利福昔明预防自发性细菌性腹膜炎有效性和安全性的Meta分析
DOI: 10.3969/j.issn.1001-5256.2021.02.015
Efficacy and safety of rifaximin in the prevention of spontaneous bacterial peritonitis: A Meta-analysis
-
摘要:
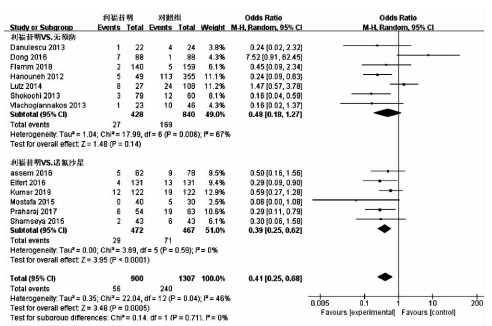
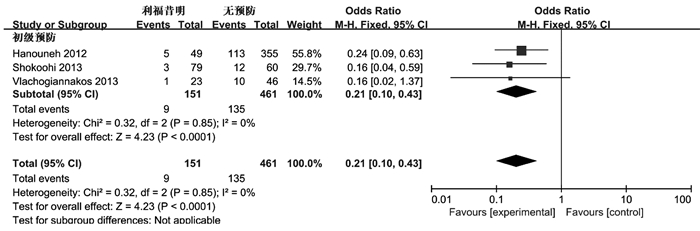
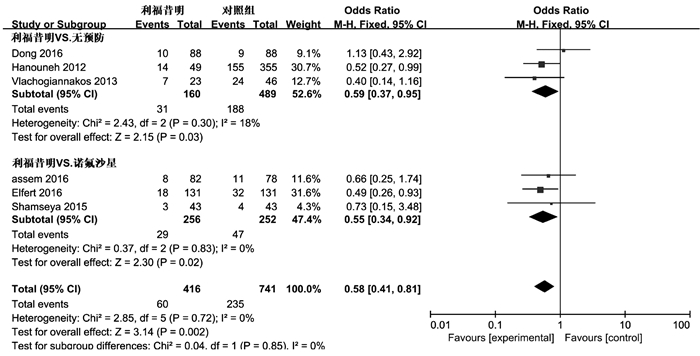
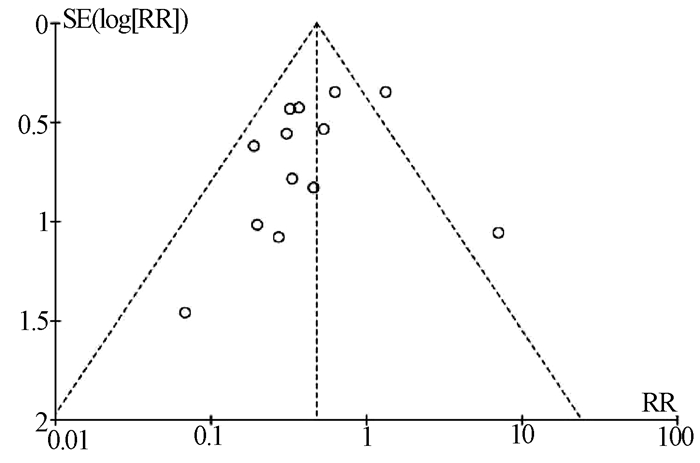
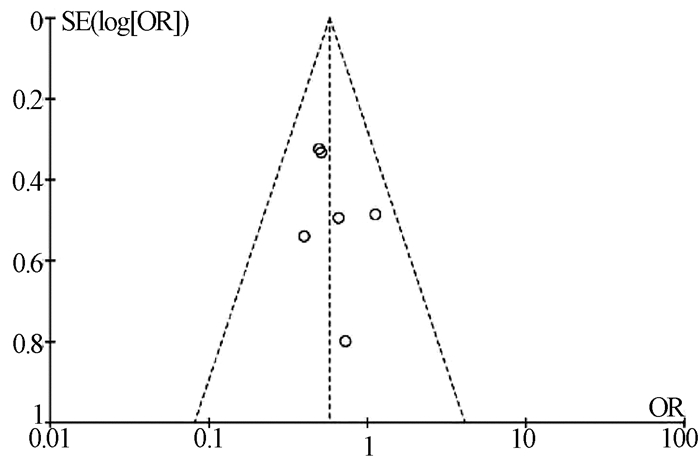
目的 评价利福昔明预防自发性细菌性腹膜炎(SBP)的有效性及安全性。 方法 通过计算机检索中国知网、万方数据、中国生物医学数据库、PubMed、Embase、Cochrane图书馆建库至2020年7月5日发表的有关利福昔明预防SBP的随机对照研究(RCT)、队列研究,根据纳入和排除标准对文献进行筛选,并对文献进行提取数据和质量评估,采用RevMan 5.3软件进行Meta分析。 结果 最终纳入13项研究,共2207例患者,其中6项为RCT,7项为队列研究。Meta分析结果显示,与无预防组相比,利福昔明组的SBP发病率(OR=0.36, 95%CI:0.14~0.96, P=0.04)、死亡率(OR=0.59, 95%CI:0.37~0.95, P=0.03)均明显下降;与诺氟沙星组相比,利福昔明组的SBP发病率(OR=0.39, 95%CI:0.25~0.62, P<0.001)、死亡率(OR=0.55, 95%CI:0.34~0.92, P=0.02)、不良反应(OR=0.36, 95%CI:0.22~0.59, P<0.001)均明显降低,根据预防类型进行亚组分析,两组在初级预防无显著差异(OR=0.56, 95%CI:0.23~1.35, P=0.20),二级预防时利福昔明组的SBP发病率(OR=0.18, 95%CI:0.08~0.43, P<0.001)明显降低。此外,还发现利福昔明可以明显降低肝肾综合征(OR=0.34, 95%CI:0.15~0.77, P=0.01)和肝性脑病(OR=0.55, 95%CI:0.32~0.95, P=0.03)的发生风险。 结论 利福昔明对SBP初级预防和二级预防安全有效,在二级预防时,利福昔明优于诺氟沙星,但仍需高质量多中心RCT进行验证。 -
关键词:
- 肝硬化 /
- 腹膜炎 /
- 利福昔明 /
- 诺氟沙星 /
- Meta分析(主题)
Abstract:Objective To evaluate the efficacy and safety of rifaximin in the prevention of spontaneous bacterial peritonitis (SBP). Methods CNKI, Wanfang Data, CBM, PubMed, Embase, and Cochrane Library were searched for randomized controlled trials (RCTs) and cohort studies on rifaximin in the prevention of SBP published up to July 5, 2020. The articles were screened according to the inclusion and exclusion criteria, and data extraction and quality assessment were performed. RevMan 5.3 software was used to conduct the meta-analysis. Results A total of 13 studies (with 2207 patients in total) were included, among which there were 6 RCTs and 7 cohort studies. The results of the meta-analysis showed that compared with the non-prevention group, the rifaximin group had significantly lower incidence rate of SBP (odds ratio [OR]=0.36, 95% confidence interval [CI]: 0.14-0.96, P=0.04) and mortality rate (OR=0.59, 95% CI: 0.37-0.95, P=0.03); compared with the norfloxacin group, the rifaximin group had significantly lower incidence rate of SBP (OR=0.39, 95% CI: 0.25-0.62, P < 0.001), mortality rate (OR=0.55, 95% CI: 0.34-0.92, P=0.02), and adverse reactions (OR=0.36, 95% CI: 0.22-0.59, P < 0.001). The subgroup analysis based on the type of prevention showed that there was no significant difference in primary prevention between the two groups (OR=0.56, 95% CI: 0.23-1.35, P=0.20), and in secondary prevention, the rifaximin group had a significantly lower incidence rate of SBP (OR=0.18, 95% CI: 0.08-0.43, P < 0.001). In addition, it was also found that rifaximin significantly reduced the incidence rate of hepatorenal syndrome (OR=0.34, 95% CI: 0.15-0.77, P=0.01) and hepatic encephalopathy (OR=0.55, 95% CI: 0.32-0.95, P=0.03). Conclusion Rifaximin is safe and effective for the primary and secondary prevention of SBP. Rifaximin is superior to norfloxacin in secondary prevention, which still needs to be confirmed by high-quality multicenter RCTs. -
Key words:
- Liver Cirrhosis /
- Peritonitis /
- Rifaximin /
- Norfloxacin /
- Meta-Analysis as Topic
-
全世界约有一半新诊断的肝细胞癌(hepatocellular cell carcinoma,HCC)病例发生在我国,其中HBV感染是主要危险因素,占全球HCC病例的50%~80%[1]。HBV通过多种机制在宿主细胞中持续存在,从而导致慢性HBV感染[2],并且诱导HCC发生[3]。超过80%的HCC在肝硬化基础上发展,这表明肝硬化在肝癌前环境中起着重要作用[4]。因此慢性HBV感染和肝硬化是肝癌发生的高风险因素。由于缺乏明显的症状和有效的筛查策略,80%的HCC患者被诊断时已为中晚期,其中仅有30%~40%患者符合当前有效治疗方案的条件[5],故在早期将慢性HBV感染、肝硬化与HCC区分诊断是延长患者生存期甚至根治HCC的关键。现急需一种检测方式能够在患有良性肝病的肝癌高危人群中早期筛查出HCC,从而降低病死率。影像学检查作为有效筛查方法被应用于临床,但是受限于设备以及检测人员。实验室检测则更能筛查大量人群,目前主要的早期实验室筛查方法为检测患者血清AFP,但单一指标检测的敏感度与特异度存在限制。考虑联合指标检测能够提高诊断效率,本文将探究AFP与GGT/AST联合检测在良性肝病和HCC区别诊断中发挥的作用,以期为临床诊断提供新的参考依据。
1. 资料与方法
1.1 研究对象
选取2019年1月15日—6月15日于本院诊治的慢性乙型肝炎患者(CHB组)、乙型肝炎肝硬化患者(LC组)、HBV相关HCC患者(HCC组),另选取同期健康体检者作为对照(HC组)。纳入标准:(1)HCC组患者HBsAg为阳性且诊断完全符合《原发性肝癌诊疗规范(2017年版)》[6]; (2)HCC组、CHB组和LC组患者均为首次诊断,在接受治疗前收集生化指标; (3)临床资料完整。排除标准:(1)排除HCC之外的恶性肿瘤; (2)排除HBV以外的其他肝炎病毒感染; (3)排除患有严重糖尿病、甲状腺亢进以及心血管疾病等患者; (4)排除妊娠患者。研究对象均自愿参与本项研究。
1.2 研究方法
采集所有研究对象空腹6~8 h后的静脉血5 ml,以转速3500 r/min(离心半径=16 cm)离心5 min,分离血清后-80 ℃冷冻备用。采用Siemens公司ADVIACENTUAR XP全自动化学发光免疫分析仪及配套试剂盒检测血清AFP水平,参考区间为0~8.0 ng/ml。使用Siemens公司ADVIA2400全自动生化分析仪检测AST、ALT、GGT水平,参考区间分别为15~40、5~40、10~60 U/L。以上操作严格依据说明书进行,所有检测均在试剂盒说明书规定时间内完成,且严格遵守试验相关操作规程。
1.3 伦理学审查
本研究方案经由武汉大学人民医院临床研究伦理委员会审批,批号:WDRY2018-K047。
1.4 统计学方法
采用SPSS 23.0软件、GraphPad Prism 6.0和MedCalc 18.2.1对数据进行统计分析。正态分布计量资料以x±s表示,多组间比较采用方差分析,进一步两两比较采用LSD-t检验。偏态分布计量资料以M(P25~P75)表示,多组间比较采用Kruskal-Wallis H检验,进一步两两比较采用Nemenyi检验。计数资料组间比较采用χ2检验。通过二元logistic回归分析,计算出预测变量,并绘制AFP、GGT/AST以及预测变量单独或联合检测的受试者工作特征曲线(ROC曲线),计算曲线下面积(AUC)及敏感度、特异度,采用Z检验对AUC进行比较。P<0.05为差异有统计学意义。
2. 结果
2.1 一般资料
共纳入研究对象352例,其中HC组86例,男42例,女44例,年龄23~82岁,平均(55.90±15.19)岁; CHB组68例,男38例,女30例,年龄22~77岁,平均(51.40±10.59)岁,HBV DNA阳性50例; LC组69例,男34例,女35例,年龄28~79岁,平均(53.49±11.35)岁,HBV DNA阳性36例,Child-Pugh A级25例,B级32例,C级12例; HCC组129例,男70例,女59例,年龄24~78岁,平均(54.60±11.17)岁,HBV DNA阳性50例,Child-Pugh A级79例,B级41例,C级9例,BCLC分期A期21例,B期31例,C期77例。4组研究对象年龄(F=1.455,P=0.227)与性别分布(χ2=1.346,P=0.718)差异均无统计学意义。
2.2 各指标检测结果比较
HCC组与HC组、CHB组、LC组之间患者的AFP、GGT/AST、GGT比较差异均有统计学意义(P值均<0.05)(表 1)。
表 1 各组AFP、GGT、AST及GGT/AST血清学水平比较组别 例数 AFP(ng/ml) GGT/AST GGT(U/L) AST(U/L) HC组 86 3.35(2.20~4.70) 0.98(0.71~1.36) 20.00(15.00~27.00) 21.00(18.00~24.00) CHB组 68 4.35(2.35~15.75) 0.82(0.46~1.25) 51.00(23.75~140.75)1) 61.00(34.25~125.00)1) LC组 69 8.60(2.70~54.20)1) 0.97(0.55~1.81) 69.00(27.00~113.00)1) 47.00(31.00~88.00)1) HCC组 129 157.10(9.90~6126.40)1)2)3) 2.00(1.19~3.11)1)2)3) 98.00(45.00~207.00)1)2)3) 46.00(29.00~88.00)1)2) H值 124.018 70.202 126.282 135.987 P值 <0.001 <0.001 <0.001 <0.001 注:与HC组比较,1)P<0.05;与CHB组比较,2)P<0.05;与LC组比较,3)P<0.05。 2.3 ROC曲线分析AFP与GGT/AST单独或联合检测诊断HCC的价值
采用二元logistic回归分析得到不同组别之间两种指标的联合回归模型,然后进一步绘制其ROC曲线(图 1),计算AUC(表 2),结果显示,在HCC组与LC组、HCC组与HC组+CHB组+LC组、HCC组与CHB组+LC组中,AFP与GGT/AST联合诊断的AUC均显著高于AFP单独诊断的AUC(Z值分别为2.684、2.241、2.415,P值分别为0.007、0.025、0.016)。
表 2 AFP与GGT/AST单独或联合检测在辅助HCC诊断中的价值分组 标志物 AUC 95%CI 敏感度(%) 特异度(%) P值 HCC组vs CHB组 AFP 0.803 0.742~0.864 72.9 72.1 <0.05 GGT/AST 0.789 0.717~0.861 67.4 83.8 <0.05 联合 0.846 0.790~0.910 79.8 77.9 <0.05 HCC组vs LC组 AFP 0.760 0.693~0.826 55.8 84.1 <0.05 GGT/AST 0.727 0.650~0.803 90.7 46.4 <0.05 联合 0.802 0.741~0.862 48.1 97.1 <0.05 HCC组vs HC组+CHB组+LC组 AFP 0.835 0.791~0.879 86.0 68.2 <0.05 GGT/AST 0.768 0.718~0.817 67.4 74.9 <0.05 联合 0.843 0.802~0.885 73.6 78.5 <0.05 HCC组vs CHB组+LC组 AFP 0.781 0.727~0.835 55.8 85.4 <0.05 GGT/AST 0.758 0.700~0.815 67.4 77.5 <0.05 联合 0.823 0.775~0.871 81.4 66.4 <0.05 3. 讨论
HCC的发生通常伴有前期的慢性HBV感染和肝硬化,在慢性HBV感染以及患有肝硬化的高危人群中,利用有效的筛查方式早期发现HCC至关重要,是患者可能获得根治的关键[7]。本文纳入352例研究对象,基于相关纳排标准将其分为4组,统计分析结果发现HCC组与HC组、CHB组、LC组AFP和GGT/AST比较,差异均有统计学意义(P值均<0.05),此结果与以往研究结果[8]相符。
AFP作为最早被发现的蛋白肿瘤标志物之一[9],其可反映肝功能情况,因此广泛用于实验室检查。综合全球多种指南,AFP在我国可作为良好的血清学指标[10],但其特异度低,易引起误判[11]。在CHB、LC患者中AFP水平也会升高,因此鉴别诊断HBV相关HCC患者时,AFP具有一定局限性,且部分HBV相关HCC患者的AFP水平并未达到筛查标准,因此容易漏查[12]。
GGT在哺乳动物组织中广泛分布,是一种质膜结合蛋白,血清中的GGT主要来源于肝胆系统[13]。已有研究[14]表明GGT通过诱导DNA损伤来促进肿瘤进展和不良预后,释放活性氧以激活与入侵相关的信号通路,与HCC发展关联密切。AST主要分布于肝细胞线粒体内,当肝脏严重病变坏死时,血清中AST水平会显著升高[15],作为肝功能检查常用生化指标,GGT与AST在临床中应用广泛[16]。本研究计算GGT/AST比值并发现其单独或联合AFP时在HBV相关HCC的诊断中具有一定价值,且该指标计算简单,不用另增检验项目,便于在HCC诊断过程中应用。
本研究ROC曲线分析结果显示,在HCC组与LC组中,GGT/AST联合AFP后AUC明显提高,表明GGT/AST与AFP联合在鉴别HCC与LC时有良好的诊断效果; 在HCC组与HC组+CHB组+LC组以及CHB组+LC组分别区别诊断时,二者联合的AUC均明显高于AFP单独诊断的AUC,进一步说明GGT/AST联合AFP在鉴别HCC与良性肝病患者中有较好的诊断价值。
综上所述,本研究表明GGT/AST联合AFP在HBV相关HCC的临床诊断中具有一定价值,提高了AFP的单独诊断效率。由于本研究样本量较小,因此相关研究结果仍需未来增加样本量予以验证。
-
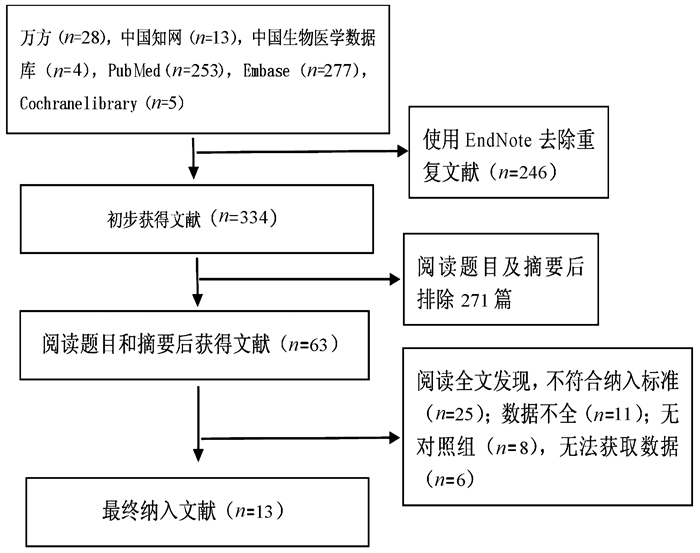
表 1 纳入文献基本信息
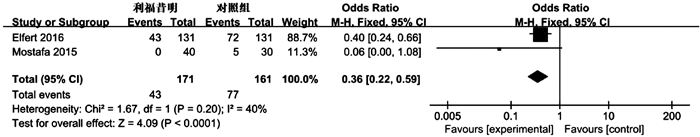
作者、时间及国家 研究类型 预防级别 试验组 对照组 随访时间 结局指标 病因 Child评分或A/B/C 年龄(岁) 例数 干预措施 病因 Child评分或A/B/C 年龄(岁) 例数 干预措施 Elfert 2016[8]埃及 RCT 二级 未提供 0/58/73 53.8±7.5 131 利福昔明1200 mg/d 未提供 0/63/68 54.1±7.1 131 诺氟沙星400 mg/d 6个月 ①②④⑤⑥ Assem 2016[9]埃及 RCT 初级 90%以上为丙型肝炎 10.2±3.1 55±18 82 利福昔明1100 mg/d 90%以上为丙型肝炎 10.1±1.6 58±15 78 诺氟沙星400 mg/d 6个月 ①②③⑤⑦ Mostafa 2015[10]埃及 RCT 二级 丙型肝炎 10.3±1.1 55.8±4.8 40 利福昔明800 mg/d 丙型肝炎 10.7±1.8 56.5±4.1 30 诺氟沙星400 mg/d 6个月 ①⑥⑦ Praharaj(1) 2017[11]印度 RCT 初级 未提供 未提供 38.8±8.8 28 利福昔明1100 mg/d 未提供 未提供 37.7±8.7 30 诺氟沙星400 mg/d 6个月 ① Praharaj(2) 2017[11]印度 二级 未提供 26 未提供 33 ① Shamseya 2015[12]埃及 队列研究 初级和二级 丙型肝炎 11.5±2.1 52.7±8.5 43 利福昔明1200 mg/d 丙型肝炎 11.5±2 50.3±9.0 43 诺氟沙星400 mg/d 12个月 ①②③④⑤⑥ Kumar 2019[13]巴基斯坦 RCT 未知 未提供 未提供 38.8±8.8 122 利福昔明1200 mg/d 未提供 未提供 37.7±8.7 122 诺氟沙星400 mg/d 6个月 ① Flamm 2018[14]美国 RCT 初级和二级 未提供 未提供 55.5 140 利福昔明1100 mg/d 未提供 未提供 56.8 159 安慰剂 6个月 ①②③④ Hanouneh 2012[15]美国 队列研究 初级 未提供 11 55 49 利福昔明1200 mg/d 未提供 10 55 355 无 4.2个月 ①⑤⑥ Lutz 2014[16]德国 队列研究 初级和二级 74%酒精性肝炎,19%病毒性肝炎 未提供 61 27 利福昔明1200 mg/d 57%酒精性肝炎,27%病毒性肝炎 未提供 60 108 无 4周 ①⑥ Vlachogiannakos 2013[17]希腊 队列研究 初级 酒精性肝炎 0/11/12 64.2±9.0 23 利福昔明1200 mg/d 酒精性肝炎 0/22/24 64.1±9.1 46 无 5年 ①②③④⑤ Shokoohi 2013[18]美国 队列研究 初级 未提供 未提供 53.5 79 利福昔明 未提供 未提供 58.2 60 无 20.2个月 ① Dong 2016[19]美国 队列研究 未知 酒精性及丙型肝炎共占60%以上 未提供 59.5 88 利福昔明550 mg/d 酒精性及丙型肝炎共占70%以上 未提供 57.5 88 无 3个月 ①③⑤ Danulescu 2013[20]罗马尼亚 队列研究 未知 未提供 0/0/22 未提供 22 利福昔明 未提供 0/0/24 未提供 24 无 6个月 ① 注:①SBP发生率;②消化道出血;③HRS;④HE;⑤死亡率;⑥腹水细菌培养;⑦不良反应。 表 2 RCT文献质量评价
纳入文献 随机方法 分配隐藏 盲法 资料完整 选择报告偏倚 其他偏倚 Jadad评分 Assem 2016 计算机随机序列 不透明的密封信封 不清楚 完整 无 无 5 Mostafa 2015 随机 不清楚 单盲 完整 无 无 5 Elfert 2016 计算机随机序列 不透明的密封信封 不清楚 完整 无 无 5 Praharaj 2017 随机 不清楚 不清楚 完整 无 无 3 Flamm 2018 随机 不清楚 不清楚 完整 无 无 3 Kumar 2019 随机 不清楚 不清楚 完整 无 无 3 表 3 队列研究文献质量评价
纳入文献 队列的选择 可比性 结果 NOS评分 暴露组代表性 非暴露组代表性 暴露因素确定方法 确定结局指标 基于设计所得队列的可比性 评价是否充分 随访是否充分 随访完整性 Shamseya 2015 1 1 1 1 2 1 1 1 9 Hanouneh 2012 1 1 1 1 1 1 1 1 8 Lutz 2014 1 1 1 1 1 1 0 0 6 Vlachogiannakos 2013 1 1 1 1 2 1 1 1 8 Shokoohi 2013 1 1 1 1 1 1 1 0 7 Dong 2016 1 1 1 1 1 1 1 1 8 Danulescu 2013 0 0 1 1 1 1 1 1 6 表 4 利福昔明对其他肝硬化并发症Meta分析结果
并发症 预防措施 纳入研究 异质性分析 效应模型 OR 95%CI Z值 P值 I2(%) P值 消化道出血 利福昔明vs无 [14][17] 0 0.33 固定 0.46 0.19~1.13 1.68 0.09 利福昔明vs诺氟沙星 [8][9][12] 0 0.93 固定 0.99 0.49~2.01 0.02 0.99 HRS 利福昔明vs无 [14][17][19] 0 0.77 固定 0.34 0.15~0.77 2.58 0.01 利福昔明vs诺氟沙星 [9][12] 0 0.47 固定 0.40 0.10~1.57 1.31 0.19 HE 利福昔明vs无 [14][17] 0 0.52 固定 0.55 0.32~0.95 2.13 0.03 利福昔明vs诺氟沙星 [8][12] 0 0.70 固定 0.35 0.15~0.82 2.43 0.01 表 5 利福昔明关于死亡原因Meta分析
表 6 利福昔明与对照组SBP腹水细菌培养信息
研究 预防级别 试验组 对照组 SBP 阳性 G+ G- 耐药 SBP 阳性 G+ G- 耐药 Mostafa 2015 二级 0 0 0 0 不清楚 5 5 5 0 不清楚 Elfert 2016 二级 4 2 0 2 2例诺氟沙星 13 8 4 4 8例诺氟
沙星耐药Shamseya 2015 初级和二级 2 0 0 0 不清楚 6 1 0 1 不清楚 Hanouneh 2012 初级 5 0 0 0 不清楚 113 35 18 17 不清楚 Lutz 2014 初级和二级 8 4 0 4 1例三代头孢,1例多重耐药 24 11 4 7 5例三代头孢耐药,1例多重耐药 -
[1] DEVER JB, SHEIKH MY. Review article: Spontaneous bacterial peritonitis-bacteriology, diagnosis, treatment, risk factors and prevention[J]. Aliment Pharmacol Ther, 2015, 41(11): 1116-1131. DOI: 10.1111/apt.13172 [2] FERNANDEZ J, NAVASA M, PLANAS R, et al. Primary prophylaxis of spontaneous bacterial peritonitis delays hepatorenal syndrome and improves survival in cirrhosis[J]. Gastroenterology, 2007, 133(3): 818-824. DOI: 10.1053/j.gastro.2007.06.065 [3] European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis[J]. J Hepatol, 2010, 53(3): 397-417. DOI: 10.1016/j.jhep.2010.05.004 [4] TANDON P, DELISLE A, TOPAL JE, et al. High prevalence of antibiotic-resistant bacterial infections among patients with cirrhosis at a US liver center[J]. Clin Gastroenterol Hepatol, 2012, 10(11): 1291-1298. DOI: 10.1016/j.cgh.2012.08.017 [5] GOEL A, RAHIM U, NGUYEN LH, et al. Systematic review with meta-analysis: Rifaximin for the prophylaxis of spontaneous bacterial peritonitis[J]. Aliment Pharmacol Ther, 2017, 46(11): 1029-1036. [6] MARCIANO S, DIRCHWOLF M, DIAZ JM, et al. Spontaneous bacterial peritonitis recurrence in patients with cirrhosis receiving secondary prophylaxis with norfloxacin[J]. Eur J Gastroenterol Hepatol, 2019, 31(4): 540-546. DOI: 10.1097/MEG.0000000000001331 [7] OLIVER A, WONG M, SANCHEZ C. Role of rifaximin in spontaneous bacterial peritonitis prevention[J]. South Med J, 2018, 111(11): 660-665. DOI: 10.14423/SMJ.0000000000000887 [8] ELFERT A, ABO ALIL, SOLIMAN S, et al. Randomized-controlled trial of rifaximin versus norfloxacin for secondary prophylaxis of spontaneous bacterial peritonitis[J]. Eur J Gastroenterol Hepatol, 2016, 28(12): 1450-1454. DOI: 10.1097/MEG.0000000000000724 [9] ASSEM M, ELSABAAWY M, ABDELRASHED M, et al. Efficacy and safety of alternating norfloxacin and rifaximin as primary prophylaxis for spontaneous bacterial peritonitis in cirrhotic ascites: A prospective randomized open-label comparative multicenter study[J]. Hepatol Int, 2016, 10(2): 377-385. DOI: 10.1007/s12072-015-9688-z [10] MOSTAFA T, BADRA G, ABDALLAH M. The efficacy and the immunomodulatory effect of rifaximin in prophylaxis of spontaneous bacterial peritonitis in cirrhotic Egyptian patients[J]. Turk J Gastroenterol, 2015, 26(2): 163-169. DOI: 10.5152/tjg.2015.7782 [11] PRAHARAJ D, TANEJA S, DUSEJA A, et al. Randomized control trial of rifaximin and norfloxacin in primary and secondary prophylaxis of spontaneous bacterial peritonitis (SBP) in cirrhotic patients[J]. J Clin Exp Hepatol, 2017, 7(S2): s71. [12] SHAMSEYA MM, MADKOUR MA. Rifaximin: A reasonable alternative for norfloxacin in the prevention of spontaneous bacterial peritonitis in patients with HCV-related liver cirrhosis[J]. Alex J Med, 2015, 52(3): 219-226. [13] KUMAR A, SHAIKH BA, SHAIKH ZA, et al. Effectiveness of rifaximin versus norfloxacin in prevention of spontaneous bacterial peritonitis in cirrhotic patients[J]. Med Forum, 2019, 30(8): 90-94. [14] FLAMM SL, MULLEN KD, HEIMANSON Z, et al. Rifaximin has the potential to prevent complications of cirrhosis[J]. Therap Adv Gastroenterol, 2018, 11: 1-10. [15] HANOUNEH MA, HANOUNEH IA, HASHASH JG, et al. The role of rifaximin in the primary prophylaxis of spontaneous bacterial peritonitis in patients with liver cirrhosis[J]. J Clin Gastroenterol, 2012, 46(8): 709-715. DOI: 10.1097/MCG.0b013e3182506dbb [16] LUTZ P, PARCINA M, BEKEREDJIAN-DING I, et al. Impact of rifaximin on the frequency and characteristics of spontaneous bacterial peritonitis in patients with liver cirrhosis and ascites[J]. PLoS One, 2014, 9(4): e93909. DOI: 10.1371/journal.pone.0093909 [17] VLACHOGIANNAKOS J, VIAZIS N, VASIANOPOULOU P, et al. Long-term administration of rifaximin improves the prognosis of patients with decompensated alcoholic cirrhosis[J]. J Gastroenterol Hepatol, 2013, 28(3): 450-455. DOI: 10.1111/jgh.12070 [18] SHOKOOHI S, ZIVONY A, LE D, et al. Rifaximin is associated with decreased incidence of spontaneous bacterial peritonitis in cirrhotics with ascites[J]. Hepatology, 2013, 58(s1): 858a. [19] DONG T, ARONSOHN A, REDDY KG, et al. Rifaximin decreases the incidence and severity of acute kidney injury and hepatorenal syndrome in cirrhosis[J]. Dig Dis Sci, 2016, 61(12): 3621-3626. DOI: 10.1007/s10620-016-4313-0 [20] DANULESCU RM, CIOBICA A, STANCIU C, et al. The role of rifaximine in the prevention of the spontaneous bacterial peritonitis[J]. Rev Med Chir Soc Med Nat Iasi, 2013, 117(2): 315-320. [21] MENSHAHAWY A, MATTAR O, BARSSOUM K, et al. Safety and efficacy of rifaximin in prophylaxis of spontaneous bacterial peritonitis: A systematic review and Meta-analysis[J]. Curr Drug Targets, 2019, 20(4): 380-387. DOI: 10.2174/1389450119666180924145156 [22] SIDHU GS, GO A, ATTAR BM, et al. Rifaximin versus norfloxacin for prevention of spontaneous bacterial peritonitis: A systematic review[J]. BMJ Open