沉默促癌基因Yap1联合丹参酮ⅡA对肝癌细胞Huh-7的影响
DOI: 10.3969/j.issn.1001-5256.2021.02.020
Effect of oncogene Yap1 silencing combined with tanshinone ⅡA on Huh-7 hepatoma cells
-
摘要:
目的 研究基因Yap1与丹参酮ⅡA对肝癌细胞Huh-7增殖、迁移侵袭的影响。 方法 选用武汉大学中南医院10对人肝细胞癌及癌旁组织标本,收集时间2019年6月1日—2019年12月1日。实时荧光定量法和蛋白质印迹法用于检测基因Yap1的表达以及表型相关分子的变化。采用MTT细胞增殖检测试剂检测不同浓度丹参酮ⅡA处理后细胞的增殖抑制率。蛋白质印迹实验检测不同干预下细胞凋亡、迁移相关标志物表达变化。流式细胞检测和Transwell实验分别检测细胞凋亡和细胞迁移以及侵袭。计量资料两组间比较采用t检验;计数资料两组间比较采用χ2检验。 结果 10对人肝癌组织标本中有8对Yap1的表达明显高于癌旁组织。相对于人正常肝上皮细胞L02,Yap1在肝癌细胞株Huh-7和HCCL-M3中表达增高。si-Yap1-3转染细胞蛋白水平沉默效率达87.004%。MTT结果显示丹参酮ⅡA能够有效抑制Huh-7细胞增殖,其半数抑制浓度为8.683 μmol/L。si-Yap1-3与丹参酮ⅡA联合处理Huh-7肝癌细胞,下游增殖迁移标志物E-钙粘蛋白表达增加,波形蛋白表达降低。Transwell实验结果示,相较于si-NC组,丹参酮ⅡA联合si-Yap1-3处理组细胞迁移、侵袭能力明显降低(43.19±2.88 vs 132.20±10.03,t=8.527,P=0.001;53.95±4.20 vs 179.10±11.11,t=4.484,P=0.011)。si-Yap1-3与丹参酮ⅡA联合处理组细胞凋亡相关标志物Bax表达增加,Bcl-2蛋白表达降低,且联合处理组细胞早期凋亡率为25.98%,高于si-NC组的9.21%(χ2=4.078,P<0.05)。 结论 沉默促癌基因Yap1联合丹参酮ⅡA能够促进肝癌细胞Huh-7凋亡,并抑制其迁移侵袭,为临床用药提供一定的指导意义。 Abstract:Objective To investigate the effect of the Yap1 gene and tanshinone ⅡA on the proliferation, migration, and invasion abilities of Huh-7 hepatoma cells. Methods A total of 10 pairs of human hepatocellular carcinoma (HCC) samples and adjacent tissue samples were collected in Zhongnan Hospital of Wuhan University from June 1 to December 1, 2019. Quantitative real-time PCR and Western blotting were used to measure the expression of the Yap1 gene and phenotype-related molecules. MTT cell proliferation detection reagent was used to measure the inhibition rate of cell proliferation after the treatment with different concentrations of tanshinone ⅡA. Western blotting was used to measure the changes in the expression of apoptosis-and migration-related markers after different interventions. Flow cytometry and Transwell assay were used to measure apoptosis and cell migration and invasion abilities. The data of 375 