利用CRISPR/Cas9慢病毒载体构建敲除大鼠肝星状细胞COX-2基因的细胞模型
DOI: 10.3969/j.issn.1001-5256.2021.02.018
Application of CRISPR/Cas9 lentiviral vector in construction of rat hepatic stellate cells with COX-2 gene knockout
-
摘要:
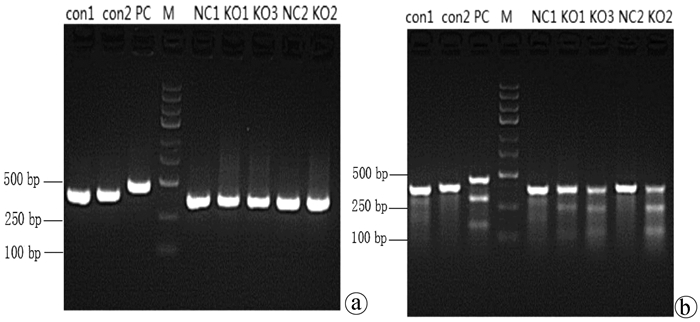
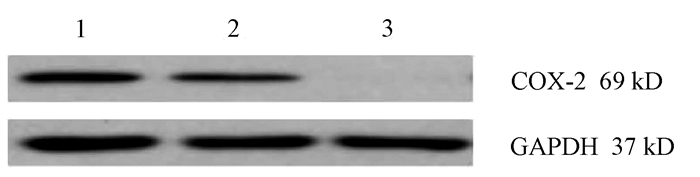
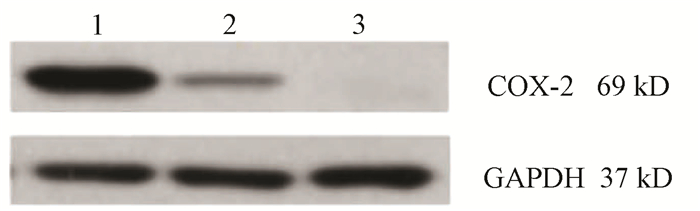
目的 通过构建CRISPR/Cas9慢病毒载体,转染HSC-T6细胞,获得能稳定表达Cas9蛋白的HSC-T6细胞和COX-2基因缺陷的HSC-T6-COX-2-/-细胞,为后期的功能研究提供良好的工具和手段,为临床上治疗肝纤维化提供新策略。 方法 设计合成COX-2基因特异性的sgRNA(COX-2-sgRNA-1、COX-2-sgRNA-2、COX-2-sgRNA-3),并将其连接至GV371载体上,提取重组后的质粒,将其与包装质粒共同转染到293T细胞,形成慢病毒颗粒,荧光法检测病毒滴度。按照MOI值计算病毒最适用量,先将Lenti-Cas9-puro转染至HSC-T6细胞,嘌呤霉素筛选得到HSC-T6-Cas9细胞,再用Lenti-COX-2-sgRNA-EGFP转染至HSC-T6-Cas9细胞获得HSC-T6-COX-2-/-细胞,通过Cruiser酶切检测及Western Blot等方法在基因和蛋白水平上进行敲除验证。计量资料多组间比较采用方差分析,进一步两两比较采用LSD-t检验。 结果 通过测序验证COX-2-sgRNA表达载体构建成功。重组表达质粒和包装质粒共同转染到293T细胞,形成慢病毒颗粒,荧光法检测病毒滴度均在1×108以上。成功构建能稳定传代的Cas9蛋白的HSC-T6细胞和COX-2基因缺陷的HSC-T6-COX-2-/-细胞模型。HSC-T6-Cas9细胞中LV-Cas9-Puro mRNA相对表达量(541.93±105.76)高于CON组细胞(1.00±0.02),差异具有统计学意义(t=12.995,P<0.01)。通过Cruiser酶切检测及Western Blot试验,结果提示CRISPR/Cas9慢病毒表达载体能在靶点起作用,其中COX-2-sgRNA-2敲除作用最明显,并且COX-2蛋白表达水平较CON组和NC组相比显著下降(P值均<0.05),提示COX-2-sgRNA有活性。 结论 成功构建了针对COX-2靶基因的CRISPR/Cas9慢病毒载体,获得稳定的COX-2基因敲除的HSC-T6-COX-2-/-细胞。 -
关键词:
- 肝硬化 /
- 规律成簇间隔短回文重复序列 /
- 慢病毒感染 /
- 基因敲除技术
Abstract:Objective To obtain HSC-T6 cells with stable expression of Cas9 protein and HSC-T6-COX-2-/- cells with COX-2 gene defect by transfecting HSC-T6 cells with CRISPR/Cas9 lentiviral vector, and to provide a good method for further functional research and new strategies for the clinical treatment of liver fibrosis. Methods The COX-2 gene-specific sgRNAs (COX-2-sgRNA-1, COX-2-sgRNA-2, COX-2-sgRNA-3) were designed, synthesized, and connected to the GV371 vector, and the recombinant plasmid and the packaging plasmid were transfected into 293T cells to form lentivirus particles; the fluorescence method was used to measure virus titer. The most appropriate amount of the virus was calculated based on MOI. Lenti-Cas9-puro was transfected into HSC-T6 cells, and HSC-T6-Cas9 cells were screened out by puromycin; Lenti-COX-2-sgRNA-EGFP was transfected into HSC-T6-Cas9 cells to obtain HSC-T6-COX-2-/- cells. Cruiser enzyme digestion and Western blot were used to verify gene knockout at the gene and protein levels. An analysis of variance was used for comparison of continuous data between multiple groups, and the least significant difference t-test was used for further comparison between two groups. Results Sequencing verified that the COX-2-sgRNA expression vector was constructed successfully. Recombinant expression plasmids and packaging plasmids were transfected into 293T cells to form lentivirus particles, and the fluorescence method showed a virus titer of > 1×108. HSC-T6 cells with stable expression of Cas9 protein and HSC-T6-COX-2-/- cells with COX-2 gene defect were successfully constructed. The HSC-T6-Cas9 group had significantly higher relative mRNA expression of LV-Cas9-Puro than the CON group (541.93±105.76 vs 1.00±0.02, t=12.995, P < 0.01). Cruiser enzyme digestion and Western blot showed that the CRISPR/Cas9 lentivirus expression vectors played a role in the target, among which COX-2-sgRNA-2 knockout had the most significant effect, and this group had a significant reduction in the protein expression level of COX-2 compared with the CON group and the NC group (both P < 0.05), suggesting that COX-2-sgRNA was active. Conclusion A CRISPR/Cas9 lentivirus vector is successfully constructed for COX-2 target gene, and HSC-T6-COX-2-/- cells with stable COX-2 gene knockout are obtained. -
表 1 LV-Cas9-Puro基因的引物序列
基因 引物序列(5′-3′) 扩增片段大小(bp) LV-Cas9-Puro 上游GCTGAAAACCTATGCCCACC 133 下游GATTGTCTTGCCGGACTGCT GAPDH 上游TTCAACGGCACAGTCAAGG 114 下游CTCAGCACCAGCATCACC 表 2 PCR引物序列
引物名称 引物序列(5′-3′) COX-2-E1-SVF AGTGACTGACAGGCTGTCTTGTC COX-2-E1-SVR CACAATGTTCCAGACTCCCTTG COX-2-E2-SVF GTGAAAACTGTACTACGCGTAAG COX-2-E2-SVR CTTCAAGGCGTTTTGAAAAGGTC COX-2-E3-SVF AGTGACTGACAGGCTGTCTTGTC COX-2-E3-SVR CACAATGTTCCAGACTCCCTTG 表 3 转染72 h、96 h各组COX-2蛋白质相对表达情况
组别 72 h COX-2 96 h COX-2 CON组 0.57±0.02 1.14±0.21 KO组 0.11±0.021) 0.09±0.031) NC组 0.55±0.022) 0.49±0.152) F值 586.926 143.643 P值 <0.01 <0.01 注:与CON组比较,1)P<0.01;与KO组比较,2)P<0.01。 -
[1] DUVAL F, MORENO-CUEVAS JE, GONZÁLEZ-GARZA MT, et al. Liver fibrosis and mechanisms of the protective action of medicinal plants targeting inflammation and the immune response[J]. Int J Inflam, 2015, 2015: 943497. [2] XIE AZ, LYU C, SHI QL, et al. Research progress on prevention and treatment of liver fibrosis by traditional Chinese medicine[J]. China Med Herald, 2020, 17(17): 34-37. (in Chinese) https://www.cnki.com.cn/Article/CJFDTOTAL-YYCY202017009.htm谢爱泽, 吕超, 石清兰, 等. 中医药防治肝纤维化机制的研究进展[J]. 中国医药导报, 2020, 17(17): 34-37. https://www.cnki.com.cn/Article/CJFDTOTAL-YYCY202017009.htm [3] YIN W, YANG XF. Effects of phospholipase Cγ and cyclooxygenase-2 on the proliferation of hepatic stellate cells[J]. Mod Med Health, 2014, 30(3): 389-391. (in Chinese) DOI: 10.3969/j.issn.1009-5519.2014.03.030尹微, 阳学风. 磷脂酶Cγ和环氧合酶-2对肝星状细胞增殖的影响[J]. 现代医药卫生, 2014, 30(3): 389-391. DOI: 10.3969/j.issn.1009-5519.2014.03.030 [4] XIA HS, CHEN SR, ZHONG YC, et al. Current status of pathogenesis and drug treatment of liver fibrosis[J]. China Med Herald, 2014, 11(18): 162-165, 168.(in Chinese) https://www.cnki.com.cn/Article/CJFDTOTAL-YYCY201418051.htm夏海珊, 陈少茹, 钟月春, 等. 肝纤维化的发病机制和药物治疗现况[J]. 中国医药导报, 2014, 11(18): 162-165, 168. https://www.cnki.com.cn/Article/CJFDTOTAL-YYCY201418051.htm [5] CHENG J, IMANISHI H, ⅡJIMA H, et al. Expression of cyclooxygenase 2 and cytosolic phospholipase A(2) in the liver tissue of patients with chronic hepatitis and liver cirrhosis[J]. Hepatol Res, 2002, 23(3): 185-195. DOI: 10.1016/S1386-6346(01)00177-2 [6] TANG SH, GAO JH, WEN SL, et al. Expression of cyclooxygenase-2 is correlated with lncRNA-COX-2 in cirrhotic mice induced by carbon tetrachloride[J]. Mol Med Rep, 2017, 15(4): 1507-1512. DOI: 10.3892/mmr.2017.6161 [7] WANG CJ.Effect of aspirin on proliferation and activation of cultured HSC-T6 cells and its molecular mechanism[D]. Changsha: Central South University, 2012. (in Chinese)王春江. 阿司匹林对培养的HSC-T6细胞增殖与活化的影响及分子机制研究[D]. 长沙: 中南大学, 2012. [8] ZHANG S. COX-2 regulates the proliferation of HSC-T6 cells and the expression of ACSL family genes through AA[D]. Hengyang: The University of South China, 2014. (in Chinese)章烁. COX-2通过AA对HSC-T6细胞增殖及Acsl家族基因表达的调控[D]. 衡阳: 南华大学, 2014. [9] BITENCOURT S, de MESQUITA FC, CABERLON E, et al. Capsaicin induces de-differentiation of activated hepatic stellate cell[J]. Biochem Cell Biol, 2012, 90(6): 683-690. DOI: 10.1139/o2012-026 [10] MOU H, SMITH JL, PENG L, et al. CRISPR/Cas9-mediated genome editing induces exon skipping by alternative splicing or exon deletion[J]. Genome Biol, 2017, 18(1): 108. DOI: 10.1186/s13059-017-1237-8 [11] HUANG XL, LIU YY, CONG M. Research advances in drug carrier systems targeting hepatic stellate cells[J]. J Clin Hepatol, 2020, 36(1): 208-212. (in Chinese) DOI: 10.3969/j.issn.1001-5256.2020.01.050黄小莉, 刘莹莹, 丛敏. 以肝星状细胞为靶向的药物载体系统[J]. 临床肝胆病杂志, 2020, 36(1): 208-212. DOI: 10.3969/j.issn.1001-5256.2020.01.050 [12] CHEN Y, WANG Y, YANG Y, et al. A molecular-logic gate for COX-2 and NAT based on conformational and structural changes: Visualizing the progression of liver disease[J]. Chem Sci, 2020, 11(24): 6209-6216. DOI: 10.1039/D0SC00574F [13] HE J, LIAO HW, YANG XF. Construction of PSCSI-GFP lentiviral for the endothelin-converting enzyme-like 1 gene[J]. J Clin Hepatol, 2019, 35(6): 1286-1292. (in Chinese) DOI: 10.3969/j.issn.1001-5256.2019.06.021何剑, 廖红伍, 阳学风. ECEL1基因PSCSI-GFP慢病毒载体的构建[J]. 临床肝胆病杂志, 2019, 35(6): 1286-1292. DOI: 10.3969/j.issn.1001-5256.2019.06.021 [14] LE C, RAN FA, COX D, et al. Multiplex genome engineering using CRISPR/Cas systems[J]. Trends in Genetics Tig, 2013, 32(12): 815. [15] RAN FA, HSU PD, WRIGHT J, et al. Genome engineering using the CRISPR-Cas9 system[J]. Nat Protoc, 2013, 8(11): 2281-2308. DOI: 10.1038/nprot.2013.143 [16] KONERMANN S, BRIGHAM MD, TREVINO AE, et al. Genome-scale transcriptional activation by an engineered CRISPR-Cas9 complex[J]. Nature, 2015, 517(7536): 583-588. DOI: 10.1038/nature14136 [17] LI SN, WANG MX, HU WD, et al. Construction and identification of miR-186 over expression lentiviral vector[J]. J Jilin Univ(Med Edit), 2019, 45(5):997-1002, 1193. (in Chinese) https://www.cnki.com.cn/Article/CJFDTOTAL-BQEB201905004.htm李胜男, 王梦旭, 胡伟东, 等. miR-186过表达慢病毒载体的构建及鉴定[J]. 吉林大学学报(医学版), 2019, 45(5): 997-1002, 1193. https://www.cnki.com.cn/Article/CJFDTOTAL-BQEB201905004.htm [18] VOIT RA, McMAHON MA, SAWYER SL, et al. Generation of an HIV resistant T-cell line by targeted "stacking" of restriction factors[J]. Mol Ther, 2013, 21(4): 786-795. DOI: 10.1038/mt.2012.284 -



 PDF下载 ( 6689 KB)
PDF下载 ( 6689 KB)


 下载:
下载: