant fibrosis in chronic hepatitis B[J]. PLoS One, 2012, 7(2): e32622. DOI: 10.1371/journal.pone.0032622 [44] LIAO B, WANG Z, LIN S, et al. Significant fibrosis is not rare in Chinese chronic hepatitis B patients with persistent normal ALT[J]. PLoS One, 2013, 8(10): e78672. DOI: 10.1371/journal.pone.0078672 [45] CHAN HL, CHAN CK, HUI AJ, et al. Effects of tenofovir disoproxil fumarate in hepatitis B e antigen-positive patients with normal levels of alanine aminotransferase and high levels of hepatitis B virus DNA[J]. Gastroenterology, 2014, 146(5): 1240-1248. DOI: 10.1053/j.gastro.2014.01.044 [46] PAN CQ, DUAN Z, DAI E, et al. Tenofovir to prevent hepatitis B transmission in mothers with high viral load[J]. N Engl J Med, 2016, 374(24): 2324-2334. DOI: 10.1056/NEJMoa1508660 [47] JOURDAIN G, NGO-GIANG-HUONG N, HARRISON L, et al. Tenofovir versus placebo to prevent perinatal transmission of hepatitis B[J]. N Engl J Med, 2018, 378(10): 911-923. DOI: 10.1056/NEJMoa1708131 [48] CHANG Y, CHOE WH, SINN DH, et al. Nucleos(t)ide analogue treatment for patients with hepatitis B virus (HBV) e antigen-positive chronic HBV genotype C infection: A nationwide, multicenter, retrospective study[J]. J Infect Dis, 2017, 216(11): 1407-1414. DOI: 10.1093/infdis/jix506 [49] CHEN CJ, YANG HI, SU J, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level[J]. JAMA, 2006, 295(1): 65-73. DOI: 10.1001/jama.295.1.65 [50] Chinese Society of Infectious Diseases, Chinese Medical Association; Chinese Society of Hepatology, Chinese Medical Association. Guidelines for the prevention and treatment of chronic hepatitis B (version 2019)[J]. J Clin Hepatol, 2019, 35(12): 2648-2669. (in Chinese) DOI: 10.3969/j.issn.1001-5256.2019.12.007中华医学会感染病学分会, 中华医学会肝病学分会. 慢性乙型肝炎防治指南(2019年版)[J]. 临床肝胆病杂志, 2019, 35(12): 2648-2669. DOI: 10.3969/j.issn.1001-5256.2019.12.007 [51] LAMPERTICO P, CHAN HL, JANSSEN HL, et al. Review article: Long-term safety of nucleoside and nucleotide analogues in HBV-monoinfected patients[J]. Aliment Pharmacol Ther, 2016, 44(1): 16-34. DOI: 10.1111/apt.13659 [52] de VRIES-SLUIJS TE, REIJNDERS JG, HANSEN BE, et al. Long-term therapy with tenofovir is effective for patients co-infected with human immunodeficiency virus and hepatitis B virus[J]. Gastroenterology, 2010, 139(6): 1934-1941. DOI: 10.1053/j.gastro.2010.08.045 [53] TENNEY DJ, ROSE RE, BALDICK CJ, et al. Long-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naïve patients is rare through 5 years of therapy[J]. Hepatology, 2009, 49(5): 1503-1514. DOI: 10.1002/hep.22841 [54] KITRINOS KM, CORSA A, LIU Y, et al. No detectable resistance to tenofovir disoproxil fumarate after 6 years of therapy in patients with chronic hepatitis B[J]. Hepatology, 2014, 59(2): 434-442. DOI: 10.1002/hep.26686 [55] LIU Y, CORSA AC, BUTI M, et al. No detectable resistance to tenofovir disoproxil fumarate in HBeAg+ and HBeAg- patients with chronic hepatitis B after 8 years of treatment[J]. J Viral Hepat, 2017, 24(1): 68-74. DOI: 10.1111/jvh.12613 [56] LIU Y, MILLER MD, KITRINOS KM. Tenofovir alafenamide demonstrates broad cross-genotype activity against wild-type HBV clinical isolates and maintains susceptibility to drug-resistant HBV isolates inïvitro[J]. Antiviral Res, 2017, 139: 25-31. DOI: 10.1016/j.antiviral.2016.12.012 [57] AGARWAL K, BRUNETTO M, SETO WK, et al. 96 weeks treatment of tenofovir alafenamide vs. tenofovir disoproxil fumarate for hepatitis B virus infection[J]. J Hepatol, 2018, 68(4): 672-681. DOI: 10.1016/j.jhep.2017.11.039 [58] CATHCART AL, CHAN HL, BHARDWAJ N, et al. No resistance to tenofovir alafenamide detected through 96 weeks of treatment in patients with chronic hepatitis B infection[J]. Antimicrob Agents Chemother, 2018, 62(10): e01064-18. [59] CHAN HLY, MARCELLIN P, PAN CQ, et al. No resistance to tenofovir alafenamide detected through 144 weeks of treatment in patients with chronic hepatitis B[J]. Hepatology, 2018, 68(Suppl 1): 231A. [60] COOKE GS, ANDRIEUX-MEYER I, APPLEGATE TL, et al. Accelerating the elimination of viral hepatitis: A Lancet Gastroenterology & Hepatology Commission[J]. Lancet Gastroenterol Hepatol, 2019, 4(2): 135-184. DOI: 10.1016/S2468-1253(18)30270-X [61] SCHRÖEDER SE, PEDRANA A, SCOTT N, et al. Innovative strategies for the elimination of viral hepatitis at a national level: A country case series[J]. Liver Int, 2019, 39(10): 1818-1836. DOI: 10.1111/liv.14222 [62] ENRIQUEZ AD, CAMPBELL MS, REDDY KR. Cost-effectiveness of suppressing hepatitis B virus DNA in immune tolerant patients to prevent hepatocellular carcinoma and cirrhosis[J]. Aliment Pharmacol Ther, 2007, 26(3): 383-391. DOI: 10.1111/j.1365-2036.2007.03382.x [63] FREW PM, ALHANTI B, VO-GREEN L, et al. Multilevel factors influencing hepatitis B screening and vaccination among Vietnamese Americans in Atlanta, Georgia[J]. Yale J Biol Med, 2014, 87(4): 455-471. [64] VEDIO A, LIU E, LEE A, et al. Improving access to health care for chronic hepatitis B among migrant Chinese populations: A systematic mixed methods review of barriers and enablers[J]. J Viral Hepat, 2017, 24(7): 526-540. DOI: 10.1111/jvh.12673 [65] KENNEDY P, LITWIN S, DOLMAN GE, et al. Immune tolerant chronic hepatitis B: The unrecognized risks[J]. Viruses, 2017, 9(5): 96. DOI: 10.3390/v9050096 [66] World Health Organization. Global Hepatitis Report[R/OL]. Geneva: WHO, 2017. http://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/10.11.19. -



 PDF下载 ( 2175 KB)
PDF下载 ( 2175 KB)


 下载:
下载: