真实世界中不同ALT、AST水平慢性丙型肝炎患者对直接抗病毒药物治疗的病毒学应答及肝纤维化指标变化情况
DOI: 10.3969/j.issn.1001-5256.2021.02.014
Virological response to direct-acting antiviral therapy and changes in liver fibrosis indices in chronic hepatitis C patients with different alanine aminotransferase and aspartate aminotransferase levels in a real-world setting
-
摘要:
目的 探讨真实世界中不同ALT、AST水平的慢性丙型肝炎患者对直接抗病毒药物(DAA)治疗的病毒学应答,以及治疗后肝硬度测定(LSM)值、4项因素的肝纤维化指数(FIB-4)和AST/PLT比值指数(APRI)的变化情况。 方法 纳入2017年12月—2020年5月在北京大学第一医院感染疾病科门诊就诊的慢性丙型肝炎患者,计算患者治疗病毒学应答率。采用Wilcoxon秩和检验对比不同组间基线及治疗结束第12周LSM、FIB-4和APRI的变化;计数资料组间比较采用χ2检验。 结果 共纳入48例慢性丙型肝炎患者,其中基线ALT或AST出现异常的患者为33.3%。所有患者DAA治疗第4周病毒学应答率为85.4%,治疗结束时、治疗结束12、24、48周均为100%;治疗结束第12周较基线LSM[6.1(5.1~12.4) kPa vs 8.6(5.7~16.9) kPa,Z=-1.676,P=0.043]、APRI[0.24(0.19~0.48) vs 0.42(0.23~1.17),Z=-2.050,P=0.027]差异有统计学意义。ALT或AST异常的患者治疗结束12周与基线LSM[8.9(5.6~13.1) kPa vs 14.4(8.0~28.2) kPa,Z=-1.679,P=0.047]、APRI[0.44(0.25~0.50) vs 1.29(0.99~2.09),Z=-3.427,P=0.001]差异有统计学意义。 结论 慢性丙型肝炎患者DAA治疗后持续病毒学应答率高,基线ALT或AST有异常较无异常的患者在治疗后LSM及APRI改善更明显。 Abstract:Objective To investigate the virologic response to direct-acting antiviral (DAA) therapy and the changes in liver stiffness measurement (LSM), fibrosis-4 (FIB-4), and aspartate aminotransferase-to-platelet ratio index (APRI) after treatment in chronic hepatitis C (CHC) patients with different alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels at baseline in a real-world setting. Methods CHC patients who attended the outpatient service of Department of Infectious Diseases, Peking University First Hospital, from December 2017 to May 2020 were enrolled, and virologic response rate was calculated. The Wilcoxon rank-sum test was used to compare LSM, FIB-4, and APRI between groups at baseline and at 12 weeks after treatment, and the chi-square test was used for comparison of categorical data between groups. Results A total of 48 CHC patients were enrolled, among whom 33.3% had abnormal ALT or AST at baseline. Among these patients, the virologic response rate was 85.4% at week 4 of treatment and 100% at the end of treatment and at 12, 24, and 48 weeks after treatment, and there were significant changes from baseline to 12 weeks after treatment in LSM [6.1 (5.1-12.4) kPa vs 8.6 (5.7-16.9) kPa, Z=-1.676, P=0.043] and APRI [0.24(0.19-0.48) vs 0.42(0.23-1.17), Z=-2.050, P=0.027]. From baseline to 12 weeks after treatment, the patients with abnormal ALT or AST at baseline had significant changes in LSM [8.9(5.6-13.1) kPa vs 14.4(8.0-28.2) kPa, Z=-1.679, P=0.047] and APRI [0.44(0.25-0.50) vs 1.29(0.99-2.09), Z=-3.427, P=0.001]. Conclusion CHC patients achieve a high sustained virologic response rate after DAA therapy, and the patients with abnormal ALT or AST at baseline tend to have more significant improvements in LSM and APRI than those without such abnormality. -
Key words:
- Hepatitis C, Chronic /
- Liver Cirrhosis /
- Antiviral Agents /
- Sustained Virologic Response
-
原发性肝癌是肝脏最常见的恶性肿瘤之一,其在我国发病率居恶性肿瘤的第4位,肿瘤相关死亡第2位[1]。肝细胞癌(HCC)是肝癌中的最常见的类型,是目前全球第五大恶性肿瘤。慢性肝脏疾病患者,如患有病毒性肝炎、酒精性肝病、非酒精性脂肪性肝炎等,是HCC的主要高危人群。中国的新发病例数及死亡病例数均占了全球的50%左右,因此HCC严重影响我国人民的健康[2]。
对于肝癌患者的根治性治疗,目前主要有肝切除术(liver resection, LR)和肝移植术(liver transplantation, LT)两种方法[3-4],2种方法的选择取决于医院和医生的水平、患者的意愿及身体状况等。为了提高对这2种治疗方法结果的认识,更好的指导临床,本文回顾性分析了首都医科大学附属北京佑安医院171例HCC患者治疗后3年的随访资料,比较LR和LT 2种方法的临床治疗效果。
1. 资料与方法
1.1 研究对象
选取2009年3月—2014年3月在本院首次接受LR或LT的HCC患者171例,依据治疗方式的不同分为LR组(n=83)和LT组(n=88)。LT组患者肝癌的分期依照UICC/AJCC第7版及巴塞罗那分期(BCLC)进行。
1.2 纳入与排除标准
纳入标准:(1)病理确诊符合HCC的诊断标准;(2)LR及LT治疗均在适应证内。排除标准:(1)既往有恶性肿瘤病史;(2)术前曾行抗肿瘤治疗;(3)术后有肿瘤残留;(4)合并严重糖尿病、高血压、心脏病、肾脏病等可能影响生存期的疾病。
1.3 随访情况
患者经过门诊及电话随访,随访主要终点为死亡或随访满3年(36个月),次要终点为检测到肿瘤复发。
1.4 伦理学审查
本研究获得首都医科大学附属北京佑安医院伦理委员会审批,批号:LL-2018-031-K。受试者均签署知情同意书。
1.5 统计学方法
采用SPSS 25.0统计学软件进行数据分析。分类资料组间比较使用χ2检验。比较亚组间无瘤生存期和总生存期的差异使用Kaplan-Meier和log-rank检验。检测影响预后的因素使用单因素和多因素Cox比例风险模型。P<0.05为差异有统计学意义。
2. 结果
2.1 一般资料
171例HCC患者中男142例(83.04%),女29例(16.96%)。95例(55.56%)患者在50岁以下。3年随访期间共有20例(11.70%)患者失访。3年随访结束时,69例(40.35%)患者肿瘤复发,32例(18.71%)患者发生HCC相关死亡(表 1)。
表 1 肝癌患者的基本资料指标 例数(n=171) 手术类型 χ2值 P值 LR组(n=83) LT组(n=88) 性别(例) 0.192 0.661 男 142 70 72 女 29 13 16 年龄(例) 0.117 0.732 ≤50岁 95 45 50 >50岁 76 38 38 潜在肝病(例) 2.333 0.311 HBV 153 76 77 HCV 17 6 11 其他 1 1 0 肿瘤位置(例) 1.161 0.281 右叶 119 61 58 非右叶或多叶 52 22 30 肿瘤数目(例) 29.649 <0.001 单发 113 38 75 多发 58 45 13 肿瘤大小(例) 46.383 <0.001 <3 cm 72 13 59 3~5 cm 62 43 19 >5 cm 37 27 10 AFP (例) 3.350 0.187 <20 ng/ml 82 42 40 20~400 ng/ml 52 20 32 >400 ng/ml 37 21 16 TNM分期(例) 0.174 0.677 Ⅰ+Ⅱ 117 57 63 Ⅲ+Ⅳ 54 26 25 BCLC肝癌分期(例) 0.057 0.972 A 87 42 45 B 44 22 22 C 40 19 21 Child-Pugh分级(例) 7.833 0.005 C 140 75 65 B+C 31 8 23 病理分级(例) 2.573 0.276 低分化 29 18 11 中分化 113 52 61 高分化 29 13 16 肿瘤复发(例) 4.121 0.042 否 102 43 59 是 69 40 29 HCC所致死亡(例) 0.937 0.333 否 139 65 74 是 32 18 14 从基础数据上可以看出,LT组较LR组的单发肿瘤比例更多、肿瘤更小、Child-Pugh分期更高、复发率更低(P值均<0.05),而其他的指标如性别、年龄、潜在肝病、肿瘤位置、AFP水平、TNM分期、BCLC肝癌分期和病理分级2组间比较差异均无统计学意义(P值均>0.05)(表 1)。
