不同肝病基础的重症酒精性肝炎患者短期预后评估及其影响因素
DOI: 10.3969/j.issn.1001-5256.2021.02.024
Evaluation and influencing factors of the short-term prognosis of severe alcoholic hepatitis with different underlying liver diseases
-
摘要:
目的 探讨不同肝病基础的重症酒精性肝炎(AH)患者临床特征及其短期预后的评估和影响因素。 方法 回顾性分析天津市第三中心医院2004年8月—2018年8月收治的170例重症AH患者临床资料,按不同肝病基础分为A型(无肝硬化,n=27)、B型(代偿期肝硬化,n=52)和C型(失代偿期肝硬化,n=91)。计算Maddrey判别函数(MDF)评分、慢性肝衰竭序贯性器官衰竭评估(CLIF-SOFA)评分、终末期肝病模型(MELD)评分、ABIC评分(年龄、胆红素、国际标准化比值、肌酐)以及Glasgow酒精性肝炎评分(GAHS)。计量资料多组间比较采用方差分析或Kruskal-Wallis H检验;计数资料多组间比较采用χ2检验。采用单因素和多因素Cox回归分析筛选影响重症AH患者短期预后的独立危险因素。应用Kaplan-Meier法绘制生存曲线,生存差异组间比较采用log-rank检验。受试者工作特征曲线计算各预测模型的曲线下面积(AUC)及95%CI、敏感度、特异度,并应用DeLong法进行比较。 结果 A、B、C型患者28 d生存率分别为88.9%、80.8%和51.6%,3组比较差异有统计学意义(χ2=19.83,P < 0.001)。MELD评分、MDF评分、GAHS评分、ABIC评分和CLIF-SOFA评分预测28 d病死率的AUC(95%CI)分别为0.584(0.493~0.676)、0.696(0.605~0.786)、0.644(0.554~0.735)、0.745(0.662~0.827)和0.795(0.726~0.863);CLIF-SOFA评分与MDF评分、MELD评分、GAHS评分相比,差异均有统计学意义(P值均 < 0.05);CLIF-SOFA评分预测28 d病死率的最佳阈值为8.50分,敏感度为79.0%,特异度为67.9%。发病时不同肝病基础(HR=2.296,95% CI:1.356~3.887,P=0.002)以及合并肝性脑病(HR=1.911,95% CI:1.059~3.449,P=0.031)是28 d预后的危险因素。 结论 不同肝病基础的重症AH患者具有不同的临床特征和短期预后,发病时不同肝病基础及合并肝性脑病与重症AH患者28 d预后密切相关。CLIF-SOFA评分能够较好地预测重症AH患者28 d预后。 Abstract:Objective To investigate the clinical features of patients with severe alcoholic hepatitis (AH) with different underlying liver diseases and the influencing factors for short-term prognosis. Methods A retrospective analysis was performed for the clinical data of 170 patients with severe AH who were admitted to Tianjin Third Central Hospital from August 2004 to August 2018, and according to the underlying liver disease, they were divided into group A (27 patients without liver cirrhosis), group B (52 patients with compensated liver cirrhosis), and group C (91 patients with decompensated liver cirrhosis). Related scores were calculated, including Maddrey's discriminant function (MDF) score, Chronic Liver Failure-Sequential Organ Failure Assessment (CLIF-SOFA) score, Model for End-Stage Liver Disease (MELD) score, age-bilirubin-international normalized ratio-creatinine (ABIC) score, and Glasgow alcoholic hepatitis score (GAHS). An analysis of variance or the Kruskal-Wallis H test was used for comparison of continuous data between multiple groups, and the chi-square test was used for comparison of categorical data between multiple groups. Univariate and multivariate Cox regression analyses were used to screen out the independent influencing factors for the short-term prognosis of patients with severe AH. The Kaplan-Meier method was used to plot survival curves, and the log-rank test was used for comparison of survival rate between groups. The receiver operating characteristic (ROC) curve was used to calculate the area under the ROC curve (AUC) and 95% confidence interval (CI), sensitivity, and specificity for each predictive model, and the DeLong method was used for comparison. Results The 28-day survival rates of patients in groups A, B, and C were 88.9%, 80.8%, and 51.6%, respectively, with a significant difference between the three groups (χ2=19.83, P < 0.001). The AUCs (95% CIs) of MELD score, MDF score, GAHS score, ABIC score, and CLIF-SOFA score were 0.584 (0.493-0.676), 0.696 (0.605-0.786), 0.644 (0.554-0.735), 0.745 (0.662-0.827), and 0.795 (0.726-0.863), respectively, in predicting 28-day mortality rate, and there were significant differences between CLIF-SOFA score and MDF, MELD, and GAHS scores (all P < 0.05); CLIF-SOFA score had a sensitivity of 79.0% and a specificity of 67.9% at the optimal cut-off value of 8.50 points in predicting 28-day mortality rate. Different underlying liver diseases (hazard ratio [HR]=2.296, 95% CI: 1.356-3.887, P=0.002) and hepatic encephalopathy (HR=1.911, 95% CI: 1.059-3.449, P=0.031) at disease onset were risk factors for 28-day prognosis. Conclusion Patients with severe AH with different underlying liver diseases have different clinical features and short-term prognoses. Different underlying liver diseases and hepatic encephalopathy at disease onset are closely associated with the 28-day prognosis of patients with severe AH. CLIF-SOFA score can predict the 28-day prognosis of patients with severe AH. -
Key words:
- Hepatitis, Alcoholic /
- Prognosis /
- Infection /
- Risk Factors
-
酒精性肝病是长期饮酒引起的肝脏损伤,包括酒精性脂肪肝、酒精性肝炎(alcoholic hepatitis,AH)、肝纤维化、肝硬化。有大量饮酒史的AH患者迅速出现黄疸和肝功能衰竭,短期病死率高达30%~40%[1-2]。来自丹麦的一项研究[3]调查显示,与无肝硬化的AH患者相比,有肝硬化的AH患者28 d病死率和84 d病死率均有所上升,且无肝硬化的AH患者5年生存率明显高于有肝硬化的AH患者。鉴于国内对于重症AH的诊断尚无具体量化标准,本研究为了使研究对象更好的同质化,采用2018年美国胃肠病学会酒精性肝病诊治临床指南[4]中的重症AH诊断标准来探讨不同肝病基础的重症AH患者的临床特征及其短期预后的评估和影响因素。
1. 资料与方法
1.1 研究对象
选取2004年8月—2018年8月天津市第三中心医院收治的符合重症AH诊断标准[4]的患者,即:出现短期进行性加重的黄疸及肝脏相关并发症,血清TBil>3 mg/dl;ALT和AST水平升高>1.5倍,但 < 400 U/L,AST/ALT比值>1.5;发病前8周有持续大量饮酒史;排除其他原因肝病;Maddrey判别函数(MDF)≥32分。
1.2 数据采集
阅读患者病历资料,回顾性采集数据,包括人口学特点、合并感染类型、实验室检验指标、影像学资料及相关并发症。根据病史和影像学资料,分为A型(无肝硬化)、B型(代偿期肝硬化)和C型(失代偿期肝硬化)。计算MDF评分、慢性肝衰竭序贯性器官衰竭评估(CLIF-SOFA)评分、终末期肝病模型(MELD)评分、ABIC评分[年龄、胆红素、国际标准化比值(INR)、肌酐(Cr)]以及Glasgow酒精性肝炎评分(GAHS)。
1.3 伦理学审查
本研究方案经由天津市第三中心医院伦理委员会审批,批号:IRB 2018-045-36,所纳入患者在治疗前均签署知情同意书。
1.4 统计学方法
采用SPSS 21.0和MedCalc 11.4.2.0统计软件进行数据分析。正态分布的计量资料用x±s表示,多组间比较采用方差分析;非正态分布的计量资料用M(P25~P75)表示,多组间比较采用Kruskal-Wallis H检验。计数资料多组间比较采用χ2检验。采用单因素和多因素Cox回归分析筛选影响重症AH患者短期预后独立危险因素。应用Kaplan-Meier法绘制生存曲线,生存差异组间比较采用log-rank检验。受试者工作特征曲线(ROC曲线)计算各预测模型的曲线下面积(AUC)及95%CI、敏感度、特异度,并应用DeLong法进行比较。P < 0.05为差异有统计学意义。
2. 结果
2.1 纳入患者临床特征
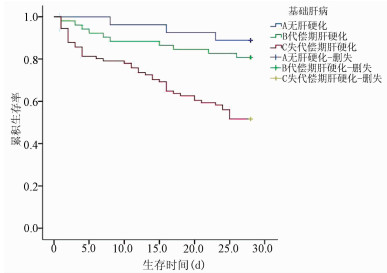
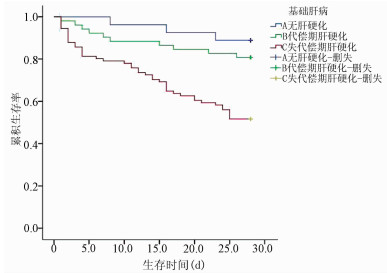
共纳入重症AH患者170例,其中A型27例(15.9%),B型52例(30.6%),C型91例(53.5%)。本研究中男166例,女4例;年龄29~70岁,平均(49.07±9.19)岁。不同肝病基础患者的性别构成比差异无统计学意义(P>0.05),均以男性为主;年龄、外周血WBC、中性粒细胞计数(NEU)、淋巴细胞计数(LYM)、PLT、Alb、GGT、TBil、INR、PT、尿素氮(BUN)、感染、肝性脑病和腹水发生率、28 d病死率在不同肝病基础患者中比较,差异均有统计学意义(P值均 < 0.05)(表 1)。共81例患者存在感染,占全部患者的47.6%,其中48例(28.2%)患者在确诊为重症AH时即存在感染,33例(19.4%)在住院期间出现感染。肺部感染最常见(36.21%),其次是自发性细菌性腹膜炎(31.09%)和菌血症(18.97%)。A、B、C型患者感染发生率比较,差异有统计学意义(P < 0.05)(表 1)。3组患者在28 d住院期间的感染人数分别为1例(3.70%)、10例(19.23%)和25例(27.47%)(χ2=19.10,P=0.027),但不同肝病基础患者入院时的感染情况无差异。A、B、C型患者的28 d生存率分别为88.9%、80.8%和51.6%,差异有统计学意义(χ2=19.83,P < 0.001)(图 1)。
表 1 不同肝病基础重症AH患者的临床特征比较临床特征 A型(n=27) B型(n=52) C型(n=91) 统计值 P值 性别[例(%)] χ2=4.26 0.119 男 25(92.6) 52(100.0) 89(97.8) 女 2(7.4) 0 2(2.2) 年龄(岁) 44.26±9.18 48.56±8.53 50.79±9.11 F=5.67 0.004 WBC(×109/L) 11.81(6.92~16.55) 8.10(5.46~10.23) 8.54(5.73~13.30) χ2=7.23 0.027 NEU(×109/L) 10.52(5.08~15.05) 5.81(3.90~8.95) 7.04(4.08~11.26) χ2=6.88 0.032 LYM(×109/L) 1.05(0.75~1.33) 0.76(0.54~1.05) 0.77(0.43~1.05) χ2=9.60 0.008 PLT(×109/L) 182.00(100.00~199.000) 71.00(45.75~97.00) 50.00(46.00~68.50) χ2=22.22 < 0.001 Alb(g/L) 29.10(25.80~31.00) 26.15(26.15~28.05) 26.35(23.35~28.63) χ2=0.85 0.011 ALT(U/L) 51.00 (46.00~72.00) 59.50(46.00~75.00) 50.00(46.00~68.50) χ2=0.50 0.780 AST(U/L) 87.00(75.50~136.00) 96.50(74.75~125.00) 87.00(76.00~128.00) χ2=0.23 0.891 GGT(U/L) 214.00(152.50~366.50) 196.50(95.50~332.50) 76.00(34.50~172.50) χ2=25.42 < 0.001 TBil(μmol/L) 284.40(207.70~353.90) 235.80(135.15~346.86) 190.80(130.50~277.55) χ2=6.41 0.041 BUN(mmol/L) 5.72(4.70~8.54) 6.55(4.51~9.68) 7.92(5.03~15.91) χ2=6.12 0.047 Cr(μmol/L) 58.00(48.50~75.50) 71.00(50.00~96.85) 78.00(53.50~124.50) χ2=5.10 0.078 Na+(mmol/L) 133.30(131.40~136.90) 132.00(125.70~135.90) 131.80(127.75~135.15) χ2=2.38 0.305 K+(mmol/L) 3.53(3.25~3.95) 3.63(3.11~4.27) 3.88(3.20~4.69) χ2=2.46 0.293 INR 1.79(1.63~2.19) 2.14(1.79~2.40) 2.36(1.90~3.05) χ2=14.87 0.001 PT(s) 20.50(19.20~24.15) 23.30(20.68~25.73) 25.70(21.56~32.30) χ2=15.01 0.001 MDF评分(分) 60.24(37.82~61.24) 49.24(38.54~65.12) 60.24(37.82~86.06) χ2=5.37 0.068 感染[例(%)] 7(25.9) 22(42.3) 52(57.1) χ2=8.99 0.011 消化道出血[例(%)] 4(14.8) 13(25.0) 26(28.6) χ2=0.38 0.352 腹水[例(%)] 11(40.7) 35(67.3) 62(68.1) χ2=7.20 0.027 肝性脑病[例(%)] 1(3.7) 13(25.0) 23(25.3) χ2=6.15 0.046 肝肾综合征[例(%)] 4(14.8) 11(21.2) 28(30.8) χ2=3.48 0.175 28 d病死率[例(%)] 3(11.1) 10(19.2) 44(48.4) χ2=19.83 < 0.001 2.2 5种评分模型的ROC曲线分析
MELD评分、MDF评分、GAHS评分、ABIC评分和CLIF-SOFA评分预测28 d病死率的AUC详见表 2。CLIF-SOFA评分与MDF评分、MELD评分、GAHS评分相比,差异均有统计学意义(P值均 < 0.05),ABIC评分与CLIF-SOFA评分比较无显著差异(P=0.179)。CLIF-SOFA评分预测28 d病死率的最佳阈值为8.50分,敏感度为79.0%,特异度为67.9%。
表 2 不同评分模型的ROC曲线分析评分模型 AUC(95% CI) 最佳阈值(分) 特异度 敏感度 MELD评分 0.584(0.493~0.676) 30.74 0.708 0.456 MDF评分 0.696(0.605~0.786) 64.66 0.805 0.632 GAHS评分 0.644(0.554~0.735) 10.50 0.858 0.421 ABIC评分 0.745(0.662~0.827) 8.92 0.903 0.526 CLIF-SOFA评分 0.795(0.726~0.863) 8.50 0.679 0.790 2.3 单因素和多因素Cox回归分析
单因素Cox分析结果显示,基础肝病、年龄、WBC、NEU、Alb、GGT、BUN、Cr、INR、PT、合并感染、合并肝性脑病、合并肝肾综合征是重症AH患者28 d预后的影响因素;进一步行多因素Cox回归分析显示,基础肝病(HR=2.296,95% CI:1.356~3.887,P=0.002)和肝性脑病(HR=1.911,95% CI:1.059~3.449,P=0.031)是重症AH患者28 d预后的危险因素(表 3)。
表 3 单因素Cox回归分析结果变量 单因素分析 HR(95% CI) P值 基础肝病 2.644(1.625~4.304) < 0.001 性别 1.391(0.193~10.052) 0.743 年龄(岁) 1.042(1.012~1.072) 0.005 WBC(×109/L) 1.076(1.041~1.113) < 0.001 NEU(×109/L) 1.085(1.045~1.127) < 0.001 LYM(×109/L) 0.886(0.543~1.444) 0.626 PLT(×109/L) 0.996(0.991~1.001) 0.086 Alb(g/L) 0.939(0.882~0.999) 0.046 ALT(U/L) 1.004(0.998~1.011) 0.216 AST(U/L) 1.003(0.999~1.007) 0.100 GGT(U/L) 0.997(0.995~0.999) 0.003 TBil(μmol/L) 1.001(0.999~1.003) 0.390 BUN(mmol/L) 1.059(1.034~1.084) < 0.001 Cr(μmol/L) 1.003(1.002~1.005) < 0.001 Na+(mmol/L) 0.989(0.955~1.023) 0.519 K+(mmol/L) 1.256(0.945~1.669) 0.116 INR 1.474(1.291~1.684) < 0.001 PT(s) 1.057(1.037~1.077) < 0.001 消化道出血 0.871(0.469~1.618) 0.663 感染 3.127(1.770~5.521) < 0.001 腹水 1.156(0.666~2.005) 0.606 肝性脑病 3.028(1.773~5.171) < 0.001 肝肾综合征 3.758(2.226~6.344) < 0.001 3. 讨论
本研究显示不同肝病基础重症AH患者在部分人口学指标、实验室指标、并发症发生率和短期预后方面存在显著差异。重症AH患者具有明显的男女性别差异,男性占97.6%;平均发病年龄为49.07岁,C型患者平均年龄最大,提示具有长期大量饮酒史的中年男性是我国重症AH的高发人群。本研究分析结果显示,A型患者的WBC、PLT高于B、C型患者,可能与B、C型患者肝硬化基础疾病有关,肝硬化门静脉压力增高引起脾脏充血性肿大继发脾功能亢进;外周血血细胞减少症在肝硬化患者中常见,以PLT和WBC减少为主。A型患者GGT、TBil水平较B、C型患者高,这与既往文献[5]报道AH患者GGT、胆红素水平高于酒精性肝硬化患者一致。与其他类型患者相比,C型患者INR、PT、BUN水平升高,感染、肝性脑病和腹水发生率以及28 d病死率更高,考虑与C型患者为失代偿期肝硬化基础有关,肝功能相对较差,发生并发症概率更高。
感染是重症AH的主要并发症之一,12%~26%的重症AH患者在入院时就存在感染[6],而伴有感染的重症AH患者60 d病死率可增加30%,生存率与糖皮质激素无应答者相似[7]。本研究中近半数(47.6%)的重症AH患者存在感染,且B型和C型的感染率显著高于A型(χ2=8.99,P=0.011),C型患者入院后发生感染的风险更是远远高于A型,推测可能与B型和C型患者存在肝硬化基础有关。肝硬化本身可引起系统性免疫功能障碍,致机体抗感染及免疫调节能力下降。肝硬化相关免疫功能障碍主要表现为获得性免疫缺陷和持续系统性炎症共存[8]。肝硬化相关免疫功能障碍越严重,发生严重细菌感染的风险就越高,患者病死率增加[9]。在STOPAH(Steroids or Pentoxifylline for Alcoholic Hepatitis)试验[10]中观察到大多数感染都与应用糖皮质激素有关,而接受激素治疗的患者中,只有大约一半的患者能从中获益。有研究[11]显示,接受激素治疗的重症AH患者比未接受激素治疗的患者更容易发生严重感染,这可能会抵消其激素治疗带来的生存获益。一项246例应用糖皮质激素治疗重症AH患者的临床研究[7]发现,未应用糖皮质激素治疗的感染者和无感染者的2个月生存率并无统计学差异,但感染者和无感染者在糖皮质激素治疗后的2个月生存率差异显著,提示糖皮质激素治疗可能会增加重症AH感染者的病死率。正因如此,激素治疗重症AH仍存在较大争议,主要聚焦在感染风险、临床获益及应用指征等方面。本研究显示不同肝病基础的重症AH患者感染风险不同,同时还发现基础肝病和发生肝性脑病是重症AH患者28 d预后的危险因素。对于重症AH患者的激素治疗,应该采取个体化治疗,特别是对于失代偿期肝硬化者,慎用激素。
本研究显示重症AH患者28 d病死率为33.5%。目前有多种方法可用于评价AH的严重程度及短期预后,主要包括MDF、MELD、GAHS、ABIC、Lille等评分。MDF评分是首个AH的评分方法,也是目前应用范围最广的预后评估模型。Forrest等[12]利用STOPAH数据分别测定泼尼松龙、己酮可可碱(PTX)、泼尼松龙联合PTX或安慰剂组的MDF、ABIC、MELD及GAHS评分,发现MELD、ABIC和GAHS评分系统在评估AH患者的预后方面优于MDF。Sandahl等[13]研究也强调了各项预测模型预后评分的局限性,在早期预测能力不足。慢加急性肝衰竭(ACLF)是发生在慢性肝病基础上因急性诱因出现的肝功能急性失代偿临床综合征,短期病死率高,有25%的ACLF与酒精相关[14],且酒精性肝病相关ACLF患者(ALD-ACLF)发生多器官衰竭的比例更高[15]。对于重症AH和ALD-ACLF的关系,也一直存在争议。重症AH与ALD-ACLF的临床表现相似,有学者[16]认为重症AH可能是ACLF中一种与酒精有关的类型;也有学者[17]认为ACLF既可在诊断重症AH时出现,也可以在其随访中发生,而若ACLF在重症AH过程中发生,则可显著增加重症AH的病死率[18]。CLIF-SOFA评分系统通过评估中枢神经系统、循环系统、呼吸系统、肝脏、凝血系统、肾脏6个系统的功能来准确预测ACLF患者的预后。Kim等[19]研究认为CLIF-SOFA评分相对于其他ACLF的预后评分模型,可以更好地预测AH合并ACLF患者的短期预后。本研究将CLIF-SOFA评分和其他AH的评分方法进行比较,结果显示评估所有器官衰竭的状况比仅仅单独评估肝衰竭的状况更能准确预测短期预后:CLIF-SOFA评分的AUC最高,其具有更好的预测性能;该评分预测28 d预后的截断值为8.50分,敏感度为79.0%,特异度为67.9%;虽然CLIF-SOFA评分与ABIC评分在AUC上无显著差异,但ABIC评分的特异度为90.3%,敏感度为52.6%,适合用于排除低病死率,不适合作为筛查高病死率。
既往本院对于重症AH患者的治疗中很少应用糖皮质激素,主要考虑此类人群免疫功能低下,为感染高发人群,应用激素可能进一步增加感染风险,因此本研究入选的患者在治疗过程中均未使用糖皮质激素,本研究所得到的结论需要在大样本的激素治疗中予以进一步验证。总之,本研究显示不同肝病基础的重症AH具有不同的临床特征和短期预后,慢性肝病基础和发生肝性脑病是重症AH患者28 d预后的危险因素;同时CLIF-SOFA评分系统能够较好地预测重症AH患者28 d预后。
-
表 1 不同肝病基础重症AH患者的临床特征比较
临床特征 A型(n=27) B型(n=52) C型(n=91) 统计值 P值 性别[例(%)] χ2=4.26 0.119 男 25(92.6) 52(100.0) 89(97.8) 女 2(7.4) 0 2(2.2) 年龄(岁) 44.26±9.18 48.56±8.53 50.79±9.11 F=5.67 0.004 WBC(×109/L) 11.81(6.92~16.55) 8.10(5.46~10.23) 8.54(5.73~13.30) χ2=7.23 0.027 NEU(×109/L) 10.52(5.08~15.05) 5.81(3.90~8.95) 7.04(4.08~11.26) χ2=6.88 0.032 LYM(×109/L) 1.05(0.75~1.33) 0.76(0.54~1.05) 0.77(0.43~1.05) χ2=9.60 0.008 PLT(×109/L) 182.00(100.00~199.000) 71.00(45.75~97.00) 50.00(46.00~68.50) χ2=22.22 < 0.001 Alb(g/L) 29.10(25.80~31.00) 26.15(26.15~28.05) 26.35(23.35~28.63) χ2=0.85 0.011 ALT(U/L) 51.00 (46.00~72.00) 59.50(46.00~75.00) 50.00(46.00~68.50) χ2=0.50 0.780 AST(U/L) 87.00(75.50~136.00) 96.50(74.75~125.00) 87.00(76.00~128.00) χ2=0.23 0.891 GGT(U/L) 214.00(152.50~366.50) 196.50(95.50~332.50) 76.00(34.50~172.50) χ2=25.42 < 0.001 TBil(μmol/L) 284.40(207.70~353.90) 235.80(135.15~346.86) 190.80(130.50~277.55) χ2=6.41 0.041 BUN(mmol/L) 5.72(4.70~8.54) 6.55(4.51~9.68) 7.92(5.03~15.91) χ2=6.12 0.047 Cr(μmol/L) 58.00(48.50~75.50) 71.00(50.00~96.85) 78.00(53.50~124.50) χ2=5.10 0.078 Na+(mmol/L) 133.30(131.40~136.90) 132.00(125.70~135.90) 131.80(127.75~135.15) χ2=2.38 0.305 K+(mmol/L) 3.53(3.25~3.95) 3.63(3.11~4.27) 3.88(3.20~4.69) χ2=2.46 0.293 INR 1.79(1.63~2.19) 2.14(1.79~2.40) 2.36(1.90~3.05) χ2=14.87 0.001 PT(s) 20.50(19.20~24.15) 23.30(20.68~25.73) 25.70(21.56~32.30) χ2=15.01 0.001 MDF评分(分) 60.24(37.82~61.24) 49.24(38.54~65.12) 60.24(37.82~86.06) χ2=5.37 0.068 感染[例(%)] 7(25.9) 22(42.3) 52(57.1) χ2=8.99 0.011 消化道出血[例(%)] 4(14.8) 13(25.0) 26(28.6) χ2=0.38 0.352 腹水[例(%)] 11(40.7) 35(67.3) 62(68.1) χ2=7.20 0.027 肝性脑病[例(%)] 1(3.7) 13(25.0) 23(25.3) χ2=6.15 0.046 肝肾综合征[例(%)] 4(14.8) 11(21.2) 28(30.8) χ2=3.48 0.175 28 d病死率[例(%)] 3(11.1) 10(19.2) 44(48.4) χ2=19.83 < 0.001 表 2 不同评分模型的ROC曲线分析
评分模型 AUC(95% CI) 最佳阈值(分) 特异度 敏感度 MELD评分 0.584(0.493~0.676) 30.74 0.708 0.456 MDF评分 0.696(0.605~0.786) 64.66 0.805 0.632 GAHS评分 0.644(0.554~0.735) 10.50 0.858 0.421 ABIC评分 0.745(0.662~0.827) 8.92 0.903 0.526 CLIF-SOFA评分 0.795(0.726~0.863) 8.50 0.679 0.790 表 3 单因素Cox回归分析结果
变量 单因素分析 HR(95% CI) P值 基础肝病 2.644(1.625~4.304) < 0.001 性别 1.391(0.193~10.052) 0.743 年龄(岁) 1.042(1.012~1.072) 0.005 WBC(×109/L) 1.076(1.041~1.113) < 0.001 NEU(×109/L) 1.085(1.045~1.127) < 0.001 LYM(×109/L) 0.886(0.543~1.444) 0.626 PLT(×109/L) 0.996(0.991~1.001) 0.086 Alb(g/L) 0.939(0.882~0.999) 0.046 ALT(U/L) 1.004(0.998~1.011) 0.216 AST(U/L) 1.003(0.999~1.007) 0.100 GGT(U/L) 0.997(0.995~0.999) 0.003 TBil(μmol/L) 1.001(0.999~1.003) 0.390 BUN(mmol/L) 1.059(1.034~1.084) < 0.001 Cr(μmol/L) 1.003(1.002~1.005) < 0.001 Na+(mmol/L) 0.989(0.955~1.023) 0.519 K+(mmol/L) 1.256(0.945~1.669) 0.116 INR 1.474(1.291~1.684) < 0.001 PT(s) 1.057(1.037~1.077) < 0.001 消化道出血 0.871(0.469~1.618) 0.663 感染 3.127(1.770~5.521) < 0.001 腹水 1.156(0.666~2.005) 0.606 肝性脑病 3.028(1.773~5.171) < 0.001 肝肾综合征 3.758(2.226~6.344) < 0.001 -
[1] O'SHEA RS, DASARATHY S, McCULLOUGH AJ, et al. Alcoholic liver disease[J]. Hepatology, 2010, 51(1): 307-328. DOI: 10.1002/hep.23258 [2] ZHAI QH, SONG FJ, XU TJ, et al. Nutritional status of 156 patients with severe alcoholic liver disease[J/CD]. Chin J Liver Dis (Electronic Version), 2020, 12(1): 44-49. (in Chinese)翟庆慧, 宋芳娇, 徐天娇, 等. 156例重症酒精性肝病患者营养现况调查[J/CD].中国肝脏病杂志(电子版), 2020, 12(1): 44-49. [3] SANDAHL TD, JEPSEN P, THOMSEN KL, et al. Incidence and mortality of alcoholic hepatitis in Denmark 1999-2008: A nationwide population based cohort study[J]. J Hepatol, 2011, 54(4): 760-764. DOI: 10.1016/j.jhep.2010.07.016 [4] SINGAL AK, BATALLER R, AHN J, et al. ACG clinical guideline: Alcoholic liver disease[J]. Am J Gastroenterol, 2018, 113(2): 175-194. DOI: 10.1038/ajg.2017.469 [5] Cooperative Group of Alcoholic Liver Disease. A multicenter study of alcoholic liver disease in China[J]. Chin J Dig, 2007, 27(4): 231-234. (in Chinese) DOI: 10.3760/j.issn:0254-1432.2007.04.005全国酒精性肝病调查协作组.全国酒精性肝病的多中心调查分析[J].中华消化杂志, 2007, 27(4): 231-234. DOI: 10.3760/j.issn:0254-1432.2007.04.005 [6] HMOUD BS, PATEL K, BATALLER R, et al. Corticosteroids and occurrence of and mortality from infections in severe alcoholic hepatitis: A meta-analysis of randomized trials[J]. Liver Int, 2016, 36(5): 721-728. DOI: 10.1111/liv.12939 [7] LOUVET A, WARTEL F, CASTEL H, et al. Infection in patients with severe alcoholic hepatitis treated with steroids: Early response to therapy is the key factor[J]. Gastroenterology, 2009, 137(2): 541-548. DOI: 10.1053/j.gastro.2009.04.062 [8] JENNE CN, KUBES P. Immune surveillance by the liver[J]. Nat Immunol, 2013, 14(10): 996-1006. DOI: 10.1038/ni.2691 [9] DIRCHWOLF M, RUF AE. Role of systemic inflammation in cirrhosis: From pathogenesis to prognosis[J]. World J Hepatol, 2015, 7(16): 1974-1981. DOI: 10.4254/wjh.v7.i16.1974 [10] THURSZ M, FORREST E, RODERICK P, et al. The clinical effectiveness and cost-effectiveness of STeroids Or Pentoxifylline for Alcoholic Hepatitis (STOPAH): A 2×2 factorial randomised controlled trial[J]. Health Technol Assess, 2015, 19(102): 1-104. DOI: 10.3310/hta191020 [11] VERGIS N, ATKINSON SR, KNAPP S, et al. In patients with severe alcoholic hepatitis, prednisolone increases susceptibility to infection and infection-related mortality, and is associated with high circulating levels of