Gastroenterol, 2017, 4(1): e000154. DOI: 10.1136/bmjgast-2017-000154 [23] FIORE M, MARAOLO AE, GENTILE I, et al. Current concepts and future strategies in the antimicrobial therapy of emerging Gram-positive spontaneous bacterial peritonitis[J]. World J Hepatol, 2017, 9(30): 1166-1175. DOI: 10.4254/wjh.v9.i30.1166 [24] SALEHI S, TRANAH TH, LIM S, et al. Rifaximin reduces the incidence of spontaneous bacterial peritonitis, variceal bleeding and all-cause admissions in patients on the liver transplant waiting list[J]. Aliment Pharmacol Ther, 2019, 50(4): 435-441. DOI: 10.1111/apt.15326 [25] KAMAL F, KHAN MA, KHAN Z, et al. Rifaximin for the prevention of spontaneous bacterial peritonitis and hepatorenal syndrome in cirrhosis[J]. Eur J Gastroenterol Hepatol, 2017, 29(10): 1109-1117. DOI: 10.1097/MEG.0000000000000940 [26] NAVASA M, FOLLO A, FILELLA X, et al. Tumor necrosis factor and interleukin-6 in spontaneous bacterial peritonitis in cirrhosis: Relationship with the development of renal impairment and mortality[J]. Hepatology, 1998, 27(5): 1227-1232. DOI: 10.1002/hep.510270507 [27] BAJAJ JS. Review article: Potential mechanisms of action of rifaximin in the management of hepatic encephalopathy and other complications of cirrhosis[J]. Aliment Pharmacol Ther, 2016, 43(S1): 11-26. [28] KIMER N, KRAG A, MOLLER S, et al. Systematic review with meta-analysis: The effects of rifaximin in hepatic encephalopathy[J]. Aliment Pharmacol Ther, 2014, 40(2): 123-132. DOI: 10.1111/apt.12803 [29] LYU XY, LI L. ClinicaI effect of rifaximin in treatment of complications associated with Iiver cirrhosis[J]. J Clin Hepatol, 2018, 34(7): 1551-1554.(in Chinese) DOI: 10.3969/j.issn.1001-5256.2018.07.040吕新月, 李磊. 利福昔明在肝硬化相关并发症中的应用[J]. 临床肝胆病杂志, 2018, 34(7): 1551-1554. DOI: 10.3969/j.issn.1001-5256.2018.07.040 期刊类型引用(3)
1. 姚爱武,廖和壁,张璟. 血清AFP、AFP-L3与肝细胞癌经肝动脉化疗栓塞术后疗效的关系分析. 分子诊断与治疗杂志. 2023(04): 690-693+698 .  百度学术
百度学术2. 朱富平,刘红强,常清,冷伟业. miR-222-3p在乙型肝炎病毒相关肝细胞癌血清外泌体中的表达及临床意义. 局解手术学杂志. 2022(08): 702-708 .  百度学术
百度学术3. 高武林,韦超,郭晓烨. 术前血清MMP-9水平对HBV相关肝细胞癌患者肝切除术后生存的预测作用. 东南大学学报(医学版). 2022(05): 652-659 .  百度学术
百度学术其他类型引用(2)
-




 PDF下载 ( 2830 KB)
PDF下载 ( 2830 KB)

 下载:
下载:

 下载:
下载:

 百度学术
百度学术