2.2 无瘤生存期和总生存期
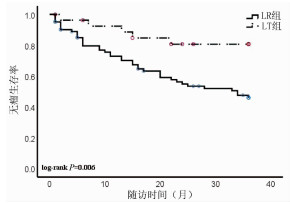
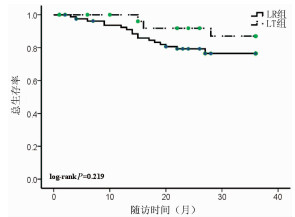
随访3年,LR组患者无瘤生存率是46.02%,而LT组患者的无瘤生存率为80.71%,两组差异具有统计学意义(P=0.006)(图 1);LR组患者的总生存率是76.44%,LT组患者的总生存率为86.99%,两组比较差异无统计学意义(P=0.219)(图 2)。
2.3 HCC患者结局的影响因素分析
使用Cox生存分析方法分析无瘤生存期、总生存期和临床特征之间的相关性。在无瘤生存期的单因素分析中,治疗方法和TNM分期是无瘤生存期的影响因素(P值均<0.05)(表 2)。多因素分析结果显示,治疗方法和TNM期是HCC患者无瘤生存期的独立预后因素[RR(95% CI)分别为0.239(0.093~0.612)、4.834(2.598~8.993),P值分别为0.003、<0.001]。在总生存期的单因素分析中,TNM分期是无瘤生存期的影响因素(P<0.001)(表 2)。多因素分析发现TNM分期是HCC患者总生存期的独立预后因素[RR(95% CI):4.970(2.052~12.037),P<0.001]。
表 2 肝癌患者无瘤生存期和总生存期的单因素分析指标 无瘤生存期 总生存期 RR 95%CI P值 RR 95%CI P值 性别 男 1.000 1.000 女 0.539 0.213~1.367 0.193 0.474 0.110~2.034 0.315 年龄 ≤50岁 1.000 1.000 >50岁 1.023 0.570~1.838 0.939 0.678 0.281~1.636 0.387 肝病 HBV 1.000 1.000 HCV/其他 0.380 0.092~1.569 0.181 0.872 0.203~3.747 0.854 肿瘤位置 肝右叶 1.000 1.000 其他位置 0.816 0.422~1.581 0.548 0.693 0.254~1.893 0.475 肿瘤数目 单发 1.000 1.000 多发 1.513 0.833~2.751 0.174 1.491 0.628~3.542 0.365 肿瘤大小 <3 cm 1.000 1.000 <3 cm 1.000 1.000 3~5 cm 1.360 0.647~2.860 0.418 2.956 0.890~9.819 0.077 >5 cm 3.877 1.924~7.812 <0.001 5.595 1.721~18.189 0.004 AFP <20 ng/ml 1.000 1.000 20~400 ng/ml 1.862 0.861~4.025 0.114 0.982 1.699~12.123 0.003 >400 ng/ml 3.625 1.804~7.285 <0.001 4.539 1.699~12.123 0.003 TNM分期 Ⅰ-Ⅱ 1.000 1.000 Ⅲ 4.084 2.237~7.455 <0.001 4.985 2.058~12.076 <0.001 BCLC HCC分期 A 1.000 1.000 B 1.778 0.887~3.564 0.003 1.235 0.349~4.378 0.001 C 3.008 1.453~6.230 0.105 0.003 5.566 2.052~15.101 0.001 Child-Pugh分级 A 1.000 1.000 B+C 0.446 0.160~1.245 0.123 1.886 0.731~4.864 0.189 病理分期 低分化 1.000 1.000 中/高分化 0.813 0.378~1.750 0.597 0.524 0.203~1.350 0.181 治疗方法 LR 1.000 1.000 LT 3.383 1.334~8.579 0.010 0.474 0.140~1.610 0.232 3. 讨论