cases of liver cancer and 50 cases of relatively normal liver tissue samples were downloaded from The Cancer Genome Atlas, including clinicopathological information. The t-test was used for comparison of continuous data between two groups, and the chi-square test was used for comparison of categorical data between two groups. Results In 8 of the 10 pairs of HCC samples and adjacent tissue samples, HCC samples had significantly higher expression of Yap1 than the adjacent tissue samples. Compared with the normal human liver epithelial cells L02, the Huh-7 and HCCL-M3 hepatoma cells had a significant increase in the expression of Yap1. The silencing efficiency of si-Yap1-3 transfection reached 87.004% at the protein level. MTT results showed that tanshinone ⅡA effectively inhibited the proliferation of Huh-7 cells, with a half inhibitory concentration of 8.683 μmol/L. After the cells were treated with si-Yap1-3 and tanshinone ⅡA, there was an increase in the expression of the downstream marker for proliferation and migration E-cadherin and a reduction in the expression of vimentin, and the results of Transwell assay showed that compared with the si-NC group, the tanshinone ⅡA+si-Yap1-3 group had significant reductions in the migration and invasion abilities of Huh-7 cells (migration: 43.19±2.88 vs 132.20±10.03, t=8.527, P=0.001; invasion: 53.95±4.20 vs 179.10±11.11, t=4.484, P=0.011). The group treated with si-Yap1-3 and tanshinone ⅡA had an increase in the expression of the apoptosis-related marker Bax and a reduction in the expression of Bcl-2, as well as a significantly higher early apoptosis rate than the si-NC group (25.98% vs 9.21%, χ2=4.078, P < 0.05). Conclusion Oncogene Yap1 silencing combined with tanshinone ⅡA can promote the apoptosis of Huh-7 hepatoma cells and inhibit their migration and invasion, which can provide certain guiding significance for clinical medication. -
