IL-32联合终末期肝病模型对HBV相关慢加急性肝衰竭患者预后的预测价值
DOI: 10.3969/j.issn.1001-5256.2021.02.012
Value of interleukin-32 combined with Model for End-Stage Liver Disease in predicting the prognosis of patients with hepatitis B virus-related acute-on-chronic liver failure
-
摘要:
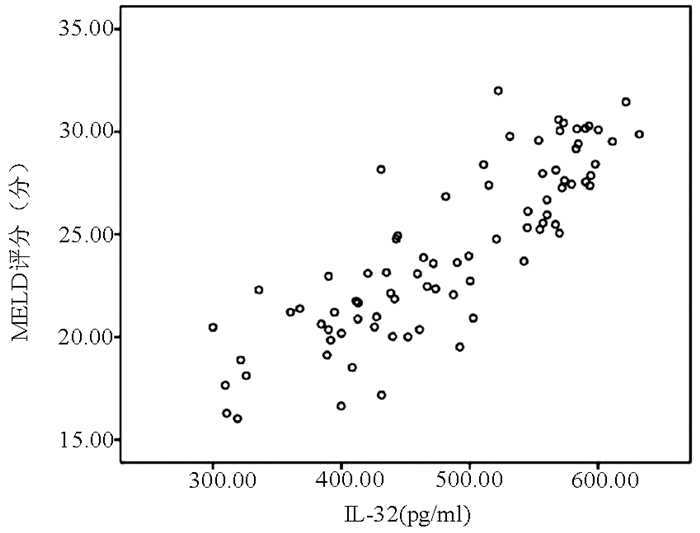
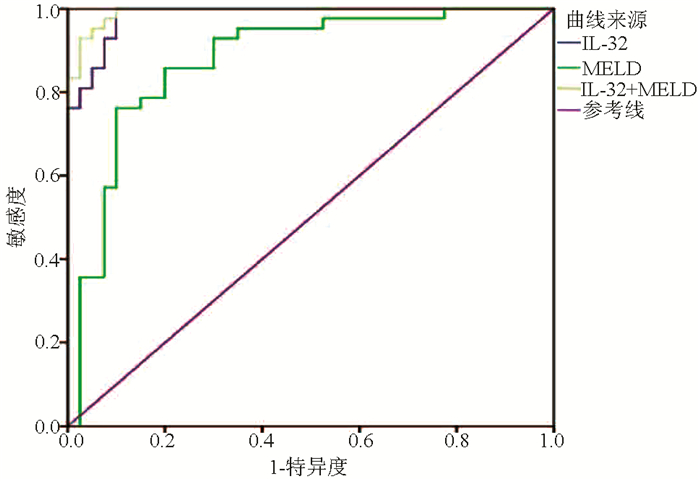
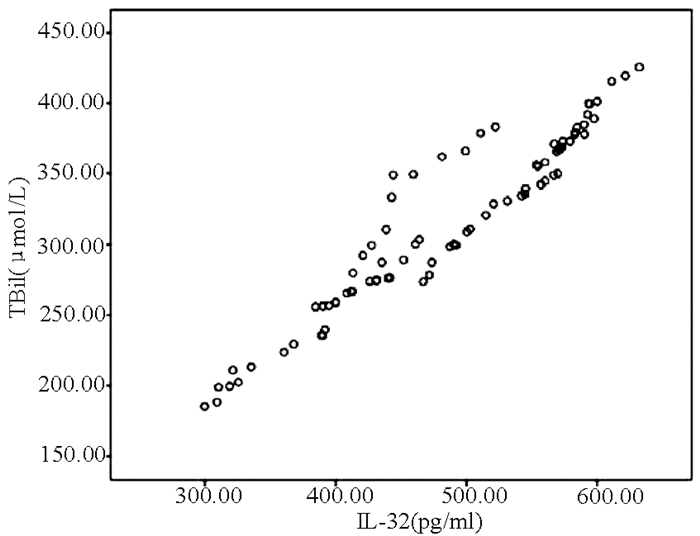
目的 探讨IL-32联合终末期肝病模型(MELD)对HBV相关慢加急性肝衰竭(HBV-ACLF)患者预后的预测价值。 方法 选取2015年1月-2018年12月在苏州大学附属第一医院住院的92例HBV-ACLF患者,根据确诊后3个月随访情况分为存活组(n=40)和死亡组(n=52)。采用酶联免疫吸附试验(ELISA)测定患者的血清IL-32水平。收集患者的临床资料,包括年龄、性别、合并基础疾病、主要并发症、WBC、PLT、红细胞比积(HCT)、TBil、ALT、AST、Alb、SCr、PT、INR、HBV DNA等。符合正态分布的计量资料2组间比较采用t检验,不符合正态分布的计量资料2组间比较采用Mann-Whitney U检验;计数资料2组间比较采用χ2检验;IL-32与其他变量进行Pearson相关性分析;采用二元logistic回归分析影响HBV-ACLF患者预后的独立危险因素;利用ROC曲线下面积(AUC)评价IL-32联合MELD评分对HBV-ACLF预后的预测价值,AUC的比较采用正态性Z检验。 结果 2组间HCT、PLT、TBil、SCr、PT、INR、HBV DNA、IL-32、MELD评分比较差异均有统计学意义(P值均<0.05);IL-32与TBil(r=0.952, P<0.001)、MELD评分(r=0.850, P<0.001)均呈显著正相关; IL-32(OR=1.137, 95%CI: 1.040~1.243, P=0.005)和MELD评分(OR=1.055,95%CI:1.001~1.109,P=0.025)是HBV-ACLF患者死亡的独立危险因素; IL-32联合MELD评分对HBV-ACLF患者预后的预测价值最高(AUC=0.992, 95%CI:0.981~1.000),优于IL-32(AUC=0.984)和MELD评分(AUC=0.877),差异均具有统计学意义(Z值分别为2.265、3.182, P值均<0.05)。 结论 IL-32、MELD评分均能预测HBV-ACLF患者预后,两者联合则预测价值更高。 Abstract:Objective To investigate the value of interleukin-32 (IL-32) combined with Model for End-Stage Liver Disease (MELD) in predicting the prognosis of patients with hepatitis B virus (HBV)-related acute-on-chronic liver failure (HBV-ACLF). Methods A total of 92 patients with HBV-ACLF who were hospitalized in The First Affiliated Hospital of Soochow University from January 2015 to December 2018 were enrolled, and according to the follow-up results at 3 months after diagnosis, the patients were divided into survival group with 40 patients and death group with 52 patients. ELISA was used to measure the serum level of IL-32. Clinical data of the patients were collected, including age, sex, underlying diseases, major complications, white blood cell count (WBC), platelet count (PLT), hematocrit (HCT), total bilirubin (TBil), alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin (Alb), serum creatinine (SCr), prothrombin time (PT), international normalized ratio (INR), and HBV DNA. The t-test was used for comparison of normally distributed continuous data between two groups, and the Mann-Whitney U test was used for comparison of non-normally distributed continuous data between two groups; the chi-square test was used