肝癌的治疗需要多学科协同、多种方法综合进行。目前临床上常用的肝癌治疗方法包括:LR、LT、局部消融治疗(射频消融治疗术为主)、经导管肝动脉化疗栓塞术、放射治疗、全身治疗(包括分子靶向治疗、免疫治疗、化疗、中医治疗等)等[3-5]。既往认为LT治疗的肝癌患者生存结局更好[5]。但是随着对HCC发生发展的探索、影像诊断水平的提高、局部和全身治疗方法的完善、手术技术的进步和术后多学科诊疗模式的形成,不同治疗下患者的最终结局,也随之而改善,有学者[6-7]提出目前LR治疗肝癌与LT同样取得理想效果。金子铮等[8]研究发现LT较LR治疗肝癌患者的5年生存率的优势,2005年—2011年较1989年—2004年明显缩小(73% vs 61%,77% vs 36%),他们认为这种优势的缩小是由于手术水平的进步、围手术期对肝硬化治疗的优化和对于复发肿瘤多手段的治疗。除了总体变化趋势外,治疗的结果还和医院的水平、患者的状况等多个因素相关。本研究发现LT较LR治疗肝癌3年后肿瘤的复发率明显降低,而总生存率差别不显著。Shen等[9]回顾分析了1218例LT和2068例LR治疗肝癌的患者得出结论,LT比LR在无瘤生存率和总生存率上都有优势。矫学黎等[7]对352例LR和37例LT治疗的患者进行了统计分析后也得到了相似的结论。本研究中LT组较LR组的肿瘤复发率明显降低,与文献一致。另一项纳入9篇关于LT和LR治疗HCC效果研究的Meta分析[10]结果显示,HCC患者术后第1年两种治疗方法的总生存率相似,术后第5年LR组较LT组总生存率明显降低。这与本研究中两组总生存率的结果稍有差别。该研究还认为,与LR组相比,HCC患者术后3年LT组的获益才开始表现出来,而本研究的随访截点正好是3年,因此本研究结果与其他中心的研究结果并不矛盾。肿瘤复发才是病死率增加的开始,如果延长随访时间,本研究的总生存率也将出现显著性差异。故本研究数据结合文献检索的结果,支持LT较LR治疗肝癌更有优势。
从笔者的基础数据上可以看出,LT组较LR组的单发肿瘤更多、肿瘤更小、Child-Pugh分期更高,而其他的指标如性别、年龄、潜在肝病、肿瘤位置、AFP水平、TNM分期、BCLC肝癌分期和病理分级2组比较差异均无统计学差异,这是因为在选择LT治疗的时候,兼顾疗效与公平,平衡考虑肿瘤患者预后,主观限制了总体的肿瘤数目和大小。LT较LR治疗肝癌患者的另一优势是不用考虑患者的肝功能,可以同时解决肿瘤和肝功能衰竭两大问题,本研究入组的LT患者中有2例是Child-Pugh C级。综上所述,LT更倾向于选择肝功能储备较差的早期肝癌患者,3年无瘤生存率令人满意。
LT也存在一定的缺点,比如供体的缺乏、术中并发症多、药物排异等,为了弥补这些缺点,目前已有较多研究在推动LT的进步,包括移植方法上的改进、治疗适应证的拓展、新型抗排异药物的研发和围手术期处理的优化等[11-14]。
本研究结果还得出TNM分期是无瘤生存期和总生存期的独立预测因素,支持TNM分期在临床上的重要作用,不但可以用于指导治疗方法的选择,还能预测术后结果。但是TNM分期也有缺点,淋巴结或远处脏器转移有时术前和术中难以准确评估,因此更好的判断预后的指标和方法也是研究的热点。
另外,本研究是单中心研究,基于现有的临床资料,还存在着样本量较小、随访时间短、纳入因素不全面等因素,而且对于手术切除范围、肝移植方法、供体类型、术后治疗、医疗成本、并发症情况等因素未纳入其中,局限了研究的意义。希望将来设计多中心、样本量更大的研究来弥补以上不足, 将更有利于临床工作的不断提升。
-
表 1 患者基线特征
变量 基线ALT或AST无异常(n=32) 基线ALT或AST异常(n=16) 统计值 P值 男/女(例) 13/19 6/10 χ2=0.044 0.835 年龄(岁) 60.5(43.3~70.3) 65.0(49.5~69.0) Z=-0.698 0.485 ALT(U/L) 22.0(15.0~28.0) 64.0(47.5~104.1) Z=-4.523 <0.001 AST(U/L) 24.0(17.4~27.3) 61.2(46.5~91.7) Z=-5.186 <0.001 TBil(μmol/L) 11.55(9.48~14.43) 17.35(14.20~26.63) Z=-1.985 0.047 DBil(μmol/L) 1.87(1.44~3.47) 4.60(2.89~10.61) Z=-2.554 0.011 血肌酐(μmol/L) 75.00(62.75~85.25) 67.05(52.02~79.50) Z=-1.019 0.308 eGFR(ml·min-1·1.73 m-2) 89.55(67.86~99.38) 90.00(64.27~108.18) Z=-0.528 0.598 PLT(g/L) 161.0(112.0~229.0) 132.0(113.5~176.5) Z=-1.504 0.133 LSM(kPa) 6.7(5.3~12.5) 14.4(8.0~28.2) Z=-2.887 0.003 FIB-4 1.68(0.93~1.93) 4.02(2.54~5.53) Z=-3.178 0.001 APRI 0.32(0.23~0.45) 1.29(0.99~2.09) Z=-4.496 <0.001 HCV RNA(lg IU/ml) 6.44(5.67~7.11) 6.87(6.48~7.30) Z=-1.973 0.049 基因型(例) χ2=0.019 0.891 1b 26 14 2a 6 2 表 2 基线ALT、AST不同状态患者DAA治疗及治疗结束后HCV RNA阴转率比较
组别 治疗4周 治疗12周 结束后12周 结束后24周 结束后48周 基线ALT或AST无异常 30/32(93.8%) 32/32(100.0%) 32/32(100.0%) 27/27(100.0%) 19/19(100.0%) 基线ALT或AST异常 11/16(68.8%) 16/16(100.0%) 16/16(100.0%) 13/13(100.0%) 9/9(100.0%) 表 3 基线不同ALT、AST水平的患者在DAA治疗后LSM值比较
组别 例数 基线LSM
(kPa)治疗12周LSM
(kPa)结束后12周LSM
(kPa)结束后24周LSM
(kPa)基线ALT或AST无异常 32 6.7(5.3~12.5) 6.7(5.2~11.7) 5.8(4.6~10.6) 7.5(4.9~17.3) 基线ALT或AST异常 16 14.4(8.0~28.2) 9.9(6.4~14.9) 8.9(5.6~13.1) 10.4(5.8~13.9) Z值 -2.887 -1.261 -0.742 0.883 P值 0.003 0.212 0.470 0.943 表 4 基线与DAA治疗结束后12周时患者各肝纤维化指标比较
肝纤维化指标 组别 例数 基线 治疗结束12周 Z值 P值 LSM (kPa) 基线ALT或AST无异常 32 6.7(5.3~12.5) 5.8(4.6~10.6) -0.232 0.817 基线ALT或AST异常 16 14.4(8.0~28.2) 8.9(5.6~13.1) -1.679 0.047 所有患者 48 8.6(5.7~16.9) 6.1(5.1~12.4) -1.676 0.043 FIB-4 基线ALT或AST无异常 32 1.68(0.93~1.93) 1.42(0.97~2.20) -0.308 0.758 基线ALT或AST异常 16 4.02(2.54~5.53) 2.71(1.26~4.10) -1.736 0.082 所有患者 48 1.74(1.00~4.16) 1.42(1.09~2.78) -0.749 0.230 APRI 基线ALT或AST无异常 32 0.32(0.23~0.45) 0.23(0.17~0.37) -1.491 0.136 基线ALT或AST异常 16 1.29(0.99~2.09) 0.44(0.25~0.50) -3.427 0.001 所有患者 48 0.42(0.23~1.17) 0.24(0.19~0.48) -2.050 0.027 -
[1] Chinese Society of Hepatology and Chinese Society of Infectious Diseases, Chinese Medical Association. The guideline of prevention and treatment for hepatitis C (2019 version)[J]. J Clin Hepatol, 2019, 35(12): 2670-2686. (in Chinese) DOI: 10.3969/j.issn.1001-5256.2019.12.008中华医学会肝病学分会, 中华医学会感染病分会. 丙型肝炎防治指南(2019)年版[J]. 临床肝胆病杂志, 2019, 35(12): 2670-2686. DOI: 10.3969/j.issn.1001-5256.2019.12.008 [2] van der MEER AJ, BERENGUER M. Reversion of disease manifestations after HCV eradication[J]. J Hepatol, 2016, 65(1 Suppl): s95-s108. [3] Chinese Society of Hepatology and Chinese Society of Infectious Diseases, Chinese Medical Association.The guideline of prevention and treatment for hepatitis C: A 2015 update[J]. J Clin Hepatol, 2015, 31(12): 1961-1979. (in Chinese) DOI: 10.3969/j.issn.1001-5256.2015.12.003中华医学会肝病学分会, 中华医学会感染病分会. 丙型肝炎防治指南(2015年更新版)[J]. 临床肝胆病杂志, 2015, 31(12): 1961-1979. DOI: 10.3969/j.issn.1001-5256.2015.12.003 [4] Chinese Society of Hepatology, Chinese Medical Association; Chinese Society of Gastroenterology, Chinese Medical Association; Chinese Society of Infectious Diseases, Chinese Medical Association. Consensus on the diagnosis and therapy of hepatic fibrosis(2019)[J]. J Clin Hepatol, 2019, 35(10): 2163-2172. (in Chinese) DOI: 10.3969/j.issn.1001-5256.2019.10.007中华医学会肝病学分会, 中华医学会消化病学分会, 中华医学会感染病学分会. 