Key words:
- Carcinoma, Hepatocellular /
- Gene Silencing /
- TANSHINONE
-
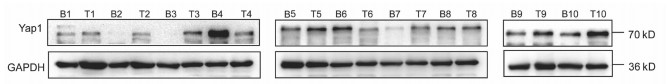
肝细胞癌具有新发病例多、死亡率高、预后极差的特征,是全球癌症相关死亡的主要事件[1]。促癌基因Yap1作为Hippo通路的经典核效应器,在细胞增殖、分化等多种生物进程中发挥重要作用的同时,在癌症的发生发展中也发挥了重要作用[2]。近年来发现Yap1在肺癌、乳腺癌、食管癌、胃肠癌等多种癌症中明显上调,发挥促癌效应[3-4]。
丹参酮ⅡA是一种提取自传统中草药丹参根茎的复合物,具有改善血液循环、血管内皮细胞修复、护肝等多种药效,并且能通过诱导细胞凋亡发挥广谱抗癌特性[5],其主要依赖p53途径发挥促癌细胞凋亡效应。例如在鼻咽癌中,丹参酮ⅡA通过p53调控细胞周期蛋B1抑制癌细胞增殖并促进其凋亡[6];急性白血病中丹参酮ⅡA通过激活p53联合伊马替尼促进细胞凋亡[7]。最近有研究[8]发现在癌症的发展阶段,丹参酮ⅡA对于癌细胞的侵袭迁移也具有抑制效应,如在结肠癌中,丹参酮ⅡA能明显抑制SW620的侵袭迁移。而其在肝癌的增殖、迁移等恶性表型中发挥作用的相关研究较少。本实验通过沉默促癌基因Yap1联合丹参酮ⅡA研究二者对肝癌细胞株Huh-7增殖、迁移能力的影响。
1. 材料与方法
1.1 细胞培养及标本收集
人肝癌细胞Huh-7、Hep-G2、HCCL-M3以及人相对正常肝上皮细胞株L02购买自中国科学院上海生物科学研究所细胞平台。采用完全培养基[含10%的胎牛血清(Gibco.美国)、DMEM(Gibco)、青-链霉素抗生素]37 ℃恒温培养箱、5% CO2培养细胞。10对人肝细胞癌及癌旁组织标本来源于武汉大学中南医院,标本经外科手术切除后立即于-80 ℃储存,以用于后续实验研究,收集时间2019年6月1日-2019年12月1日。标本疾病类型及质量经病理科校验。
1.2 药物及细胞处理
丹参酮ⅡA购买自中国药品生物制品检定所(YZ-110766),高效液谱色相法(HPLC)检测药品纯度≥ 98%。丹参酮ⅡA溶于二甲基亚砜(DMSO,Sigma)中,工作液浓度选用2 mmol/L,DMSO稀释分别得到浓度梯度为0、2.5、5、10、15、20 μmol/L,处理肝癌细胞Huh-7 24 h后进行后续实验,药物-20 ℃避光保存。
1.3 RNA的提取及相对定量
Life Trizol RNA分离试剂购买自Invitrogen公司,按说明书步骤收集RNA沉淀并用DEPC水溶解,Nanodrop2000测所得RNA的浓度与纯度。RNA的反转录试剂盒购买自Toyobo公司(日本),按说明书方法得到cDNA,UltraSYBR Mixture购买自北京康为生物科技有限公司,按2-ΔΔCt法处理数据。实验所涉及的基因引物如下,Yap1:GAACTCGGCTTCAGGTCCTC(F),GGTTCATGGCAAAACGAGGG(R);GAPDH:AGAAGGCTGGGGCTCATTTG(F),GCAGGAGGCATTGCTGATGA(R)。
1.4 蛋白的提取及蛋白质印迹
蛋白提取试剂采用含有PMSF的Ripa裂解缓冲液(碧云天),按说明书步骤进行操作。蛋白浓度测定采用BCA蛋白测定试剂盒(碧云天)。蛋白上样缓冲液变性蛋白用于蛋白质印迹上样,通过SDS-聚丙烯酰胺凝胶电泳分离蛋白,转移至聚偏二氟乙烯膜(PVDF膜),5%的脱脂牛奶封闭2 h,一抗孵育过夜,二抗封闭1.5 h,采用普通ECL显影液GENESys显影仪显影。实验涉及的抗体如下:Anti-Yap1(CST) 1:1000,Anti-E-cad (CST) 1:1000,Anti-Vimentin(CST) 1:1000,Anti-GAPDH(abcam) 1:1000,Anti-Bcl2(abcam) 1:1000,Anti-Bax(abcam) 1:1000,山羊抗兔IgG(Abbkine) 1:4000,山羊抗鼠IgG(Biovison) 1:8000。
1.5 细胞增殖试验(MTT法)
MTT细胞增殖及细胞毒性检测试剂盒购买自上海碧云天公司(C0009),取处于对数生长期的细胞,96孔板每孔加入200 μl完全培养基,大约4000个细胞,贴壁培养24 h,用浓度梯度0、2.5、5、10、15、20 μmol/L处理细胞24 h,同时设置对照孔与空白孔用于计算调零。24 h后换液,每孔加入10 μl MTT溶液(5 mg/ml),继续37 ℃培养大约4 h,每孔加入100 μl Formazan溶解液,适当振摇,37 ℃培养箱孵育4 h充分溶解紫色结晶。于570 nm下测定吸光度。计算如下:细胞增殖抑制率(%)=[(1-实验组OD值/对照组OD值)/(对照组OD值-空白孔OD值)]×100%。
1.6 细胞凋亡实验
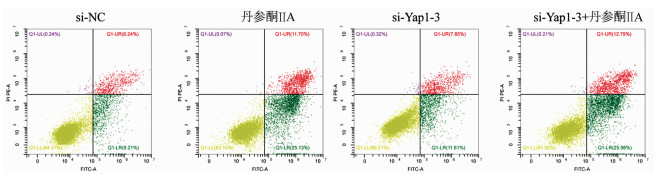
细胞凋亡检测试剂盒购买自贝博(#BB-4101)。取对数生长期细胞铺于6孔板,24 h后待细胞融合至70%左右,分别给予转染si-NC、丹参酮ⅡA、si-Yap1以及si-Yap1联合丹参酮ⅡA处理,24 h后收集细胞,按说明书步骤操作,进行FITC及PI双染,立即上流式细胞仪检测。
1.7 细胞迁移侵袭实验
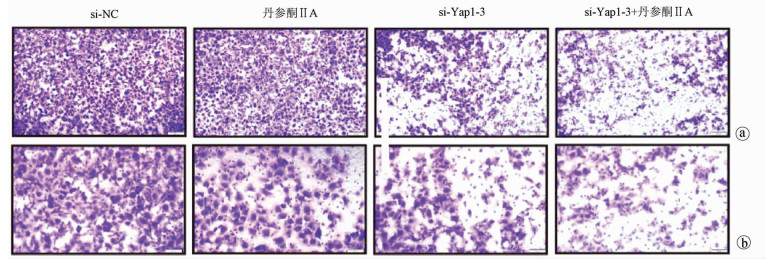
细胞迁移实验(康宁)选用滤膜孔径8.0 μm的小室,取处于对数生长期的细胞铺于6孔板,24 h后转染si-Yap1与7.8 μmol/L丹参酮ⅡA共处理细胞,si-NC组作为对照,8 h换液,(给药24 h后)胰酶消化细胞铺于小室,大约4万个细胞/小室。细胞侵袭实验同迁移实验区别在于小室需要用0.3 mg/ml的Matrigel(康宁)预处理,于37 ℃恒温培养箱中静置凝固3~4 h后小室方可进行后续实验。24 h后4%多聚甲醛室温固定10 min,0.1%的结晶紫染色20 min,棉签擦去小室上层细胞,100倍、200倍视野下随机选取3个视野拍照计数分析。
1.8 伦理学审查
人体标本获取获得湖北省实验研究伦理委员会批准,批号:科伦快审2018078。人肝细胞癌及癌旁组织标本所涉及患者均已签署知情同意书。
1.9 统计学方法
所有数据采用GraphPad Prism 7进行统计分析。计量资料以x±s表示,两组间比较采用t检验。计数资料两组间比较采用χ2检验。P<0.05为差异有统计学意义。
2. 结果
2.1 肝癌中Yap1表达上调以及转染si-Yap的沉歪默抑制效率
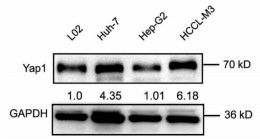
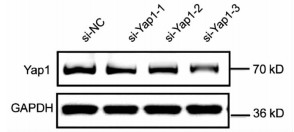
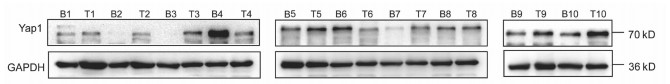
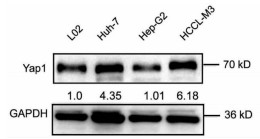
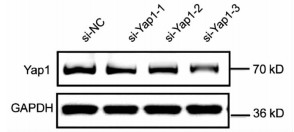
10对人肝细胞癌和对应癌旁标本进行蛋白印迹检测,8对标本Yap1在癌中的表达高于癌旁组织(图 1)。对比人正常肝细胞L02和肝癌细胞株HepG2、Huh-7、HCCL-M3中Yap1的表达发现,Yap1在肝癌细胞中表达增高,其中在Huh-7中表达最高(图 2)。设计合成的3对si-Yap1序列分别瞬时转染Huh-7细胞,培养48 h后发现mRNA和蛋白水平si-Yap1-3沉默Yap1表达效果明显,蛋白水平沉默效率达到87.004%(表 1),mRNA水平沉默效率达到53.558%(表 2),因此选择第3条si-Yap1(si-Yap1-3)进行后续实验(图 3)。
表 1 si-Yap1瞬时转染Huh-7细胞灰度值分析指标 si-NC si-Yap1-1 si-Yap1-2 si-Yap1-3 Yap1 3745.0±131.6 2641.0±74.5 2253.0±74.7 4939.0±6.8 GAPDH 8235.0±129.6 7461.0±129.4 6716.0±108.3 8613.0±61.2 t值 15.33 21.68 25.69 P值 0.004 0.002 0.002 表 2 si-Yap转染Huh-7后mRNA水平抑制Yap1的表达指标 si-NC si-Yap1-1 si-Yap1-2 si-Yap1-3 Yap1(Ct值) 26.62±0.33 26.72±0.11 26.79±0.06 27.72±0.02 GAPDH(Ct值) 16.69±0.25 16.62±0.08 16.26±0.07 16.66±0.22 t值 0.739 10.92 98.97 P值 0.501 <0.001 <0.001 2.2 丹参酮ⅡA抑制肝癌细胞增殖、迁移侵袭
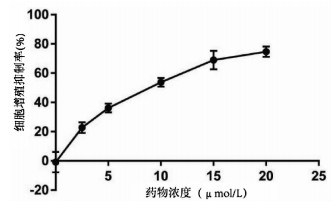
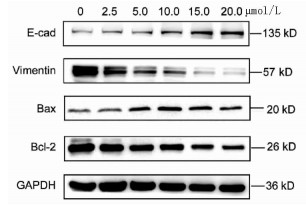
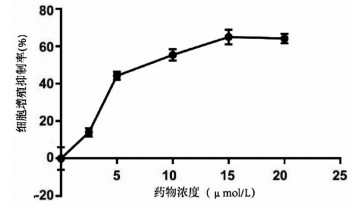
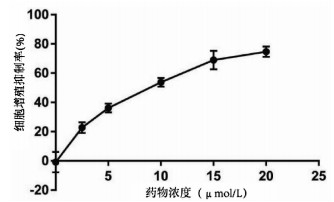
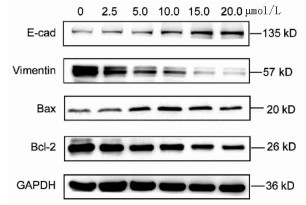
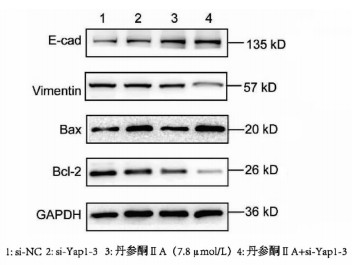
实验分别采用0、2.5、5、10、15、20 μmol/L 6个浓度处理肝癌细胞Huh-7,培养24 h后进行MTT检测,结果显示随着丹参酮ⅡA浓度增高,肝癌细胞Huh-7的细胞增殖被逐渐抑制,药物处理24 h后丹参酮ⅡA的半数抑制浓度(IC50)为8.683 μmol/L(图 4)。上皮间质转换(EMT)作为肿瘤细胞获得迁移侵袭能力的一大重要途径,其特征在于细胞表面上皮E-钙黏蛋白(E-cad)的表达减少,间质细胞标志物波形蛋白(Vimentin)的表达增高。不同浓度的丹参酮ⅡA处理细胞24 h收集蛋白,检测下游EMT相关指标,结果显示随着药物浓度的增加,E-cad表达增加,Vimentin表达减少(图 5)。同时,Bcl-2和Bax作为细胞凋亡过程中一对作用相反的明星蛋白分子,其比例决定了细胞的存亡。不同药物处理下,抑制凋亡分子Bcl-2的表达逐渐减少,而促凋亡分子Bax的表达增高(图 5)。
2.3 沉默促癌基因Yap1联合丹参酮ⅡA对于肝癌细胞株Huh-7增殖、凋亡、迁移的影响
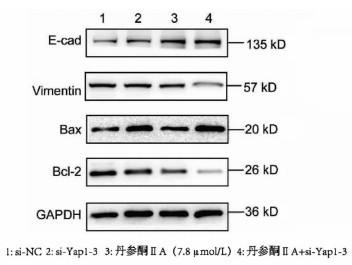
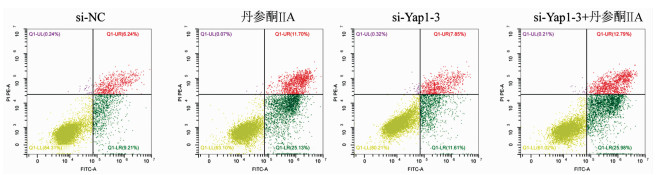
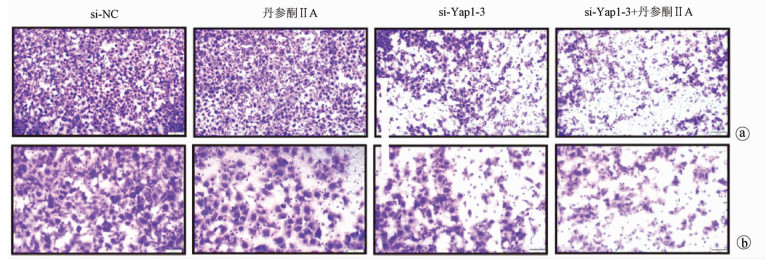
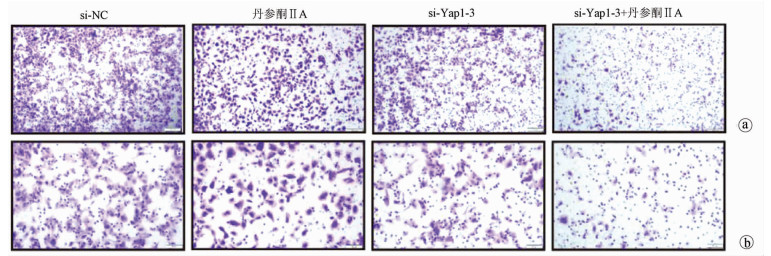
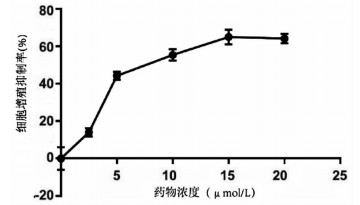
采用si-Yap1联合不同浓度的丹参酮ⅡA处理细胞24 h之后进行MTT检测,结果显示,联合处理细胞其IC50为7.867 μmol/L(图 6)。联合浓度为7.867 μmol/L的丹参酮ⅡA和si-Yap1-3共同处理肝癌细胞Huh-7。检测下游凋亡情况如图 7所示,si-Yap1联合丹参酮ⅡA处理组细胞Bax蛋白表达增高,而Bcl-2表达降低。流式细胞检测结果显示,与si-NC组比较,联合处理组早凋细胞占比率增高(25.98% vs 9.21%,χ2=4.078,P<0.05)(图 8)。联合组E-cad表达增高,而Vimentin表达减少证实联合处理组细胞的迁移侵袭能力降低(图 7)。Transwell细胞迁移及侵袭实验结果显示,联合用药组比si-NC组细胞迁移(43.19±2.88 vs 132.20±10.03,t=8.527,P=0.001)及侵袭(53.95±4.20 vs 179.10±11.11,t=4.484,P=0.011)能力明显降低(图 9、10)。
3. 讨论
肝癌作为全世界排名第二的致死癌症,其有效的治疗手段十分有限。超过半数的肝癌患者由于确诊时已经处于疾病的晚期阶段,而丧失了接受如肝切除、肝移植等治愈性治疗措施的机会[9]。索拉非尼和乐伐替尼作为多激酶抑制剂是目前公认的肝细胞癌治疗靶向药物,延长患者中位生存期3~4个月,疗效难以满意[10]。肝癌的复发与转移是缩短肝细胞癌患者生存期与治疗后复发的主要原因,肿瘤的微环境有助于肿瘤细胞获得浸润型表型,EMT这一过程是公认的赋予癌细胞侵袭与迁移能力的重要途径,尤其是E-cad的缺失[11]。监测相关指标的变化对于判断癌细胞的迁移侵袭能力有指导性意义。Hippo通路的经典核效应器Yap1不仅在正常细胞增殖、器官发育中维持细胞形态与细胞骨架,同时与Wnt通路以及TGFβ等多条通路相互联合,参与肝癌的发生发展[12]。在癌症的发展中,Yap1的表达变化以及核质穿梭协同癌细胞的变形和迁移[13]。丹参酮ⅡA作为中草药的提取混合物,以往研究[6]证实其在促进癌细胞凋亡方面发挥重要作用,可以通过诱导肿瘤细胞死亡并抑制肿瘤生长。本研究通过联合沉默促癌基因Yap1和丹参酮ⅡA观察其对于肝癌细胞增殖、凋亡、迁移、侵袭能力的影响。其中MTT的实验结果显示,联合用药组的细胞增殖减慢且药物的有效半数抑制浓度降低。下游的凋亡相关标志物以及流式细胞检测发现联合用药组细胞早期凋亡率明显增加,凋亡进程被促进。通过检测EMT相关指标,结果显示Yap1沉默联合药物丹参酮ⅡA明显下调Vimentin表达,E-cad表达增加,提示细胞迁移侵袭能力减弱。Transwell细胞迁移及侵袭实验进一步证实了这一点。综上所述,沉默促癌基因Yap1联合丹参酮ⅡA能够抑制肝癌细胞Huh-7增殖、凋亡及侵袭迁移的进展,为肝癌药物的进一步临床研究提供了方向。
-
表 1 si-Yap1瞬时转染Huh-7细胞灰度值分析
指标 si-NC si-Yap1-1 si-Yap1-2 si-Yap1-3 Yap1 3745.0±131.6 2641.0±74.5 2253.0±74.7 4939.0±6.8 GAPDH 8235.0±129.6 7461.0±129.4 6716.0±108.3 8613.0±61.2 t值 15.33 21.68 25.69 P值 0.004 0.002 0.002 表 2 si-Yap转染Huh-7后mRNA水平抑制Yap1的表达
指标 si-NC si-Yap1-1 si-Yap1-2 si-Yap1-3 Yap1(Ct值) 26.62±0.33 26.72±0.11 26.79±0.06 27.72±0.02 GAPDH(Ct值) 16.69±0.25 16.62±0.08 16.26±0.07 16.66±0.22 t值 0.739 10.92 98.97 P值 0.501 <0.001 <0.001 -
[1] RAWLA P, SUNKARA T, MURALIDHARAN P, et al. Update in global trends and aetiology of hepatocellular carcinoma[J]. Contemp Oncol (Pozn), 2018, 22(3): 141-150. [2] XIAO Y, ZHANG H, MA Q, et al. YAP1-mediated pancreatic stellate cell activation inhibits pancreatic cancer cell proliferation[J]. Cancer Lett, 2019, 462: 51-60. DOI: 10.1016/j.canlet.2019.07.015 [3] LU T, LI Z, YANG Y, et al. The Hippo/YAP1 pathway interacts with FGFR1 signaling to maintain stemness in lung cancer[J]. Cancer Lett, 2018, 423: 36-46. DOI: 10.1016/j.canlet.2018.02.015 [4] GUO L, CHEN Y, LUO J, et al. YAP1 overexpression is associated with poor prognosis of breast cancer patients and induces breast cancer cell growth by inhibiting PTEN[J]. FEBS Open Bio, 2019, 9(3): 437-445. DOI: 10.1002/2211-5463.12597 [5] HU Y, WANG S, WU X, et al. Chinese herbal medicine-derived compounds for cancer therapy: A focus on hepatocellular carcinoma[J]. J Ethnopharmacol, 2013, 149(3): 601-612. DOI: 10.1016/j.jep.2013.07.030 [6] LIU B, LIU L, ZANG A, et al. Tanshinone ⅡA inhibits proliferation and induces apoptosis of human nasopharyngeal carcinoma cells via p53-cyclin B1/CDC2[J]. Oncol Lett, 2019, 18(3): 3317-3322. [7] GUO Y, LI Y, XIANG B, et al. Nutlin-3 plus tanshinone ⅡA exhibits synergetic anti-leukemia effect with imatinib by reactivating p53 and inhibiting the AKT/mTOR pathway in Ph+ ALL[J]. Biochem J, 2017, 474(24): 4153-4170. DOI: 10.1042/BCJ20170386 [8] XUE J, JIN X, WAN X, et al. Effects and mechanism of tanshinone Ⅱ A in proliferation, apoptosis, and migration of human colon cancer cells[J]. Med Sci Monit, 2019, 25: 4793-4800. DOI: 10.12659/MSM.914446 [9] FAIVRE S, RIMASSA L, FINN RS. Molecular therapies for HCC: Looking outside the box[J]. J Hepatol, 2020, 72(2): 342-352. DOI: 10.1016/j.jhep.2019.09.010 [10] KUDO M, FINN RS, QIN S, et al. Lenvatinib versus sorafenib B in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial[J]. Lancet, 2018, 391(10126): 1163-1173. DOI: 10.1016/S0140-6736(18)30207-1 [11] CHO ES, KANG HE, KIM NH, et al. Therapeutic implications of cancer epithelial-mesenchymal transition (EMT)[J]. Arch Pharm Res, 2019, 42(1): 14-24. DOI: 10.1007/s12272-018-01108-7 [12] CHEN X, LI Y, LUO J, et al. Molecular mechanism of Hippo-YAP1/TAZ pathway in heart development, disease, and regeneration[J]. Front Physiol, 2020, 11: 389. DOI: 10.3389/fphys.2020.00389 [13] EGE N, DOWBAJ AM, JIANG M, et al. Quantitative analysis reveals that actin and Src-family kinases regulate nuclear YAP1 and its export[J]. Cell Syst, 2018, 6(6): 692-708. DOI: 10.1016/j.cels.2018.05.006 期刊类型引用(4)
1. 张学莲,安世芝,杜堂彩,郭欣,姜晨. miR-599靶向YAP1对卵巢癌细胞迁移及侵袭的影响. 武汉大学学报(医学版). 2023(08): 923-927 .  百度学术
百度学术2. 苗津东,张丹英,程雯,俞瑾,俞超芹. YAP1和HIF-1α表达对宫颈鳞癌患者预后的评估价值. 局解手术学杂志. 2022(02): 98-102 .  百度学术
百度学术3. 雷雅婷,唐浪,吴广阳. 基于网络药理学与分子对接探讨丹参-川芎药对改善肝癌癌前病变的作用机制. 大众科技. 2022(07): 96-101 .  百度学术
百度学术4. 丘佩容,陈泽山,朱文琳,彭佩纯,吴纪添,李玉莲,邓鑫. 中药对肝细胞癌相关信号通路的影响及潜在机制. 中国实验方剂学杂志. 2022(23): 264-272 .  百度学术
百度学术其他类型引用(1)
-




 PDF下载 ( 3256 KB)
PDF下载 ( 3256 KB)


 下载:
下载:









 下载:
下载:

 百度学术
百度学术