for comparison of categorical data between two groups; a Pearson correlation analysis was performed for IL-32 and other variables; a binary logistic regression analysis was performed to investigate the independent risk factors for the prognosis of patients with HBV-ACLF. The receiver operating characteristic(ROC) curve(AUC) was used to evaluate the value of IL-32 combined with MELD score in predicting the prognosis of patients with HBV-ACLF. The normal Z test was used for comparison of AUC. Results There were significant differences between the two groups in HCT, PLT, TBil, SCr, PT, INR, HBV DNA, IL-32, and MELD score (all P < 0.05). IL-32 was positively correlated with TBil (r=0.952, P < 0.001) and MELD score (r=0.850, P < 0.001). IL-32 (odds ratio [OR]=1.137, 95% confidence interval [CI]: 1.040-1.243, P=0.005) and MELD score (OR=1.055, 95% CI: 1.001-1.109, P=0.025) were independent risk factors for the death of HBV-ACLF patients. IL-32 combined with MELD score had the highest value in predicting the prognosis of patients with HBV-ACLF (AUC=0.992, 95% CI: 0.981-1.000), with a significantly higher AUC than IL-32 (0.992 vs 0.984, Z=2.265, P < 0.05) and MELD score (0.992 vs 0.877, Z=3.182, P < 0.05). Conclusion Both IL-32 and MELD score can predict the prognosis of patients with HBV-ACLF, and the combination of these two indicators has a better predictive value. -
Key words:
- Hepatitis B Virus /
- Acute-On-Chronic Liver Failure /
- Interleukins /
- Prognosis
-
表 1 两组患者一般资料比较
指标 死亡组(n=52) 存活组(n=40) 统计值 P值 男性[例(%)] 32(61.54) 28(70.00) χ2=0.714 0.398 年龄(岁) 51.76±10.23 52.55±11.69 t=0.325 0.746 WBC(×109) 6.76±2.08 7.06±2.33 t=0.610 0.543 HCT(%) 0.36±0.05 0.39±0.05 t=2.391 0.019 PLT(×109) 70.67±13.69 92.70±16.69 t=6.550 <0.001 TBil(μmol/L) 354.30±38.55 272.75±52.55 t=-8.039 <0.001 ALT(U/L) 679.51±164.75 576.28±139.22 t=-3.057 0.503 AST(U/L) 667.26±157.49 551.04±141.88 t=-3.480 0.071 Alb(g/L) 32.76±2.74 33.34±2.78 t=0.952 0.344 SCr(μmol/L) 78.00(70.50~89.33) 73.65(65.48~78.58) U=611.000 0.034 PT(s) 32.30(29.73~33.25) 26.25(25.55~27.48) U=98.000 <0.001 INR 2.60(2.20~2.80) 1.70(1.60~1.90) U=106.000 <0.001 HBV DNA(×107IU/ml) 2.79±0.69 2.28±0.97 t=-2.736 0.008 IL-32(pg/ml) 555.80±42.18 408.99±55.56 t=-13.517 <0.001 MELD评分(分) 26.87±3.01 21.54±3.35 t=-7.596 <0.001 有肝硬化基础[例(%)] 31(59.62) 19(47.50) χ2=1.338 0.247 合并基础疾病[例(%)] 糖尿病 21(40.38) 18(45.00) χ2=0.197 0.657 心血管疾病 20(38.46) 18(45.00) χ2=0.399 0.528 慢性肺部疾病 19(36.54) 17(42.50) χ2=0.337 0.561 慢性肾病 16(30.77) 13(32.50) χ2=0.031 0.859 主要并发症[例(%)] 消化道出血 28(53.85) 16(40.00) χ2=1.737 0.188 腹水 41(78.85) 30(75.00) χ2=0.190 0.663 肝性脑病 37(71.15) 21(52.50) χ2=3.377 0.066 肝肾综合征 23(44.23) 15(37.50) χ2=0.422 0.516 表 2 HBV-ACLF患者预后相关因素分析
指标 单因素分析 多因素分析 P值 OR 95%CI P值 OR 95%CI 年龄 0.742 0.993 0.954~1.034 性别 0.853 1.087 0.451~2.618 WBC 0.538 0.939 0.770~1.147 HCT 0.023 0.647 0.266~1.575 0.363 0.668 0.280~1.595 PLT <0.001 0.909 0.872~0.948 0.987 1.013 0.213~4.826 TBil <0.001 1.036 1.021~1.050 0.831 1.397 0.065~29.828 ALT 0.515 1.004 1.001~1.008 AST 0.062 1.005 1.002~1.008 Alb 0.340 0.925 0.788~1.086 SCr 0.282 1.014 0.988~1.041 PT <0.001 2.640 1.745~3.996 0.092 5.852 0.750~45.685 INR <0.001 683.865 39.105~11 959.338 0.430 647.954 39.695~14 093.261 HBV DNA 0.011 2.102 1.182~3.736 0.430 1.582 0.506~4.949 IL-32 <0.001 1.062 1.030~1.094 0.005 1.137 1.040~1.243 MELD评分 <0.001 1.592 1.317~1.925 0.025 1.055 1.001~1.109 -
[1] SARIN SK, CHOUDHURY A, SHARMA MK, et al. Acute-on-chronic liver failure: Consensus recommendations of the Asian Pacific association for the study of the liver (APASL): An update[J]. Hepatol Int, 2019, 13(4): 353-390. DOI: 10.1007/s12072-019-09946-3 [2] Liver Failure and Artificial Liver Group, Chinese Society of Infectious Diseases, Chinese Medical Association; Severe Liver Disease and Artifical Liver Group, Chinese Society of Hepatology, Chinese Medical Association. Guideline for diagnosis and treatment of liver failure(2018)[J]. J Clin Hepatol, 2019, 35(1): 38-44. (in Chinese) DOI: 10.3969/j.issn.1001-5256.2019.01.007中华医学会感染病学分会肝衰竭与人工肝学组, 中华医学会肝病学分会重型肝病与人工肝学组. 肝衰竭诊治指南(2018年版)[J].临床肝胆病杂志, 2019, 35(1): 38-44. DOI: 10.3969/j.issn.1001-5256.2019.01.007 [3] LI H, JIA YN, HE Q, et al. Progress of immunosuppressant management, infection prevention and treatment after liver transplantation in severe liver disease[J]. Ogran Transplantation, 2020, 11(3): 344-349. (in Chinese) DOI: 10.3969/j.issn.1674-7445.2020.03.004李瀚, 贾亚男, 贺强, 等. 危重症肝病肝移植术后免疫抑制剂管理和感染的防治进展[J]. 器官移植, 2020, 11(3): 344-349. DOI: 10.3969/j.issn.1674-7445.2020.03.004 [4] SUNDARAM V, JALAN R, WU T, et al. Factors associated with survival of patients with severe acute-on-chronic liver failure before and after liver transplantation[J]. Gastroenterology, 2019, 156(5): 1381-1391. DOI: 10.1053/j.gastro.2018.12.007 [5] XU J, HUANG M. Predictive value of plasma diamine oxidase concentration combined with iMELD score on short-term prognosis of hepatitis B virus-related acute-on-chronic liver failure[J/CD].Chin J Liver Dis (Electronic Version), 2020, 12(2): 68-75. (in Chinese)许俊, 黄敏. 