肝纤维化诊断及治疗共识(2019年)[J]. 临床肝胆病杂志, 2019, 35(10): 2163-2172. DOI: 10.3969/j.issn.1001-5256.2019.10.007 [5] SHAHID M, IDREES M, NASIR B, et al. Correlation of biochemical markers and HCV RNA titers with fibrosis stages and grades in chronic HCV-3a patients[J]. Eur J Gastroenterol Hepatol, 2014, 26(7): 788-794. DOI: 10.1097/MEG.0000000000000109 [6] BACHOFNER JA, VALLI PV, KROGER A, et al. Direct antiviral agent treatment of chronic hepatitis C results in rapid regression of transient elastography and fibrosis markers fibrosis-4 score and aspartate aminotransferase-platelet ratio index[J]. Liver Int, 2017, 37(3): 369-376. DOI: 10.1111/liv.13256 [7] SNYDER N, GAJULA L, XIAO SY, et al. APRI: An easy and validated predictor of hepatic fibrosis in chronic hepatitis C[J]. J Clin Gastroenterol, 2006, 40(6): 535-542. DOI: 10.1097/00004836-200607000-00013 [8] LIANG J, ZHANG YP, LIU F, et al. Efficacy of direct-acting antiviral agents in treatment of chronic hepatitis C and its effect on liver stiffness and aspartate aminotransferase-to-platelet ratio index[J]. J Clin Hepatol, 2020, 36(6): 1263-1267. (in Chinese) DOI: 10.3969/j.issn.1001-5256.2020.06.015梁静, 张亚苹, 刘芳, 等. 直接抗病毒药物治疗慢性丙型肝炎的效果及对肝硬度、APRI的影响[J]. 临床肝胆病杂志, 2020, 36(6): 1263-1267. DOI: 10.3969/j.issn.1001-5256.2020.06.015 [9] PARCZEWSKI M, KORDEK J, JANCZEWSKA E, et al. Hepatitis C virus (HCV) genotype 1 NS5A resistance-associated variants are associated with advanced liver fibrosis independently of HCV-transmission clusters[J]. Clin Microbiol Infect, 2019, 25(4): 513. [10] MAYLIN S, LAOUENAN C, MARTINOT-PEIGNOUX M, et al. Role of hepatic HCV-RNA level on the severity of chronic hepatitis C and response to antiviral therapy[J]. J Clin Virol, 2012, 53(1): 43-47. DOI: 10.1016/j.jcv.2011.09.029 [11] IOANNOU GN, FELD JJ. What are the benefits of a sustained virologic response to direct-acting antiviral therapy for hepatitis C virus infection?[J]. Gastroenterology, 2019, 156(2): 446-460. DOI: 10.1053/j.gastro.2018.10.033 期刊类型引用(1)
1. 宋林泉,梁志宏. 肝门再阻断法在肝切除术后胆漏中的预防效果. 吉林医学. 2024(02): 364-367 .  百度学术
百度学术其他类型引用(3)
-

 本文二维码
本文二维码
计量
- 文章访问数: 727
- HTML全文浏览量: 156
- PDF下载量: 49
- 被引次数: 4



 PDF下载 ( 1858 KB)
PDF下载 ( 1858 KB)

 下载:
下载:


 下载:
下载:  百度学术
百度学术