脂毒性在非酒精性脂肪性肝病发生发展中的作用
DOI: 10.3969/j.issn.1001-5256.2021.02.045
作者贡献声明:张丽慧负责查询文献,撰写论文;刘鸣昊参与修改论文;赵文霞负责指导撰写论文,修改论文并最后定稿。
Role of lipotoxicity in the development and progression of nonalcoholic fatty liver disease
-
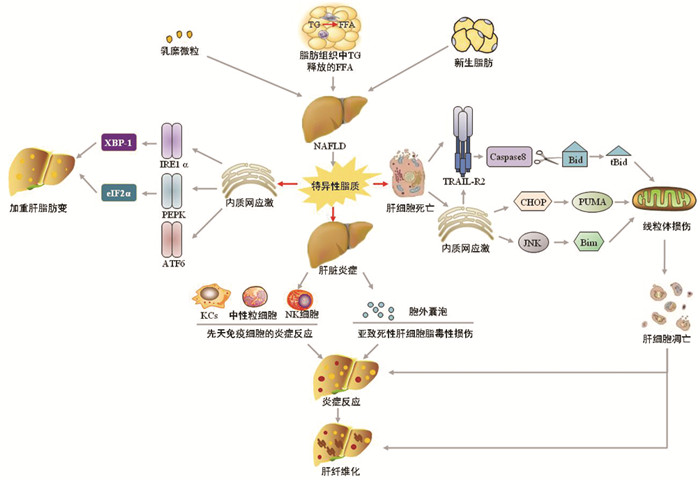
摘要: 非酒精性脂肪性肝病(NAFLD)目前是体检时肝脏生化学指标异常的首要原因,但其发生发展的机制仍不十分明确,尚缺乏有效的治疗方法。简述了脂毒性通过触发肝脏内质网应激、细胞死亡、炎症三种病理反应驱动NAFLD向非酒精性脂肪性肝炎、肝硬化的发生及转化,认为脂毒性是促进NAFLD向炎症、纤维化发展的重要因素,为NAFLD的防治提供一种新的途径。Abstract: Nonalcoholic fatty liver disease (NAFLD) is currently the leading cause of abnormal liver biochemical parameters, but the mechanism of its development and progression remains unclear and there is a lack of effective treatment methods. This article reviews that lipotoxicity drives the transformation of NAFLD to nonalcoholic steatohepatitis and liver cirrhosis by triggering the three pathological responses in the liver, i.e., endoplasmic reticulum stress, cell death, and inflammation. It is believed that lipotoxicity is an important factor that promotes the progression of NAFLD to inflammation and fibrosis, which provides a new method for the prevention and treatment of NAFLD.
-
[1] DIEHL AM, DAY C. Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis[J]. N Engl J Med, 2017, 377(21): 2063-2072. DOI: 10.1056/NEJMra1503519 [2] ZHOU Q, SU J, JI MY. Progress in the treatment of nonalcoholic fatty liver disease[J]. China Med Herald, 2020, 17(6): 26-29. (in Chinese) https://www.cnki.com.cn/Article/CJFDTOTAL-YYCY202006008.htm周谦, 苏娟, 季梦遥. 非酒精性脂肪性肝病的治疗研究进展[J]. 中国医药导报, 2020, 17(6): 26-29. https://www.cnki.com.cn/Article/CJFDTOTAL-YYCY202006008.htm [3] SHU JR, LI JQ, LIU Q. Epidemiology of nonalcoholic fatty liver disease and related risk factors[J]. J Clin Hepatol, 2019, 35(9): 2085-2090. (in Chinese) DOI: 10.3969/j.issn.1001-5256.2019.09.045舒筠然, 李俊琪, 刘琼. 非酒精性脂肪性肝病的流行病学和危险因素分析[J]. 临床肝胆病杂志, 2019, 35(9): 2085-2090. DOI: 10.3969/j.issn.1001-5256.2019.09.045 [4] YOUNOSSI ZM, KOENIG AB, ABDELATIF D, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes[J]. Hepatology, 2016, 64(1): 73-84. DOI: 10.1002/hep.28431 [5] AHMED A, WONG RJ, HARRISON SA. Nonalcoholic fatty liver disease review: Diagnosis, treatment, and outcomes[J]. Clin Gastroenterol Hepatol, 2015, 13(12): 2062-2070. DOI: 10.1016/j.cgh.2015.07.029 [6] TILG H, MOSCHEN AR. Evolution of inflammation in nonalcoholic fatty liver disease: The multiple parallel hits hypothesis[J]. Hepatology, 2010, 52(5): 1836-1846. DOI: 10.1002/hep.24001 [7] SCHAFFER JE. Lipotoxicity: Many roads to cell dysfunction and cell death: Introduction to a thematic review series[J]. J Lipid Res, 2016, 57(8): 1327-1328. DOI: 10.1194/jlr.E069880 [8] ANGULO P, KLEINER DE, DAM-LARSEN S, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease[J]. Gastroenterology, 2015, 149(2): 389-397. DOI: 10.1053/j.gastro.2015.04.043 [9] MUSSO G, CASSADER M, PASCHETTA E, et al. Bioactive lipid species and metabolic pathways in progression and resolution of nonalcoholic steatohepatitis[J]. Gastroenterology, 2018, 155(2): 282-302. DOI: 10.1053/j.gastro.2018.06.031 [10] CUSI K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: Pathophysiology and clinical implications[J]. Gastroenterology, 2012, 142(4): 711-725. DOI: 10.1053/j.gastro.2012.02.003 [11] MACHADO MV, DIEHL AM. Pathogenesis of nonalcoholic steatohepatitis[J]. Gastroenterology, 2016, 150(8): 1769-1777. DOI: 10.1053/j.gastro.2016.02.066 [12] PARTHASARATHY G, REVELO X, MALHI H. Pathogenesis of nonalcoholic steatohepatitis: An overview[J]. Hepatol Commun, 2020, 4(4): 478-492. DOI: 10.1002/hep4.1479 [13] IBRAHIM SH, HIRSOVA P, GORES GJ. Non-alcoholic steatohepatitis pathogenesis: Sublethal hepatocyte injury as a driver of liver inflammation[J]. Gut, 2018, 67(5): 963-972. DOI: 10.1136/gutjnl-2017-315691 [14] LEBEAUPIN C, VALLÉE D, HAZARI Y, et al. Endoplasmic reticulum stress signalling and the pathogenesis of non-alcoholic fatty liver disease[J]. J Hepatol, 2018, 69(4): 927-947. DOI: 10.1016/j.jhep.2018.06.008 [15] ZHANG B, LI M, ZOU Y, et al. NFκB/Orai1 facilitates endoplasmic reticulum stress by oxidative stress in the pathogenesis of non-alcoholic fatty liver disease[J]. Front Cell Dev Biol, 2019, 7: 202. DOI: 10.3389/fcell.2019.00202 [16] YANG MT, CHEN ZJ, XIAO CX, et al. Effects of sera of rats fed with Huganqingzhi tablets on endoplasmic reticulum stress in a HepG2 cell model of nonalcoholic fatty liver disease[J]. J South Med Univ, 2018, 38(11): 1277-1287. (in Chinese) https://www.cnki.com.cn/Article/CJFDTOTAL-DYJD201811002.htm杨妙婷, 陈芝娟, 肖淳欣, 等. 护肝清脂片药物血清对非酒精性脂肪肝细胞模型内质网应激的影响[J]. 南方医科大学学报, 2018, 38(11): 1277-1287. https://www.cnki.com.cn/Article/CJFDTOTAL-DYJD201811002.htm [17] WANG L, CHEN J, NING C, et al. Endoplasmic reticulum stress related molecular mechanisms in nonalcoholic fatty liver disease (NAFLD)[J]. Curr Drug Targets, 2018, 19(9): 1087-1094. DOI: 10.2174/1389450118666180516122517 [18] SANO R, REED JC. ER stress-induced cell death mechanisms[J]. Biochim Biophys Acta, 2013, 1833(12): 3460-3470. DOI: 10.1016/j.bbamcr.2013.06.028 [19] WANG S, CHEN Z, LAM V, et al. IRE1α-XBP1s induces PDI expression to increase MTP activity for hepatic VLDL assembly and lipid homeostasis[J]. Cell Metab, 2012, 16(4): 473-486. DOI: 10.1016/j.cmet.2012.09.003 [20] ZHANG K, WANG S, MALHOTRA J, et al. The unfolded protein response transducer IRE1α prevents ER stress-induced hepatic steatosis[J]. EMBO J, 2011, 30(7): 1357-1375. DOI: 10.1038/emboj.2011.52 [21] YE D, LI FY, LAM KS, et al. Toll-like receptor-4 mediates obesity-induced non-alcoholic steatohepatitis through activation of X-box binding protein-1 in mice[J]. Gut, 2012, 61(7): 1058-1067. DOI: 10.1136/gutjnl-2011-300269 [22] NING J, HONG T, WARD A, et al. Constitutive role for IRE1α-XBP1 signaling pathway in the insulin-mediated hepatic lipogenic program[J]. Endocrinology, 2011, 152(6): 2247-2255. DOI: 10.1210/en.2010-1036 [23] DONNELLY N, GORMAN AM, GUPTA S, et al. The eIF2α kinases: Their structures and functions[J]. Cell Mol Life Sci, 2013, 70(19): 3493-3511. DOI: 10.1007/s00018-012-1252-6 [24] LAKE AD, NOVAK P, HARDWICK RN, et al. The adaptive endoplasmic reticulum stress response to lipotoxicity in progressive human nonalcoholic fatty liver disease[J]. Toxicol Sci, 2014, 137(1): 26-35. DOI: 10.1093/toxsci/kft230 [25] XIAO G, ZHANG T, YU S, et al. ATF4 protein deficiency protects against high fructose-induced hypertriglyceridemia in mice[J]. J Biol Chem, 2013, 288(35): 25350-25361. DOI: 10.1074/jbc.M113.470526 [26] LI H, MENG Q, XIAO F, et al. ATF4 deficiency protects mice from high-carbohydrate-diet-induced liver steatosis[J]. Biochem J, 2011, 438(2): 283-289. DOI: 10.1042/BJ20110263 [27] DEZWAAN-MCCABE D, SHELDON RD, GORECKI MC, et al. ER stress inhibits liver fatty acid oxidation while unmitigated stress leads to anorexia-induced lipolysis and both liver and kidney steatosis[J]. Cell Rep, 2017, 19(9): 1794-1806. DOI: 10.1016/j.celrep.2017.05.020 [28] CHEN X, ZHANG F, GONG Q, et al. Hepatic ATF6 increases fatty acid oxidation to attenuate hepatic steatosis in mice through peroxisome proliferator-activated receptor α[J]. Diabetes, 2016, 65(7): 1904-1915. DOI: 10.2337/db15-1637 [29] ALKHOURI N, CARTER-KENT C, FELDSTEIN AE. Apoptosis in nonalcoholic fatty liver disease: Diagnostic and therapeutic implications[J]. Expert Rev Gastroenterol Hepatol, 2011, 5(2): 201-212. DOI: 10.1586/egh.11.6 [30] ZHANG J, ZHANG H, DENG X, et al. Baicalin attenuates non-alcoholic steatohepatitis by suppressing key regulators of lipid metabolism, inflammation and fibrosis in mice[J]. Life Sci, 2018, 192: 46-54. DOI: 10.1016/j.lfs.2017.11.027 [31] CAZANAVE SC, MOTT JL, BRONK SF, et al. Death receptor 5 signaling promotes hepatocyte lipoapoptosis[J]. J Biol Chem, 2011, 286(45): 39336-39348. DOI: 10.1074/jbc.M111.280420 [32] MA ZW, LIU DX. Humanin decreases mitochondrial membrane permeability by inhibiting the membrane association and oligomerization of Bax and Bid proteins[J]. Acta Pharmacol Sin, 2018, 39(6): 1012-1021. DOI: 10.1038/aps.2017.169 [33] CAZANAVE SC, ELMI NA, AKAZAWA Y, et al. CHOP and AP-1 cooperatively mediate PUMA expression during lipoapoptosis[J]. Am J Physiol Gastrointest Liver Physiol, 2010, 299(1): g236-g243. DOI: 10.1152/ajpgi.00091.2010 [34] IURLARO R, MUÑOZ-PINEDO C. Cell death induced by endoplasmic reticulum stress[J]. FEBS J, 2016, 283(14): 2640-2652. DOI: 10.1111/febs.13598 [35] SHEN X, LI LP. Progress in the relationship between apoptosis and nonalcoholic fatty liver disease[J]. Int J Dig Dis, 2014, 34(4): 245-248, 252. (in Chinese) DOI: 10.3969/j.issn.1673-534X.2014.04.006沈雪, 李良平. 非酒精性脂肪性肝病与细胞凋亡关系的研究进展[J]. 国际消化病杂志, 2014, 34(4): 245-248, 252. DOI: 10.3969/j.issn.1673-534X.2014.04.006 [36] KUBES P, MEHAL WZ. Sterile inflammation in the liver[J]. Gastroenterology, 2012, 143(5): 1158-1172. DOI: 10.1053/j.gastro.2012.09.008 [37] HIRSOVA P, GORES GJ. Death receptor-mediated cell death and proinflammatory signaling in nonalcoholic steatohepatitis[J]. Cell Mol Gastroenterol Hepatol, 2015, 1(1): 17-27. DOI: 10.1016/j.jcmgh.2014.11.005 [38] KAHRAMAN A, SCHLATTJAN M, KOCABAYOGLU P, et al. Major histocompatibility complex class I-related chains A and B (MIC A/B): A novel role in nonalcoholic steatohepatitis[J]. Hepatology, 2010, 51(1): 92-102. DOI: 10.1002/hep.23253 [39] PAN J, OU Z, CAI C, et al. Fatty acid activates NLRP3 inflammasomes in mouse Kupffer cells through mitochondrial DNA release[J]. Cell Immunol, 2018, 332: 111-120. DOI: 10.1016/j.cellimm.2018.08.006 [40] REID DT, REYES JL, MCDONALD BA, et al. Kupffer cells undergo fundamental changes during the development of experimental NASH and are critical in initiating liver damage and inflammation[J]. PLoS One, 2016, 11(7): e0159524. DOI: 10.1371/journal.pone.0159524 [41] BAECK C, WEHR A, KARLMARK KR, et al. Pharmacological inhibition of the chemokine CCL2 (MCP-1) diminishes liver macrophage infiltration and steatohepatitis in chronic hepatic injury[J]. Gut, 2012, 61(3): 416-426. DOI: 10.1136/gutjnl-2011-300304 [42] CASTANHEIRA F, KUBES P. Neutrophils and NETs in modulating acute and chronic inflammation[J]. Blood, 2019, 133(20): 2178-2185. DOI: 10.1182/blood-2018-11-844530 [43] van der WINDT DJ, SUD V, ZHANG H, et al. Neutrophil extracellular traps promote inflammation and development of hepatocellular carcinoma in nonalcoholic steatohepatitis[J]. Hepatology, 2018, 68(4): 1347-1360. DOI: 10.1002/hep.29914 [44] IBUSUKI R, UTO H, ARIMA S, et al. Transgenic expression of human neutrophil peptide-1 enhances hepatic fibrosis in mice fed a choline-deficient, L-amino acid-defined diet[J]. Liver Int, 2013, 33(10): 1549-1556. DOI: 10.1111/liv.12203 [45] GOMEZ-SANTOS L, LUKA Z, WAGNER C, et al. Inhibition of natural killer cells protects the liver against acute injury in the absence of glycine N-methyltransferase[J]. Hepatology, 2012, 56(2): 747-759. DOI: 10.1002/hep.25694 [46] HIRSOVA P, IBRAHIM SH, KRISHNAN A, et al. Lipid-induced signaling causes release of inflammatory extracellular vesicles from hepatocytes[J]. Gastroenterology, 2016, 150(4): 956-967. DOI: 10.1053/j.gastro.2015.12.037 [47] POVERO D, EGUCHI A, NIESMAN IR, et al. Lipid-induced toxicity stimulates hepatocytes to release angiogenic microparticles that require Vanin-1 for uptake by endothelial cells[J]. Sci Signal, 2013, 6(296): ra88. http://med.wanfangdata.com.cn/Paper/Detail/PeriodicalPaper_PM24106341 [48] DENG ZB, LIU Y, LIU C, et al. Immature myeloid cells induced by a high-fat diet contribute to liver inflammation[J]. Hepatology, 2009, 50(5): 1412-1420. DOI: 10.1002/hep.23148 [49] JIN J, LIU JX, YI M. Protective effect of berberine against hepatic lipid toxicity induced by high-fat diet in NAFLD mice and its related mechanism[J]. J Clin Exp Med, 2019, 18(11): 1124-1128. (in Chinese) DOI: 10.3969/j.issn.1671-4695.2019.11.002金军, 刘吉祥, 易鸣. 黄连素对高脂饮食诱导非酒精性脂肪性肝病小鼠肝脂毒性的保护作用及相关机制研究[J]. 临床和实验医学杂志, 2019, 18(11): 1124-1128. DOI: 10.3969/j.issn.1671-4695.2019.11.002 [50] IBRAHIM SH, HIRSOVA P, TOMITA K, et al. Mixed lineage kinase 3 mediates release of C-X-C motif ligand 10-bearing chemotactic extracellular vesicles from lipotoxic hepatocytes[J]. Hepatology, 2016, 63(3): 731-744. DOI: 10.1002/hep.28252 [51] KAKAZU E, MAUER AS, YIN M, et al. Hepatocytes release ceramide-enriched pro-inflammatory extracellular vesicles in an IRE1α-dependent manner[J]. J Lipid Res, 2016, 57(2): 233-245. DOI: 10.1194/jlr.M063412 -



 PDF下载 ( 1962 KB)
PDF下载 ( 1962 KB)

 下载:
下载:


