程序性细胞死亡受体1及其配体抑制剂在肝细胞癌治疗中的应用进展
DOI: 10.3969/j.issn.1001-5256.2021.02.040
Advances in the application of programmed death-1/programmed death-ligand 1 inhibitors in the treatment of hepatocellular carcinoma
-
摘要: 肝细胞癌(HCC)是我国最常见的恶性肿瘤之一,由于缺乏特异性表现,超过一半的患者在初诊时已处于进展期。靶向治疗和系统化疗是进展期HCC的主要治疗方法,疗效有限。近年来,免疫治疗迅速发展。介绍了免疫检查点抑制剂——程序性细胞死亡受体1及其配体(PD-1/PD-L1)抑制剂在HCC治疗中的现状,归纳了多项临床试验的数据结果,分析了单药治疗及联合治疗的安全性及有效性。通过分析表明,免疫治疗已成为全身系统治疗的重要方法之一,尤其是联合治疗可显著提高HCC的疗效,其安全性亦在可控范围内,是未来发展的重要方向。
-
关键词:
- 癌, 肝细胞 /
- 免疫疗法 /
- 免疫检查点抑制剂 /
- 程序性细胞死亡受体1
Abstract: Hepatocellular carcinoma (HCC) is one of the most common malignancies in China, and due to the lack of specific symptoms, more than half of these patients are in the advanced stage at the time of initial diagnosis. Targeted therapy and systemic chemotherapies are the main treatment methods for advanced HCC with limited efficacy. In recent years, immunotherapy has been developed rapidly. This article introduces the current status of the immune checkpoint inhibitors, programmed death-1 (PD-1)/programmed death-ligand 1 (PD-L1) inhibitors, in the treatment of HCC, summarizes the latest data of several clinical trials, and analyzes the safety and efficacy of monotherapy and combination therapy. The analysis shows that immunotherapy has become one of the important methods for systemic treatment, and combination therapy can significantly improve the outcome of HCC with a manageable safety profile, which is an important direction for future development. -
原发性肝癌目前是我国第4位常见恶性肿瘤及第2位肿瘤致死病因,肝细胞癌(HCC)是原发性肝癌中最常见的病理类型,占85%~90%[1]。本文中所讲的“肝癌”特指HCC。肝癌缺乏特异性症状,早期诊断困难,超过一半的患者初诊时已经处于进展期,需要行全身系统治疗。既往靶向治疗和系统化疗是进展期HCC的主要治疗方法,疗效有限。近年来,免疫治疗发展迅速,在肝癌中也展开应用。
免疫系统对肿瘤细胞具有清除作用。肿瘤免疫主要由T淋巴细胞介导,肿瘤细胞逃避免疫监视有多种途径,包括无效的肿瘤抗原递呈、免疫抑制细胞聚集、免疫抑制因子释放,以及免疫检查点的失调。调控T淋巴细胞激活或抑制的信号通路称为免疫检查点,目前研究最多的包括细胞毒性T淋巴细胞相关抗原4(cytotoxic T lymphocyte associated antigen 4, CTLA-4)通路和程序性细胞死亡受体1(programmed death-1, PD-1)通路。PD-1是一种Ⅰ型跨膜蛋白,主要表达于活化的T淋巴细胞、B淋巴细胞、自然杀伤细胞等,其配体包括PD-L1(programmed death ligand 1, PD-L1)和PD-L2。PD-1与配体结合后,可抑制T淋巴细胞活性,诱导抗原耐受,促进T淋巴细胞凋亡,从而抑制或终止免疫应答,防止自身免疫疾病的发生。肿瘤细胞可以表达PD-L1,与T淋巴细胞表面的PD-1结合后,抑制T淋巴细胞的杀伤功能,还可以抑制细胞因子如IL-2、IFNγ的产生,导致肿瘤免疫逃逸,促进肿瘤生长[2]。因此,应用特异性单克隆抗体阻断PD-1和PD-L1的结合,可增强T淋巴细胞的增殖和杀伤功能,发挥抗肿瘤作用。
PD-1或PD-L1抑制剂已广泛用于多种恶性肿瘤的治疗,在肝癌中也开展了多项临床试验,并逐步被指南推荐为系统治疗的一线或二线方案。巴塞罗那分期(BCLC)是目前最常用的肝癌分期标准,免疫治疗主要在无法切除的肝癌(unresectable HCC, uHCC)患者中进行,包括BCLC C期的进展期肝癌(advanced HCC, aHCC)和部分BCLC B期但经介入治疗后进展的患者。本文就PD-1/PD-L1抑制剂在HCC中应用的临床研究进展作一综述。
1. PD-1/PD-L1抑制剂单药治疗
PD-1或PD-L1抑制剂单药治疗aHCC在全球范围开展了多项临床试验(表 1),验证了其有效性和安全性,并逐步被多个指南推荐为系统治疗药物。
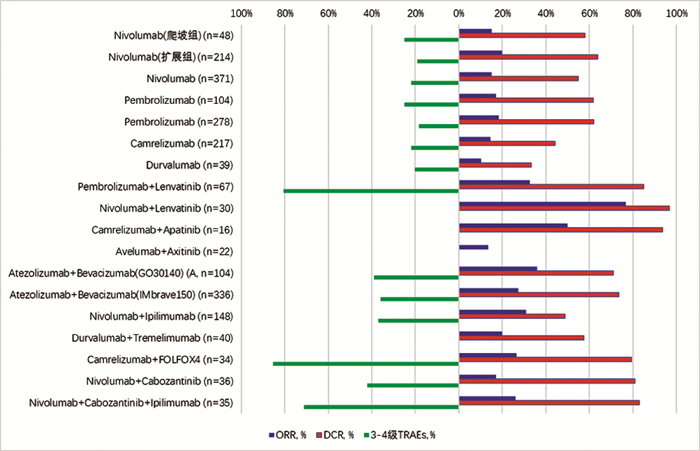
表 1 PD-1/PD-L1抑制剂单药治疗在aHCC中的临床试验药物 临床试验 分期 治疗线数 入组例数 ORR(%) CR(%) DCR(%) mDOR(月) mOS(月) mPFS(月) TRAEs(%) 任何级别 3~4级 Nivolumab CheckMate 040 Ⅰ/Ⅱ - 48 15 6 58 17 15.0 - 83 25 - 214 20 1 64 9.9 NR 4.0 74 19 一线 80 20 1 54 17.15 28.6 - 78 29 二线 ESC组,n=37 19 3 54 19.35 15.0 - 77 18 EXP组,n=145 14 1 55 16.59 15.6 - - 85(亚洲队列) 15 4 49 9.7 14.9 - 74 16 - 49(Child-Pugh B) 10.2 - 55.1 9.9 7.6 - 51 24.5 Nivolumabvs Sorafenib CheckMate 459 Ⅲ 一线 743(NIVO,n=371;SOR,n=372) 15 vs 7 4 vs 1 55 vs 58 23.3 vs 23.4 16.4 vs 14.7 3.7 vs 3.8 - 22 vs 49 Pembrolizumab KEYNOTE-224 Ⅱ 二线 104 17 1 62 NR 12.9 4.9 73 25 Pembrolizumabvs安慰剂 KEYNOTE-240 Ⅲ 二线 413(PEM,n=278;安慰剂,n=135) 18.3 vs 4.4 2.2 vs 0 62.2 vs 53.3 13.8 vs NR 13.9 vs 10.6 3.0 vs 2.8 60.9 vs 48.5 18.3 vs 7.5 Camrelizumab(SHR-1210) - Ⅱ 二线 217 14.7 0 44.2 NR 13.8 2.1 - 22 Durvalumab - Ⅰ/Ⅱ 二线 40(可评估,n=39) 10.3 (n=39) 0 33.3 (n=39) - 13.2 - 80 20 注:“-”,未提供数据;ORR,客观缓解率;CR,完全缓解;DCR,疾病控制率;mDOR,中位缓解持续时间;mOS,中位总生存期;mPFS,中位无进展生存期;TRAEs,治疗相关不良事件;NR,未达到;ESC,剂量爬坡;EXP,剂量扩展;表格中所列数据均根据实体瘤疗效评价标准1.1版(RECIST v1.1)评估所得。 1.1 纳武利尤单抗(Nivolumab)
Nivolumab是抗PD-1的全人源化IgG4型单克隆抗体。CheckMate 040是Nivolumab在aHCC中开展的Ⅰ/Ⅱ期、多队列研究[3]。队列1和队列2是Nivolumab的ESC与EXP试验,共262例患者。ESC队列(Nivolumab 0.1/0.3/1/3/10 mg/kg,静脉点滴,每2周一次)纳入48例,结果显示TRAEs的发生率与药物使用剂量无关。1例患者因药物相关毒性而停药,3例患者因TRAEs而停药,没有治疗相关死亡发生。常见的TRAEs(发生率>10%)包括AST、ALT、脂肪酶、淀粉酶的升高,以及皮疹和瘙痒。12例(25%)出现3~4级TRAEs,3例(6%)发生治疗相关严重不良事件(serious adverse events, SAEs)。疗效方面,大部分患者在3个月内出现应答,ORR为15%,DCR为58%,mOS为15.0个月。EXP队列(Nivolumab 3 mg/kg,静脉点滴,每2周一次)纳入214例,ORR为20%,DCR为64%,mPFS为4个月,mOS未达到。安全性方面,8例患者因药物毒性而停药,24例患者因TRAEs而停药,没有治疗相关死亡发生。
262例患者中,索拉非尼(Sorafenib)初治80例,Sorafenib经治182例[4]。Nivolumab作为一线治疗,ORR为20%,mOS为28.6个月,治疗有效率和长期生存均有明显改善;作为二线治疗,在ESC组和EXP组中,ORR分别为19%和14%,mOS分别为15.0和15.6个月。在Sorafenib经治的182例患者(ITT队列)中,来自亚洲的有85例[5],亚洲队列患者HBV感染、远处转移和既往接受过治疗的比例更高,对我国更具参考意义。ITT队列和亚洲队列的ORR分别为14%和15%,mOS分别为15.1和14.9个月。
CheckMate 040第5队列研究了Nivolumab用于Child-Pugh B级的aHCC[6]。49例患者ORR为10.2%,mOS为7.6个月。安全性方面与Child-Pugh A级患者相类似。另一回顾性病例分析[7]中,18例Child-Pugh B级aHCC患者应用Nivolumab,17例发生3级以上AEs,5例发生3级以上TRAEs,4例患者因TRAEs而停药,ORR为17%,mOS为5.9个月。Nivolumab用于Child-Pugh B级患者需要谨慎,并有待更多循证学证据。
CheckMate 459是对比Nivolumab与Sorafenib用于一线治疗aHCC的Ⅲ期临床试验(Nivolumab 240 mg,静脉点滴,每2周一次;Sorafenib 400 mg,口服,2次/d),在2019年欧洲肿瘤内科学会(ESMO)年会上公布了结果[8]。Nivolumab组(371例)与Sorafenib组(372例)mOS分别为16.4和14.7个月(HR=0.85,95%CI:0.72~1.02,P=0.075 2),未达到统计意义的预定阈值,两组ORR分别为15%和7%。不同于CheckMate 040中Nivolumab作为一线用药的良好结果,该Ⅲ期临床试验没有达到预期的主要研究终点(OS),但与Sorafenib相比,Nivolumab的疗效确实有所提高。
基于CheckMate 040的结果,2017年9月Nivolumab获得美国食品药品管理局(FDA)快速批准作为二线方案用于既往Sorafenib治疗后进展或无法耐受的肝癌患者。2018年中国临床肿瘤学会颁布的原发性肝癌诊疗指南[9]中将Nivolumab作为晚期HCC的二线治疗。由于CheckMate 459的失败,Nivolumab仍被推荐作为二线方案,但由于其表现出较好的生存获益和安全性,在2020年美国国立综合癌症网络(NCCN)指南[10]中指出,当不能应用酪氨酸激酶抑制剂或其他抗血管生成药时,推荐Nivolumab作为一线用药,而且Nivolumab作为二线用药时适应证也包含了Child-Pugh B级患者。
1.2 帕博利珠单抗(Pembrolizumab)
Pembrolizumab是抗PD-1的人源化IgG4型单克隆抗体。KEYNOTE-224是评估Pembrolizumab二线治疗aHCC的Ⅱ期临床试验(Pembrolizumab 200 mg,静脉点滴,每3周一次)[11]。纳入104例患者,中位随访时间12.3个月,ORR为17%,mPFS为4.9个月,mOS为12.9个月。3级TRAEs有25例(24%),最常见AST升高、ALT升高、疲劳和肾上腺功能不全,4级TRAEs有1例(高胆红素血症),治疗相关死亡1例(溃疡性食管炎)。15例(14%)发生免疫相关不良事件(immune-related AEs, irAEs),3例(3%)发生免疫相关肝炎,没有观察到HBV或HCV的活化。最新发布的KEYNOTE-224随访2年后的数据[12]显示,24个月总生存率为30.8%,ORR从17%提高到18.3%。
KEYNOTE-240是Pembrolizumab(200 mg,静脉点滴,每3周一次)对比安慰剂用于aHCC二线治疗的Ⅲ期临床试验[13]。Pembrolizumab组(278例)和安慰剂(135例)组mOS分别为13.9和10.6个月,mPFS分别为3.0和2.8个月,ORR分别为18.3%和4.4%。试验结果未达到预设的统计学终点,研究结果为阴性。在Pembrolizumab组和安慰剂组,3级以上TRAEs分别占18.3%和7.5%。irAEs在Pembrolizumab组比例较高(18.3% vs 8.2%),常见甲状腺功能减退、甲状腺功能亢进和肺炎。
基于KEYNOTE-224研究,2018年11月美国FDA批准Pembrolizumab作为二线治疗用于既往接受过Sorafenib治疗的aHCC患者。由于KEYNOTE-240的失败,NCCN在2019年第2版肝胆肿瘤指南[14]中将Pembrolizumab用于aHCC二线治疗的证据类别从2A改为2B。Pembrolizumab在亚洲人群中的Ⅲ期临床试验KEYNOTE-394正在进行,以期再次验证其疗效。
1.3 卡瑞利珠单抗(Camrelizumab)
Camrelizumab(SHR-1210)也是抗PD-1的人源化IgG4型单克隆抗体,在我国开展了二线治疗aHCC的Ⅱ期临床试验(SHR-1201 3 mg/kg,静脉点滴,每2周或3周一次)[15]。该研究中HBV感染的患者比例较高(83%)。217例患者ORR为14.7%,mPFS为2.1个月,mOS为13.8个月。研究中发现,有少部分患者出现治疗后肿瘤病灶先增大后缩小的情况。所有级别中最常见的TRAEs为反应性皮肤毛细血管增生症(67%)、AST升高(25%)、ALT升高(24%)和蛋白尿(23%)。发生反应性皮肤毛细血管增生症的患者绝大多数见于体表皮肤,无内脏出血或死亡发生,且客观缓解比例更高(ORR 19.3% vs 5.6%)。发生3~4级TRAEs者占22%。46例发生HBV DNA水平升高,但没有患者因此中断或终止治疗。
1.4 度伐利尤单抗(Durvalumab)
Durvalumab是抗PD-L1的人源化IgG1型单克隆抗体。2017年美国临床肿瘤学会(ASCO)公布了Durvalumab单药治疗实体肿瘤的Ⅰ/Ⅱ期临床试验[16]中肝癌患者的中期分析结果:40例HCC患者接受Durvalumab单药治疗(10 mg/kg,静脉点滴,每2周一次),其中93%患者既往应用过Sorafenib。TRAEs发生率80%,最常见为疲劳、瘙痒和AST升高,20%患者出现3~4级TRAEs,无TRAEs所致停药或死亡。40例患者mOS为13.2个月,39例可评估疗效,ORR为10.3%,DCR为33.3%。
2. PD-1/PD-L1抑制剂联合治疗
治疗HCC的不同药物抗肿瘤的机制不同,可能具有潜在的协同作用。系统化疗可暴露肿瘤抗原,更好地被免疫系统识别;靶向药物可减少免疫抑制性细胞数量、诱导活化的细胞毒性T淋巴细胞;抗血管生成药有助于肿瘤血管的正常化,重塑肿瘤微环境。免疫治疗与其他治疗机制的药物联合,或者两种不同机制作用通路的免疫治疗联合,是目前研究的主要方向[17-18](表 2)。
表 2 PD-1/PD-L1抑制剂联合治疗在aHCC中的临床试验药物 临床试验 分期 治疗线数 入组例数 ORR(%) CR(%) DCR(%) mDOR(月) mOS(月) mPFS(月) TRAEs(%) 任何级别 3~4级 Pembrolizumab+Lenvatinib KEYNOTE-524/Study 116 Ⅰb 一线 100 36 1 88 12.6 22.0 8.6 95 67 Nivolumab+Lenvatinib Study 117 Ⅰb 一线 30 76.7 13.3 96.7 - - - 100 - Camrelizumab(SHR-1210)+Apatinib - Ⅰ 二线 16(可评估) 50 0 93.8 NR NR 7.2 - - Avelumab+Axitinib VEGF Liver 100 Ⅰb 一线 22 13.6 - - - - 5.5 - - Atezolizumab+Bevacizumab GO30140 Ⅰb 一线 队列A,n=104 36 12 71 NE 17.1 7.3 88 39 队列F,n=119
Atezo+Bev,n=60;
Atezo,n=5920 vs 17 2 vs 5 67 vs 49 NE vs NE - 5.6 vs 3.4 68 vs 41 20 vs 5 Atezolizumab+Bevacizumabvs Sorafenib IMBrave 150 Ⅲ 一线 501Atezo+Bev,n=336;
SOR,n=16527.3 vs 11.9 5.5 vs 0 73.6 vs 55.3 NE vs 6.3 NE vs 13.2 6.8 vs 4.3 84 vs 94 36 vs 46 194(中国人群)
Atezo+Bev,n=133;
SOR,n=6125 vs 7 - - - NE vs 11.4 5.7 vs 3.2 - - Nivolumab+Ipilimumab CheckMate 040 Ⅰ/Ⅱ 二线 148 31 4.7 49 17.5 - - - 37 A组,n=50 32 8 54 17.5 22.8 B组,n=49 31 6 43 22.2 12.5 C组,n=49 31 0 49 16.6 12.7 Durvalumab+Tremelimumab - Ⅱ - T300+D,n=75 22.7 - - NR 18.7 - - 35.1 T75+D,n=84 9.5 - - 13.2 11.3 - - 24.4 Camrelizumab+FOLFOX4 - Ⅱ 一线 34 26.5 - 79.4 NR NR 5.5 - 85.3 Nivolumab+Cabozantinib±Ipilimumab CheckMate 040 Ⅰ/Ⅱ - 71二联,n=36三联,n=35 17 vs 26 0 vs 0 81 vs 83 - NR vs NR 5.5 vs 6.8 - 42 vs 71 注:NE,无法评估;表格中所列数据均根据RECIST v1.1评估所得;Atezo, Atezolizumab; Bev, Bevacizumab; SOR, Sorafenib。 2.1 与分子靶向药物联合
KEYNOTE-524/Study 116是Pembrolizumab联合仑伐替尼(Lenvatinib)一线治疗uHCC的Ⅰb期临床试验[Lenvatinib 12 mg(≥60 kg)或8 mg(<60 kg),口服,1次/d+Pembrolizumab 200 mg,静脉点滴,每3周一次],2020年ASCO年会公布了最新数据[19]:共纳入104例患者,对100例进行分析,ORR达36%,DCR达88%,mPFS为8.6个月,mOS达到22.0个月。安全性方面,3级以上TRAEs占67%(最常见的为高血压,占17%),3例因TRAEs死亡。初期分析提示联合治疗的有效率和生存期明显提高,但需警惕其安全性及药物的相互作用。Lenvatinib联合Pembrolizumab一线治疗aHCC的Ⅲ期临床试验LEAP-002已经启动[20]。
Nivolumab联合Lenvatinib用于一线治疗uHCC的Ⅰ b期临床试验数据(Study117)在2020年ASCO-GI大会公布[21],30例患者接受Lenvatinib+Nivolumab治疗[Lenvatinib 12 mg(≥60 kg)或8 mg(<60 kg),口服,1次/d+Nivolumab 240 mg,静脉点滴,每2周一次],ORR为76.7%,DCR为96.7%。30例均出现TRAEs,最常见的为手足综合征(56.7%)和发音困难(53.3%)。
Camrelizumab(SHR-1210)联合阿帕替尼(Apatinib)用于二线治疗aHCC和胃癌/胃食管交界处癌的Ⅰ期临床试验[22]结果已公布。共纳入43例患者,其中18例为肝癌(均为HBV感染)。ESC队列的给药方案为:Apatinib 125/250/500 mg,口服,1次/d+SHR-1210 1200 mg,静脉点滴,每2周一次,EXP队列中Apatinib推荐剂量为250 mg。在应用Apatinib 250 mg的33例患者中,2例因TRAEs停药,常见的3级以上TRAEs为高血压(15.2%)和AST升高(15.2%)。SHR-1210可能增加了部分Apatinib相关AEs的发生率,如高血压和ALT/AST升高,但SHR-1210相关AEs并无明显增加,最常见的反应性皮肤毛细血管增生症发生率反而有所下降。疗效方面,其中16例可评估,ORR为50%,mPFS为7.2个月,mOS未达到。
阿维鲁单抗(Avelumab)为人源化抗PD-L1的IgG1单抗。VEGF Liver 100是评估Avelumab联合阿昔替尼(Axitinib)用于初治HCC的Ⅰb期临床试验(Avelumab 10 mg/kg,静脉点滴,每2周一次+Axitinib 5 mg,口服,2次/d)[23]。22例患者中,最常见的3级TRAEs为高血压(50%)和手足综合征(22.7%),无4~5级TRAEs发生,最常见的irAEs为甲状腺功能减退(31.8%)和甲状腺功能亢进(13.6%)。采用RECIST v1.1和改良的实体瘤疗效评价标准(modified RECIST, mRECIST)评价ORR分别为13.6%和31.8%,mPFS分别为5.5和3.8个月,OS的数据尚不可评估。
卡博替尼(Cabozantinib)是酪氨酸激酶抑制剂,为HCC系统治疗的二线药物。COSMIC-312是评估Cabozantinib联合Atezolizumab对比Sorafenib用于aHCC一线治疗的Ⅲ期临床研究[24],目前正在进行。
2.2 与抗血管生成抗体联合
阿替利珠单抗(Atezolizumab)是人源化抗PD-L1的IgG1单抗,贝伐珠单抗(Bevacizumab)为抗血管生成的单抗。GO30140是研究Atezolizumab+Bevacizumab一线治疗uHCC的Ⅰb期临床试验,2019年ESMO年会上发布了最新数据[25]。A组104例患者接受Atezolizumab+Bevacizumab联合治疗(Atezolizumab 1200 mg+Bevacizumab 15 mg/kg,静脉点滴,每3周一次),ORR达到36%,mPFS为7.3个月,mOS为17.1个月;3~4级TRAEs有39%,5级TRAEs有3%。F组患者中,60例接受Atezolizumab+Bevacizumab联合治疗,59例接受Atezolizumab单药治疗,联合治疗相比于单药治疗可显著改善mPFS(5.6个月vs 3.4个月),但TRAEs发生比例亦更高。两组中,最常见的不良反应有蛋白尿、食欲下降、疲劳、皮疹、腹泻、高血压和腹痛,最常见的SAEs为肺炎和骨折。
IMbrave150是Atezolizumab+Bevacizumab对比Sorafenib一线治疗uHCC的Ⅲ期临床试验[26-27]。Atezolizumab+Bevacizumab组(336例)和Sorafenib组(165例)的mOS分别为未达到和13.2个月(HR=0.58,95%CI:0.42~0.79,P<0.001),mPFS分别为6.8和4.3个月(HR=0.59,95%CI:0.47~0.76,P<0.001),ORR分别为27.3%和11.9%,DCR分别为73.6%和55.3%。联合治疗组的有效率和生存时间均明显延长。安全性方面,在Atezolizumab+Bevacizumab组和Sorafenib组中,3~4级TRAEs分别为36%和46%。联合治疗还可以延缓患者报告生活质量发生恶化的时间(至恶化发生的中位时间:11.2个月vs 3.6个月,HR=0.63,95%CI:0.46~0.85)。2020年2月欧洲肝病学会主办的肝癌峰会上公布了IMbrave 150的中国亚群数据[28]。共194例中国患者(137例来自IMbrave150全球研究,57例中国扩展队列)。中国患者中HBV感染、BCLC C期、发生血管侵犯和/或远处转移、AFP≥400 ng/ml的比例更高。联合治疗组(133例)比Sorafenib组(61例)的mOS、mPFS、ORR均显著提高。
IMbrave150取得双终点阳性结果,联合治疗组OS和PFS都显著延长,ORR和DCR也大幅提高,提示免疫联合疗法可能成为治疗aHCC的有效方法。基于此研究结果,2020年NCCN新版指南[10]中将Atezolizumab+Bevacizumab联合治疗推荐为系统治疗的一线用药。
2.3 与CTLA-4抑制剂联合
2019年ASCO会议上,CheckMate 040第4队列数据证实Nivolumab和伊匹单抗(Ipilimumab,CTLA-4抑制剂)联合治疗可使aHCC患者显著获益,且安全性可控[29-30]。148例Sorafenib经治患者随机分为3组:A组Nivolumab 1 mg/kg+Ipilimumab 3 mg/kg,每3周一次(连续4个周期);B组Nivolumab 3 mg/kg+Ipilimumab 1 mg/kg,每3周一次(连续4个周期),随后接受Nivolumab 240 mg,每2周一次;C组Nivolumab 3 mg/kg,每2周一次+Ipilimumab 1 mg/kg,每6周一次。截至2018年9月25日,总体ORR为31%,3组mOS分别为22.8、12.5和12.7个月。安全性方面,最常见的TRAEs为瘙痒和皮疹,3~4级TRAEs占37%,多见AST和脂肪酶升高,未观察到因增加Ipilimumab出现新的不良反应。NCCN指南[10]在2020年将Nivolumab+Ipilimumab联合治疗纳入系统治疗的二线方案。
Durvalumab(D)联合Tremelimumab(T)(CTLA-4抑制剂)用于uHCC的Ⅱ临床试验在2020年ASCO年会上公布结果[31]。研究共分4组,其中两组为T+D联合治疗[T300+D组(n=75):300 mg T药+ 1500 mg D药1个疗程后使用D药,每4周一次;T75+D组(n=84):75 mg T药+1500 mg D药4个疗程后使用D药,每4周一次]。T300+D组的ORR为22.7%,mOS达18.7个月;T75+D组ORR为9.5%,mOS为11.3个月;安全性方面,两组3~4级TRAEs分别占35.1%和24.4%,因TRAEs而停药者分别占10.8%和6.1%。两药联合对比Sorafenib用于一线治疗uHCC的Ⅲ期临床研究(HIMALAYA)正在开展中[32]。
2.4 与系统化疗联合
我国秦叔逵教授团队[33]开展了Camrelizumab(SHR-1210)(3 mg/kg,静脉点滴,每2周一次)联合FOLFOX4(氟尿嘧啶+亚叶酸+奥沙利铂)或GEMOX(吉他西滨+奥沙利铂)全身性化疗用于一线治疗aHCC或胆道癌的Ⅱ期临床研究。在34例HCC患者中,27例合并HBV感染,ORR为26.5%,DCR为79.4%,mPFS为5.5个月,mOS尚未达到。3级以上TRAEs发生率为85.3%,最常见的包括中性粒细胞减少、白细胞减少和血小板减少。
2.5 多药联合
Checkmate 040的另一队列研究了Nivolumab+Cabozantinib±Ipilimumab用于aHCC的疗效和安全性,在2020年ASCO-GI会议上公布结果[34]。Sorafenib初治或经治的aHCC患者随机分为两组:Nivolumab 240 mg,静脉点滴,每2周一次+Cabozantinib 40 mg,口服,1次/d,或者Nivolumab 3 mg/kg,每2周一次+Ipilimumab 1 mg/kg,每6周一次+Cabozantinib 40 mg,口服,1次/d。二联治疗组(36例)和三联治疗组(35例)ORR分别为17%和26%,mPFS分别为5.5和6.8个月,mOS均未达到。安全性方面,二联治疗的TRAEs发生率低于三联治疗,两组发生3~4级TRAEs的患者分别有15例(42%)和25例(71%),分别导致1例和7例患者停药。
3. 小结
HCC的治疗已经进入免疫治疗时代。目前PD-1/PD-L1抑制剂在HCC治疗中已取得初步成绩。综合上述临床试验结果(图 1),PD-1/PD-L1抑制剂单药治疗的ORR为14%~20%,DCR为44%~64%,mOS大部分为13~17个月,mPFS为2~5个月,与标准Sorafenib或安慰剂治疗相比,疗效有所提高,但取得的优势有限。部分临床试验未达到统计学差异,可能与对照组患者疾病进展后加用其他二线治疗或者免疫治疗后生存期延长相关。而在免疫联合治疗中,ORR大部分可达20%~35%,DCR可达49%~74%,mOS亦明显延长,基本在13个月以上,最长可达22.8个月,且有许多临床试验尚在随访中,mPFS可达5~9个月。以免疫治疗为主的联合治疗在HCC的临床试验中显示出前所未有的疗效,正在改变HCC的治疗格局,是未来的重要研究方向。
在关注良好疗效的同时,亦需重视免疫治疗相关不良事件(图 1)。在PD-1/PD-L1抑制剂单药治疗中,3~4级TRAEs发生率为18%~29%,而在免疫联合治疗中,3~4级TRAEs发生率明显升高,达20%~80%。两种不同作用机制的药物单独不良反应,以及药物间的相互作用,可能都导致了TRAEs发生率的增加。故如何在提高疗效的同时,最大限度减少不良反应,是免疫联合治疗亟需面对的问题。对于联合治疗,合理布局免疫治疗与其他药物的用药顺序(联合或序贯),确定哪种策略安全性更好,需要进一步的探索。
除用于aHCC的系统治疗,免疫治疗在术前新辅助或术后辅助治疗中也逐步开展,以期发挥更大的临床效益。目前认为与免疫治疗效果相关的生物标志物有PD-L1表达水平、高度微卫星不稳定性或错配修复缺陷和肿瘤突变负荷等。在其他恶性肿瘤中,PD-1/PD-L1抑制剂的疗效与生物标志物的相关性已得到证实,笔者也期待在HCC中早日发现合适的生物标志物,用于筛选优势获益人群,实现精确、个体化的治疗。综上,免疫治疗有望贯穿于HCC治疗的各个阶段,多项临床试验正在进行中,将使越来越多的肝癌患者获益。
-
表 1 PD-1/PD-L1抑制剂单药治疗在aHCC中的临床试验
药物 临床试验 分期 治疗线数 入组例数 ORR(%) CR(%) DCR(%) mDOR(月) mOS(月) mPFS(月) TRAEs(%) 任何级别 3~4级 Nivolumab CheckMate 040 Ⅰ/Ⅱ - 48 15 6 58 17 15.0 - 83 25 - 214 20 1 64 9.9 NR 4.0 74 19 一线 80 20 1 54 17.15 28.6 - 78 29 二线 ESC组,n=37 19 3 54 19.35 15.0 - 77 18 EXP组,n=145 14 1 55 16.59 15.6 - - 85(亚洲队列) 15 4 49 9.7 14.9 - 74 16 - 49(Child-Pugh B) 10.2 - 55.1 9.9 7.6 - 51 24.5 Nivolumabvs Sorafenib CheckMate 459 Ⅲ 一线 743(NIVO,n=371;SOR,n=372) 15 vs 7 4 vs 1 55 vs 58 23.3 vs 23.4 16.4 vs 14.7 3.7 vs 3.8 - 22 vs 49 Pembrolizumab KEYNOTE-224 Ⅱ 二线 104 17 1 62 NR 12.9 4.9 73 25 Pembrolizumabvs安慰剂 KEYNOTE-240 Ⅲ 二线 413(PEM,n=278;安慰剂,n=135) 18.3 vs 4.4 2.2 vs 0 62.2 vs 53.3 13.8 vs NR 13.9 vs 10.6 3.0 vs 2.8 60.9 vs 48.5 18.3 vs 7.5 Camrelizumab(SHR-1210) - Ⅱ 二线 217 14.7 0 44.2 NR 13.8 2.1 - 22 Durvalumab - Ⅰ/Ⅱ 二线 40(可评估,n=39) 10.3 (n=39) 0 33.3 (n=39) - 13.2 - 80 20 注:“-”,未提供数据;ORR,客观缓解率;CR,完全缓解;DCR,疾病控制率;mDOR,中位缓解持续时间;mOS,中位总生存期;mPFS,中位无进展生存期;TRAEs,治疗相关不良事件;NR,未达到;ESC,剂量爬坡;EXP,剂量扩展;表格中所列数据均根据实体瘤疗效评价标准1.1版(RECIST v1.1)评估所得。 表 2 PD-1/PD-L1抑制剂联合治疗在aHCC中的临床试验
药物 临床试验 分期 治疗线数 入组例数 ORR(%) CR(%) DCR(%) mDOR(月) mOS(月) mPFS(月) TRAEs(%) 任何级别 3~4级 Pembrolizumab+Lenvatinib KEYNOTE-524/Study 116 Ⅰb 一线 100 36 1 88 12.6 22.0 8.6 95 67 Nivolumab+Lenvatinib Study 117 Ⅰb 一线 30 76.7 13.3 96.7 - - - 100 - Camrelizumab(SHR-1210)+Apatinib - Ⅰ 二线 16(可评估) 50 0 93.8 NR NR 7.2 - - Avelumab+Axitinib VEGF Liver 100 Ⅰb 一线 22 13.6 - - - - 5.5 - - Atezolizumab+Bevacizumab GO30140 Ⅰb 一线 队列A,n=104 36 12 71 NE 17.1 7.3 88 39 队列F,n=119
Atezo+Bev,n=60;
Atezo,n=5920 vs 17 2 vs 5 67 vs 49 NE vs NE - 5.6 vs 3.4 68 vs 41 20 vs 5 Atezolizumab+Bevacizumabvs Sorafenib IMBrave 150 Ⅲ 一线 501Atezo+Bev,n=336;
SOR,n=16527.3 vs 11.9 5.5 vs 0 73.6 vs 55.3 NE vs 6.3 NE vs 13.2 6.8 vs 4.3 84 vs 94 36 vs 46 194(中国人群)
Atezo+Bev,n=133;
SOR,n=6125 vs 7 - - - NE vs 11.4 5.7 vs 3.2 - - Nivolumab+Ipilimumab CheckMate 040 Ⅰ/Ⅱ 二线 148 31 4.7 49 17.5 - - - 37 A组,n=50 32 8 54 17.5 22.8 B组,n=49 31 6 43 22.2 12.5 C组,n=49 31 0 49 16.6 12.7 Durvalumab+Tremelimumab - Ⅱ - T300+D,n=75 22.7 - - NR 18.7 - - 35.1 T75+D,n=84 9.5 - - 13.2 11.3 - - 24.4 Camrelizumab+FOLFOX4 - Ⅱ 一线 34 26.5 - 79.4 NR NR 5.5 - 85.3 Nivolumab+Cabozantinib±Ipilimumab CheckMate 040 Ⅰ/Ⅱ - 71二联,n=36三联,n=35 17 vs 26 0 vs 0 81 vs 83 - NR vs NR 5.5 vs 6.8 - 42 vs 71 注:NE,无法评估;表格中所列数据均根据RECIST v1.1评估所得;Atezo, Atezolizumab; Bev, Bevacizumab; SOR, Sorafenib。 -
[1] Bureau of Medical Administration, National Health Commission of the People's Republic of China. Guidelines for diagnosis and treatment of primary liver cancer in China (2019 edition)[J]. J Clin Hepatol, 2020, 36(2): 277-292. (in Chinese) DOI: 10.3969/j.issn.1001-5256.2020.02.007中华人民共和国国家卫生健康委员会医政医管局. 原发性肝癌诊疗规范(2019年版)[J]. 临床肝胆病杂志, 2020, 36(2): 277-292. DOI: 10.3969/j.issn.1001-5256.2020.02.007 [2] PARDOLL DM. The blockade of immune checkpoints in cancer immunotherapy[J]. Nat Rev Cancer, 2012, 12(4): 252-264. DOI: 10.1038/nrc3239 [3] EL-KHOUEIRY AB, SANGRO B, YAU T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial[J]. Lancet, 2017, 389(10088): 2492-2502. DOI: 10.1016/S0140-6736(17)31046-2 [4] CROCENZI TS, EL-KHOUEIRY AB, YAU T, et al. Nivolumab in sorafenib-naive and-experienced patients with advanced hepatocellular carcinoma: CheckMate 040 study[J]. J Clin Oncol, 2017, 35(15 Suppl): 4013. [5] YAU T, HSU C, KIM TY, et al. Nivolumab in advanced hepatocellular carcinoma: Sorafenib-experienced Asian cohort analysis[J]. J Hepatol, 2019, 71(3): 543-552. DOI: 10.1016/j.jhep.2019.05.014 [6] KUDO M, MATILLA A, SANTORO A, et al. Checkmate-040: Nivolumab (NIVO) in patients (pts) with advanced hepatocellular carcinoma (aHCC) and Child-Pugh B (CPB) status[J]. J Clin Oncol, 2019, 37(4 Suppl): 327. [7] KAMBHAMPATI S, BAUER KE, BRACCI PM, et al. Nivolumab in patients with advanced hepatocellular carcinoma and Child-Pugh class B cirrhosis: Safety and clinical outcomes in a retrospective case series[J]. Cancer, 2019, 125(18): 3234-3241. DOI: 10.1002/cncr.32206 [8] YAU T, PARK JW, FINN RS, et al. CheckMate 459: A randomized, multi-center phase Ⅲ study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC)[J]. Ann Oncol, 2019, 30(Suppl 5): v874-v875. [9] SUN YK. Interpretation of CSCO guidelines for the diagnosis and treatment of primary liver cancer: Systemic treatment[J/CD]. Electronic J Liver Tumor, 2018, 5(3): 11-14. (in Chinese)孙永琨. 2018《CSCO原发性肝癌诊疗指南》解读——全身治疗部分[J/CD]. 肝癌电子杂志, 2018, 5(3): 11-14. [10] National Comprehensive Cancer Network(NCCN). Clinical Practice Guidelines in Oncology. Hepatobiliary Cancers, Version 1.2020.[EB/OL]. (2020-03-23)[2020-06-20]. https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf. [11] ZHU AX, FINN RS, EDELINE J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): A non-randomised, open-label phase 2 trial[J]. Lancet Oncol, 2018, 19(7): 940-952. DOI: 10.1016/S1470-2045(18)30351-6 [12] KUDO M, FINN RS, EDELINE J, et al. Updated efficacy and safety of KEYNOTE-224: A phase Ⅱ study of pembrolizumab (pembro) in patients with advanced hepatocellular carcinoma (HCC)[J]. J Clin Oncol, 2020, 38(4 Suppl): 518. [13] FINN RS, RYOO BY, MERLE P, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: A randomized, double-blind, phase Ⅲ trial[J]. J Clin Oncol, 2020, 38(3): 193-202. DOI: 10.1200/JCO.19.01307 [14] BENSON AB, D'ANGELICA MI, ABBOTT DE, et al. Guidelines insights: Hepatobiliary cancers, Version 2.2019[J]. J Natl Compr Canc Netw, 2019, 17(4): 302-310. DOI: 10.6004/jnccn.2019.0019 [15] QIN S, REN Z, MENG Z, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: A multicentre, open-label, parallel-group, randomised, phase 2 trial[J]. Lancet Oncol, 2020, 21(4): 571-580. DOI: 10.1016/S1470-2045(20)30011-5 [16] WAINBERG ZA, SEGAL NH, JAEGER D, et al. Safety and clinical activity of durvalumab monotherapy in patients with hepatocellular carcinoma (HCC)[J]. J Clin Oncol, 2017, 35(15 Suppl): 4071. [17] FENG Z, RONG P, WANG W. Meta-analysis of the efficacy and safety of PD-1/PD-L1 inhibitors administered alone or in combination with anti-VEGF agents in advanced hepatocellular carcinoma[J]. Gut, 2020, 69(10): 1904-1906. DOI: 10.1136/gutjnl-2019-320116 [18] KUDO M. Immuno-oncology therapy for hepatocellular carcinoma: Current status and ongoing trials[J]. Liver Cancer, 2019, 8(4): 221-238. DOI: 10.1159/000501501 [19] ZHU AX, FINN RS, IKEDA M, et al. A phase Ib study of lenvatinib (LEN) plus pembrolizumab (PEMBRO) in unresectable hepatocellular carcinoma (uHCC)[J]. J Clin Oncol, 2020, 38(15 Suppl): 4519. [20] LLOVET JM, KUDO M, CHENG AL, et al. Lenvatinib (len) plus pembrolizumab (pembro) for the first-line treatment of patients (pts) with advanced hepatocellular carcinoma (HCC): Phase 3 LEAP-002 study[J]. J Clini Oncol, 2019, 37(15 Suppl): TPS4152. [21] KUDO M, IKEDA M, MOTOMURA K, et al. A phase Ib study of lenvatinib (LEN) plus nivolumab (NIV) in patients (pts) with unresectable hepatocellular carcinoma (uHCC): Study 117[J]. J Clin Oncol, 2020, 38(4 Suppl): 513. [22] XU J, ZHANG Y, JIA R, et al. Anti-PD-1 antibody SHR-1210 combined with apatinib for advanced hepatocellular carcinoma, gastric, or esophagogastric junction cancer: An open-label, dose escalation and expansion study[J]. Clin Cancer Res, 2019, 25(2): 515-523. DOI: 10.1158/1078-0432.CCR-18-2484 [23] KUDO M, MOTOMURA K, WADA Y, et al. First-line avelumab + axitinib in patients with advanced hepatocellular carcinoma: Results from a phase 1b trial (VEGF Liver 100)[J]. J Clin Oncol, 2019, 37(15 Suppl): 4072. [24] RIMASSA L, CHENG AL, BRAITEH F, et al. Phase Ⅲ (COSMIC-312) study of cabozantinib (C) in combination with atezolizumab (A) vs sorafenib (S) in patients (pts) with advanced hepatocellular carcinoma (aHCC) who have not received previous systemic anticancer therapy[J]. Ann Oncol, 2019, 30(Suppl 5): v320. [25] LEE M, RYOO BY, HSU CH, et al. Randomised efficacy and safety results for atezolizumab (Atezo) + bevacizumab (Bev) in patients (pts) with previously untreated, unresectable hepatocellular carcinoma (HCC)[J]. Ann Oncol, 2019, 30(Suppl 5): v875. [26] FINN RS, QIN S, IKEDA M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma[J]. N Engl J Med, 2020, 382(20): 1894-1905. DOI: 10.1056/NEJMoa1915745 [27] CHENG AL, QIN S, IKEDA M, et al. IMbrave150: Efficacy and safety results from a ph Ⅲ study evaluating atezolizumab (atezo)+bevacizumab (bev) vs sorafenib (Sor) as first treatment (tx) for patients (pts) with unresectable hepatocellular carcinoma (HCC)[J]. Ann Oncol, 2019, 30(Suppl 9): ix186-ix187. [28] QIN SK, REN ZG, FENG YS, et al. OP02-03 efficacy and safety of atezolizumab + bevacizumab vs sorafenib in Chinese patients with unresectable HCC in the phase Ⅲ IMbrave150 study.[EB/OL]. (2020-02-06)[2020-06-20]. https://easl.eu/livercancersummit2020/wp-content/uploads/2020/01/Liver-Cancer-Summit-2020-Abstract-book.pdf. [29] YAU T, KANG YK, KIM TY, et al. Nivolumab (NIVO) + ipilimumab (IPI) combination therapy in patients (pts) with advanced hepatocellular carcinoma (aHCC): Results from CheckMate 040[J]. J Clin Oncol, 2019, 37(15 Suppl): 4012. [30] HE AR, YAU T, HSU C, et al. Nivolumab (NIVO) + ipilimumab (IPI) combination therapy in patients (pts) with advanced hepatocellular carcinoma (aHCC): Subgroup analyses from CheckMate 040[J]. J Clin Oncol, 2020, 38(4 Suppl): 512. [31] KELLEY RK, SANGRO B, HARRIS WP, et al. Efficacy, tolerability, and biologic activity of a novel regimen of tremelimumab (T) in combination with durvalumab (D) for patients (pts) with advanced hepatocellular carcinoma (aHCC)[J]. J Clin Oncol, 2020, 38(15 Suppl): 4508. [32] ABOU-ALFA GK, CHAN SL, FURUSE J, et al. A randomized, multicenter phase 3 study of durvalumab (D) and tremelimumab (T) as first-line treatment in patients with unresectable hepatocellular carcinoma (HCC): HIMALAYA study[J]. J Clin Oncol, 2018, 36(15 Suppl): TPS4144. [33] QIN S, CHEN Z, LIU Y, et al. A phase Ⅱ study of anti-PD-1 antibody camrelizumab plus FOLFOX4 or GEMOX systemic chemotherapy as first-line therapy for advanced hepatocellular carcinoma or biliary tract cancer[J]. J Clin Oncol, 2019, 37(15 Suppl): 4074. [34] YAU T, ZAGONEL V, SANTORO A, et al. Nivolumab (NIVO)+ipilimumab (IPI)+cabozantinib (CABO) combination therapy in patients (pts) with advanced hepatocellular carcinoma (aHCC): Results from CheckMate 040[J]. J Clin Oncol, 2020, 38(4 Suppl): 478. 期刊类型引用(8)
1. 董旭,张亮. 特瑞普利单抗诱发免疫相关性心肌炎1例及其机制探讨. 中国循证心血管医学杂志. 2024(07): 873-874+885 .  百度学术
百度学术2. 朱丽珍,许晓磊,阿卜杜萨拉木·艾尼,王小娟,周虎,汤睿,樊海宁,卢倩. 肝细胞癌免疫治疗研究进展. 临床肝胆病杂志. 2023(05): 1197-1203 .  本站查看
本站查看3. 喻丹,何胜利,沈婕,胡南华,蔡芸芸,曹铁留. 如意金黄散联合ICIs治疗肝癌晚期伴肝胆湿热证者的临床观察. 中国药房. 2023(12): 1488-1492 .  百度学术
百度学术4. 李竹雨,陶嘉楠. 程序性死亡受体1基因多态性及其在肝细胞癌中的研究进展. 中国临床研究. 2023(09): 1348-1351 .  百度学术
百度学术5. 张艳艳. 卡瑞利珠单抗联合阿帕替尼治疗肝癌的疗效及安全性. 实用中西医结合临床. 2023(19): 25-27+49 .  百度学术
百度学术6. 叶德宇,杨帅,赵龙龙,孟宪瑛. 炎性标志物对恶性肿瘤发生发展和预后的影响及其机制的研究进展. 吉林大学学报(医学版). 2022(04): 1079-1087 .  百度学术
百度学术7. 侯昱丞,汤睿,赵洪强,阿卜杜萨拉木·艾尼,唐炳钧,于里涵,卢倩. 肝癌肝移植术前应用免疫检查点抑制剂与术后急性排斥反应的相关性研究进展. 中华器官移植杂志. 2022(08): 508-512 .  百度学术
百度学术8. 朱金霞,刘光伟,杨培伟. 调控PD-1/PD-L1表达影响肝癌进展的分子机制研究进展. 中西医结合肝病杂志. 2022(11): 1049-1053 .  百度学术
百度学术其他类型引用(2)
-




 PDF下载 ( 2049 KB)
PDF下载 ( 2049 KB)

 下载:
下载:

 下载:
下载:
 百度学术
百度学术