bacterial DNA[J]. Gastroenterology, 2017, 152(5): 1068-1077. DOI: 10.1053/j.gastro.2016.12.019 [12] FORREST EH, ATKINSON SR, RICHARDSON P, et al. Application of prognostic scores in the STOPAH trial: Discriminant function is no longer the optimal scoring system in alcoholic hepatitis[J]. J Hepatol, 2018, 68(3): 511-518. DOI: 10.1016/j.jhep.2017.11.017 [13] SANDAHL TD, VILSTRUP H, JEPSEN P. The long-term prognosis of alcoholic hepatitis is poor and independent of disease severity for patients surviving an acute episode[J]. J Hepatol, 2018, 68(6): 1330-1331. DOI: 10.1016/j.jhep.2018.02.025 [14] HERNAEZ R, SOLÀ E, MOREAU R, et al. Acute-on-chronic liver failure: An update[J]. Gut, 2017, 66(3): 541-553. DOI: 10.1136/gutjnl-2016-312670 [15] MEHTA G, MOOKERJEE RP, SHARMA V, et al. Systemic inflammation is associated with increased intrahepatic resistance and mortality in alcohol-related acute-on-chronic liver failure[J]. Liver Int, 2015, 35(3): 724-734. DOI: 10.1111/liv.12559 [16] MICHELENA J, ALTAMIRANO J, ABRALDES JG, et al. Systemic inflammatory response and serum lipopolysaccharide levels predict multiple organ failure and death in alcoholic hepatitis[J]. Hepatology, 2015, 62(3): 762-772. DOI: 10.1002/hep.27779 [17] WANG BY. Importance of the clinical research on alcoholic hepatitis[J]. J Clin Hepatol, 2019, 35(3): 469-471. (in Chinese) DOI: 10.3969/j.issn.1001-5256.2019.03.002王炳元.重视酒精性肝炎的临床研究[J].临床肝胆病杂志, 2019, 35(3): 469-471. DOI: 10.3969/j.issn.1001-5256.2019.03.002 [18] SERSTÉ T, CORNILLIE A, NJIMI H, et al. The prognostic value of acute-on-chronic liver failure during the course of severe alcoholic hepatitis[J]. J Hepatol, 2018, 69(2): 318-324. DOI: 10.1016/j.jhep.2018.02.022 [19] KIM HY, KIM CW, KIM TY, et al. Assessment of scoring systems for acute-on-chronic liver failure at predicting short-term mortality in patients with alcoholic hepatitis[J]. World J Gastroenterol, 2016, 22(41): 9205-9213. DOI: 10.3748/wjg.v22.i41.9205 期刊类型引用(4)
1. 朱淑雅,袁通立,王旭锋. 当归芍药散加减治疗酒精性肝硬化气滞血瘀型临床观察. 云南中医中药杂志. 2023(03): 75-77 .  百度学术
百度学术2. 任玲玲,陈芳玲,陈益,王立明,陈光兰. 非诺贝特对酒精性肝病患者肝脏巨噬细胞极化及肝纤维化指标的影响. 国际消化病杂志. 2023(05): 328-333+354 .  百度学术
百度学术3. 李翠琴,彭志,李学锋,向平. MELD评分对酒精性肝病患者短期预后预测价值的研究进展. 吉首大学学报(自然科学版). 2022(02): 73-77 .  百度学术
百度学术4. 刘兴斌,梁锡阳,陈奇辉,张泽霖. 2019年东莞市石碣镇农村居民体检中彩色多普勒超声在肝胆疾病诊断中的应用研究. 中国医学创新. 2021(17): 67-71 .  百度学术
百度学术其他类型引用(2)
-




 PDF下载 ( 2142 KB)
PDF下载 ( 2142 KB)

 下载:
下载:

 下载:
下载:
 百度学术
百度学术