血浆二胺氧化酶联合iMELD评分对乙型肝炎病毒相关慢加急性肝衰竭患者近期预后的预测价值[J/CD]. 中国肝脏病杂志(电子版), 2020, 12(2): 68-75. [6] ALBILLOS A, LARIO M, ÁLVAREZ-MON M. Cirrhosis-associated immune dysfunction: Distinctive features and clinical relevance[J]. J Hepatol, 2014, 61(6): 1385-1396. DOI: 10.1016/j.jhep.2014.08.010 [7] RIBEIRO-DIAS F, SAAR GOMES R, de LIMA SILVA LL, et al. Interleukin 32: A novel player in the control of infectious diseases[J]. J Leukoc Biol, 2017, 101(1): 39-52. DOI: 10.1189/jlb.4RU0416-175RR [8] XU Q, PAN X, SHU X, et al. Increased interleukin-32 expression in chronic hepatitis B virus-infected liver[J]. J Infect, 2012, 65(4): 336-342. DOI: 10.1016/j.jinf.2012.05.009 [9] ZHANG WJ, ZHAO LJ, WU JZ. Value of MELD、AARC、COSSH scoring systems in evaluating the 90-day prognosis of hepatitis B virus-related acute-on-chronic liver failure[J]. J Clin Hepatol, 2020, 36(4): 813-817. (in Chinese) DOI: 10.3969/j.issn.1001-5256.2020.04.021张文佳, 赵丽娟, 吴基洲. MELD、AARC、COSSH评分系统对乙型肝炎相关慢加急性肝衰竭90天预后的评估价值[J]. 临床肝胆病杂志, 2020, 36(4): 813-817. DOI: 10.3969/j.issn.1001-5256.2020.04.021 [10] ZHAO RH, SHI Y, ZHAO H, et al. Acute-on-chronic liver failure in chronic hepatitis B: An update[J]. Expert Rev Gastroenterol Hepatol, 2018, 12(4): 341-350. DOI: 10.1080/17474124.2018.1426459 [11] WU W, YAN H, ZHAO H, et al. Characteristics of systemic inflammation in hepatitis B-precipitated ACLF: Differentiate it from No-ACLF[J]. Liver Int, 2018, 38(2): 248-257. DOI: 10.1111/liv.13504 [12] CLÀRIA J, STAUBER RE, COENRAAD MJ, et al. Systemic inflammation in decompensated cirrhosis: Characterization and role in acute-on-chronic liver failure[J]. Hepatology, 2016, 64(4): 1249-1264. DOI: 10.1002/hep.28740 [13] SOLÉ C, SOLÀ E. Update on acute-on-chronic liver failure[J]. Gastroenterol Hepatol, 2018, 41(1): 43-53. DOI: 10.1016/j.gastrohep.2017.05.012 [14] DALI-YOUCEF N, VIX M, COSTANTINO F, et al. Interleukin-32 contributes to human nonalcoholic fatty liver disease and insulin resistance[J]. Hepatol Commun, 2019, 3(9): 1205-1220. DOI: 10.1002/hep4.1396 [15] GUI M, ZHANG H, ZHONG K, et al. Clinical significance of interleukin-32 expression in patients with rheumatoid arthritis[J]. Asian Pac J Allergy Immunol, 2013, 31(1): 73-78. [16] YANG Z, SHI L, XUE Y, et al. Interleukin-32 increases in coronary arteries and plasma from patients with coronary artery disease[J]. Clin Chim Acta, 2019, 497: 104-109. DOI: 10.1016/j.cca.2019.07.019 [17] DI BENEDETTO P, GUGGINO G, MANZI G, et al. Interleukin-32 in systemic sclerosis, a potential new biomarker for pulmonary arterial hypertension[J]. Arthritis Res Ther, 2020, 22(1): 127. DOI: 10.1186/s13075-020-02218-8 [18] KOEKEN V, VERRALL AJ, ARDIANSYAH E, et al. IL-32 and its splice variants are associated with protection against Mycobacterium tuberculosis infection and skewing of Th1/Th17 cytokines[J]. J Leukoc Biol, 2020, 107(1): 113-118. DOI: 10.1002/JLB.4AB0219-071R [19] ZOU Y, BAO J, PAN X, et al. NKP30-B7-H6 interaction aggravates hepatocyte damage through up-regulation of interleukin-32 expression in hepatitis B virus-related acute-on-chronic liver failure[J]. PLoS One, 2015, 10(8): e0134568. DOI: 10.1371/journal.pone.0134568 [20] TIAN ZJ, SHEN Y, LI XR, et al. Increased interleukin-32, interleukin-1, and interferon-γ levels in serum from hepatitis B patients and in HBV-stimulated peripheral blood mononuclear cells from healthy volunteers[J]. J Infect Public Health, 2019, 12(1): 7-12. DOI: 10.1016/j.jiph.2018.06.006 [21] LI ZY, YANG ST, ZHAO CY, et al. Analysis of prognostic risk factors and establishment of prognosis model in patients with hepatitis B virus-related acute-on-chronic liver failure[J]. Chin J Infect Dis, 2019, 37(12): 737-741. (in Chinese) DOI: 10.3760/cma.j.issn.1000-6680.2019.12.004李子月, 杨士田, 赵彩彦, 等. 乙型肝炎病毒相关慢加急性肝衰竭患者预后危险因素分析及预后模型建立[J]. 中华传染病杂志, 2019, 37(12): 737-741. DOI: 10.3760/cma.j.issn.1000-6680.2019.12.004 [22] ZHANG H, SHI XX, ZENG YL, et al. Clinical study on short-term prognostic value of serum IL-8 level in patients with HBV related acute-on-chronic liver failure[J]. Infect Dis Info, 2020, 33(2): 136-139. (in Chinese) DOI: 10.3969/j.issn.1007-8134.2020.02.009张鸿, 石新星, 曾义岚, 等. 血清IL-8预测HBV相关慢加急性肝衰竭患者短期预后的临床研究[J]. 传染病信息, 2020, 33(2): 136-139. DOI: 10.3969/j.issn.1007-8134.2020.02.009 [23] WANG J, WANG Q, GUAN SH, et al. Expression and clinical significance of IL-26 in hepatitis B virus-associated acute-on-chronic liver failure[J]. Acta Universitatis Medicinalis Anhui, 2020, 55(4): 612-617. (in Chinese) https://www.cnki.com.cn/Article/CJFDTOTAL-YIKE202004025.htm汪静, 王琴, 管世鹤, 等. 白细胞介素-26在乙型肝炎相关慢加急性肝衰竭患者中的表达及临床意义[J]. 安徽医科大学学报, 2020, 55(4): 612-617. https://www.cnki.com.cn/Article/CJFDTOTAL-YIKE202004025.htm [24] FISCHER J, SILVA TE, SOARES E SILVA PE, et al. From stable disease to acute-on-chronic liver failure: Circulating cytokines are related to prognosis in different stages of cirrhosis[J]. Cytokine, 2017, 91: 162-169. DOI: 10.1016/j.cyto.2016.12.017 -



 PDF下载 ( 2025 KB)
PDF下载 ( 2025 KB)

 下载:
下载: