游离脂肪酸诱导的肝细胞脂肪变性对巨噬细胞极化的影响
DOI: 10.3969/j.issn.1001-5256.2021.02.027
Effect of hepatocyte fatty degeneration induced by free fatty acid on macrophage polarization
-
摘要:
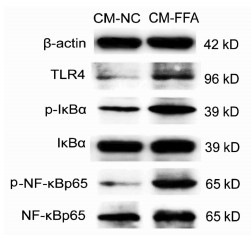
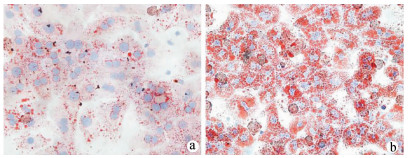
目的 探讨游离脂肪酸诱导的肝细胞脂肪变性对巨噬细胞极化的影响及可能机制。 方法 胶原酶原位灌注法分离C57BL/6小鼠原代肝细胞,将细胞分为对照(NC)组和混合游离脂肪酸(FFA)处理组,并制备肝细胞条件培养液(CM),以CM干预巨噬细胞株RAW264.7。油红O染色检测肝细胞内脂质沉积情况,Real-time PCR检测肝细胞脂质代谢相关基因和巨噬细胞M1/M2型极化基因表达,ELISA检测肝细胞培养上清细胞因子水平。Western Blot检测巨噬细胞Toll样受体4(TLR4)/核因子-κB(NF-κB)信号通路相关蛋白表达。计量资料两组间比较采用独立样本t检验;多组间比较采用单因素方差分析,进一步两两比较采用Tukey检验。 结果 与NC组相比,FFA孵育诱导肝细胞内大量脂滴沉积,细胞内甘油三酯和总胆固醇含量明显升高(t值分别为15.65、3.49,P值均 < 0.05)。FFA显著上调肝细胞脂质合成基因固醇调节元件结合蛋白1C和脂肪酸合酶的mRNA表达(t值分别为2.89、2.82,P值均 < 0.05),并降低脂质分解基因脂酰辅酶A氧化酶1和肉碱棕榈酰转移酶1A的mRNA表达(t值分别为14.30、3.36,P值均 < 0.05)。FFA诱导肝细胞培养上清炎性细胞因子IL-6、IL-1β和TNFα的水平明显增加(P值均 < 0.05)。相比CM-NC,CM-FFA显著增加巨噬细胞M1型基因诱生型一氧化氮合酶2、TNFα和IL-6 mRNA表达(P值均 < 0.05),降低M2型基因IL-10 mRNA表达水平(P < 0.05)。Western Blot检测结果显示,CM-FFA显著增加巨噬细胞TLR4、磷酸化NF-κBp65和磷酸化NF-κB抑制因子表达水平(t值分别为2.88、3.69、3.54,P值均 < 0.05)。 结论 FFA诱导的肝细胞脂肪变性和炎症反应可促进巨噬细胞M1型极化,启动和触发非酒精性脂肪性肝病发生发展。 Abstract:Objective To investigate the effect of hepatocyte fatty degeneration induced by free fatty acid on macrophage polarization and the possible mechanism. Methods Primary hepatocytes of C57BL/6 mice were isolated by in situ collagenase perfusion, and then the hepatocytes were divided into control (NC) group and mixed free fatty acid (FFA) treatment group. A conditioned medium (CM) was prepared for hepatocytes and was used for the intervention of RAW264.7 macrophages. Oil red O staining was used to observe lipid deposition in hepatocytes; real-time PCR was used to measure the mRNA expression of lipid metabolism genes and macrophage M1/M2 polarization markers; ELISA was used to measure the levels of cytokines in supernatant; Western blot was used to measure the expression of proteins involved in the Toll-like receptor 4 (TLR4)/nuclear factor-kappa B (NF-κB) pathway in macrophages. The independent samples t-test was used for comparison between two groups; a one-way analysis of variance was used for comparison between multiple groups, and the Tukey test was used for further comparison between two groups. Results Compared with the NC group, the FFA treatment group had the deposition of massive lipid droplets in hepatocytes and significant increases in triglyceride and total cholesterol (t=15.65 and 3.49, both P < 0.05). Besides, FFA significantly increased the mRNA expression of the lipid synthesis genes SREBP1C and FASN (t=2.89 and 2.82, both P < 0.05) and reduced the mRNA expression of the lipid decomposition genes ACOX1 and CPT1A (t=14.30 and 3.36, both P < 0.05) in hepatocytes. FFA also induced significant increases in the levels of the inflammatory cytokines interleukin-6 (IL-6), interleukin-1β, and tumor necrosis factor-α (TNF-α) in supernatant (all P < 0.05). Compared with the CM-NC group, the CM-FFA group had significant increases in the mRNA expression of the M1 phenotype markers iNOS2, TNF-α, and IL-6 (all P < 0.05) and a significant reduction in the mRNA expression of the M2 phenotype marker interleukin-10 (P < 0.05). Moreover, Western blot showed that CM-FFA significantly upregulated the protein expression of TLR4, p-NF-κBp65, and p-ⅠκBα in macrophages (t=2.88, 3.69, and 3.54, all P < 0.05). Conclusion FFA-induced hepatocyte fatty degeneration and inflammation can promote M1 macrophage polarization, thereby initiating and triggering the development and progression of nonalcoholic fatty liver disease. -
Key words:
- Non-alcoholic Fatty Liver Disease /
- Macrophages /
- Fatty Acids
-
表 1 Real-time PCR引物序列表
基因名称 引物序列 iNOS2 F:5′-GTGTTCCACCAGGAGATGTTG-3′ R:5′-CTCCTGCCCACTGAGTTCGTC-3′ TNFα F:5′-TCTTCTCATTCCTGCTTGTGG-3′ R:5′-GGTCTGGGCCATAGAACTGA-3′ IL-6 F:5′-GTTCTCTGGGAAATCGTGGA-3′ R:5′-GGAAATTGGGGTAGGAAGGA-3′ Arg1 F:5′-CTCCAAGCCAAAGTCCTTAGA-3′ R:5′-AGGAGCTGTCATTAGGGACATC-3′ Mrc2 F:5′-TACAGCTCCACGCTATGGATT-3′ R:5′-CACTCTCCCAGTTGAGGTACT-3′ IL-10 F:5′-GTTACTTGGGTTGCCAAG-3′ R:5′-TTGATCATCATGTATGCTTC-3′ IL-1β F:5′-TCTTTGAAGTTGACGGACCC-3′ R:5′-TGAGTGATACTGCCTGCCTG-3′ ACOX1 F:5′-AACAGCCCAACTGTGACTTC-3′ R:5′-ACAAAGGCATGTAACCCGTA-3′ CPT1A F:5′-CTTCCCATTTGACACCTTTG-3′ R:5′-ATACGTGAGGCAGAACTTGC-3′ SREBP1C F:5′-ACAGCAACCAGAAGCTCAAG-3′ R:5′-TGCCCTCCATAGACACATCT-3′ FASN F:5′-TTGGGTGCTGACTACAACCT-3′ R:5′-TGGATGATGTTGATGATGGA-3′ β-actin F:5′-TGTTACCAACTGGGACGACA-3′ R:5′-CTGGGTCATCTTTTCACGGT-3′ 注:iNOS2,诱生型一氧化氮合酶2;Arg1,精氨酸酶1;Mrc2,甘露糖受体c2;SREBP1C,固醇调节元件结合蛋白1C;FASN,脂肪酸合酶;ACOX1,脂酰辅酶A氧化酶1;CPT1A,肉碱棕榈酰转移酶1A。 表 2 两组肝细胞内脂质沉积及脂质代谢相关基因相对表达量的比较
组别 脂质沉积 脂质合成基因mRNA 脂质分解基因mRNA TG T-CHO SREBP1C FASN ACOX1 CPT1A NC组 1.00 1.00 1.00 1.00 1.00 1.00 FFA组 4.80±0.42 1.79±0.39 2.93±1.16 2.49±0.91 0.55±0.05 0.70±0.15 t值 15.65 3.49 2.89 2.82 14.30 3.36 P值 < 0.001 0.025 0.044 0.048 < 0.001 0.028 表 3 FFA对肝细胞分泌细胞因子的影响
组别 IL-6(pg/ml) IL-1β(pg/ml) TNFα(pg/ml) NC组 55.60±16.56 52.65±19.45 47.24±16.50 LPS组 307.40±69.861) 332.40±75.141) 139.20±42.981) FFA组 243.80±47.131) 246.20±34.011) 152.50±14.891) F值 20.93 25.73 12.63 P值 < 0.001 0.002 0.008 注:与NC组相比,1)P < 0.05。 表 4 3组巨噬细胞M1/M2型极化基因相对表达量的比较
组别 iNOS2 TNFα IL-6 Arg1 Mrc2 IL-10 CM-NC组 1.00 1.00 1.00 1.00 1.00 1.00 CM-FFA组 4.08±0.231) 2.78±0.171) 2.43±0.201) 1.24±0.53 0.89±0.10 0.36±0.081) 低浓度FFA组 1.33±0.292) 1.22±0.382) 1.28±0.222) 1.34±0.13 1.14±0.14 1.04±0.212) F值 191.80 50.24 58.16 0.92 4.53 25.08 P值 < 0.001 < 0.001 < 0.001 0.449 0.063 0.001 注:与CM-NC组相比,1)P < 0.05;与CM-FFA组组比,2)P < 0.05。 表 5 两组巨噬细胞TLR4/NF-κB通路相关蛋白相对表达量的比较
组别 TLR4 p-ⅠκBα/ⅠκBα p-NF-κBp65/NF-κBp65 CM-NC组 1.00 1.00 1.00 CM-FFA组 2.50±0.52 3.53±1.24 5.95±1.34 t值 2.88 3.54 3.69 P值 0.045 0.024 0.021 -
[1] YOUNOSSI ZM. Non-alcoholic fatty liver disease - A global public health perspective[J]. J Hepatol, 2019, 70(3): 531-544. DOI: 10.1016/j.jhep.2018.10.033 [2] TACKE F. Targeting hepatic macrophages to treat liver diseases[J]. J Hepatol, 2017, 66(6): 1300-1312. DOI: 10.1016/j.jhep.2017.02.026 [3] WANG Q, XU QY, WU HM, et al. Effect of lipid-induced macrophage M1/M2 polarization on lipid metabolism in hepatocytes[J]. Chin J Hepatol, 2018, 26(4): 276-281. (in Chinese) DOI: 10.3760/cma.j.issn.1007-3418.2018.04.009王祺, 许钦瑜, 吴惠敏, 等.脂质诱导的巨噬细胞M1/M2型极化对肝细胞脂质代谢的影响[J].中华肝脏病杂志, 2018, 26(4): 276-281. DOI: 10.3760/cma.j.issn.1007-3418.2018.04.009 [4] GÓMEZ-LECHÓN MJ, DONATO MT, MARTÍNEZ-ROMERO A, et al. A human hepatocellular in vitro model to investigate steatosis[J]. Chem Biol Interact, 2007, 165(2): 106-116. DOI: 10.1016/j.cbi.2006.11.004 [5] XIAO WS, LE YY, ZENG SL, et al. Research advances in the pathogenesis of nonalcoholic fatty liver disease[J]. J Clin Hepatol, 2020, 36(8): 1874-1879. (in Chinese) DOI: 10.3969/j.issn.1001-5256.2020.08.043肖伟松, 乐滢玉, 曾胜澜, 等.非酒精性脂肪性肝病的发病机制研究进展[J].临床肝胆病杂志, 2020, 36(8): 1874-1879. DOI: 10.3969/j.issn.1001-5256.2020.08.043 [6] HOLLAND WL, BIKMAN BT, WANG LP, et al. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice[J]. J Clin Invest, 2011, 121(5): 1858-1870. DOI: 10.1172/JCI43378 [7] KAZANKOV K, JØRGENSEN S, THOMSEN KL, et al. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis[J]. Nat Rev Gastroenterol Hepatol, 2019, 16(3): 145-159. DOI: 10.1038/s41575-018-0082-x [8] TOSELLO-TRAMPONT AC, LANDES SG, NGUYEN V, et al. Kuppfer cells trigger nonalcoholic steatohepatitis development in diet-induced mouse model through tumor necrosis factor-α production[J]. J Biol Chem, 2012, 287(48): 40161-40172. DOI: 10.1074/jbc.M112.417014 [9] WU HM, NI XX, XU QY, et al. Regulation of lipid-induced macrophage polarization through modulating peroxisome proliferator-activated receptor-gamma activity affects hepatic lipid metabolism via a Toll-like receptor 4/NF-κB signaling pathway[J]. J Gastroenterol Hepatol, 2020, 35(11): 1998-2008. DOI: 10.1111/jgh.15025 [10] KOU XN, XIE XK, HAO MX, et al. Effects and mechanism of microRNA-140 inhibition on the development of non-alcoholic fatty liver disease in mice[J/CD]. Chin J Liver Dis (Electronic Version), 2020, 12(3): 34-40. (in Chinese)寇小妮, 解新科, 郝明霞, 等.微小RNA-140抑制对小鼠非酒精性脂肪性肝病进展的影响及机制[J/CD].中国肝脏病杂志(电子版), 2020, 12(3): 34-40. [11] ZHENG Y, WANG JR, LIU LL, et al. Molecular mechanism of the anti -liver fibrosis effect of curcumol: An analysis based on the TLR4 /NF-κB signaling pathway[J]. J Clin Hepatol, 2020, 36(7): 1508-1513. (in Chinese) DOI: 10.3969/j.issn.1001-5256.2020.07.013郑洋, 王嘉孺, 刘露露, 等.基于Toll样受体4 /核因子-κB信号通路研究莪术醇抗肝纤维化的分子机制[J].临床肝胆病杂志, 2020, 36(7): 1508-1513. DOI: 10.3969/j.issn.1001-5256.2020.07.013 [12] ZHANG CH, LI Y, LI ZY, et al. Protective effect of procyanidine B1 on LPS-induced injury of mouse macrophages RAW264.7 and its mechanism[J]. J Jilin Univ(Med Edit), 2019, 45(6): 1243-1247. (in Chinese) http://www.cnki.com.cn/Article/CJFDTotal-BQEB201906008.htm张宸豪, 李瑶, 李正祎, 等.原花青素B1对LPS诱导小鼠巨噬细胞RAW264.7损伤的保护作用及其机制[J].吉林大学学报(医学版), 2019, 45(6): 1243-1247. http://www.cnki.com.cn/Article/CJFDTotal-BQEB201906008.htm [13] GORDON S, MARTINEZ F O. Alternative activation of macrophages: Mechanism and functions[J]. Immunity, 2010, 32(5): 593-604. DOI: 10.1016/j.immuni.2010.05.007 -



 PDF下载 ( 2265 KB)
PDF下载 ( 2265 KB)

 下载:
下载: