血清C肽在2型糖尿病合并非酒精性脂肪性肝病患者肝纤维化进展中的作用
DOI: 10.3969/j.issn.1001-5256.2021.05.030
Association between liver fibrosis and C-peptide in patients with type 2 diabetes and nonalcoholic fatty liver disease
-
摘要:
目的 探究血清C肽水平对2型糖尿病(T2DM)合并非酒精性脂肪性肝病(NAFLD)患者肝纤维化进展的影响。 方法 收集2018年12月—2020年7月就诊于兰州大学第二医院老年病科的484例T2DM患者,依据腹部彩超检查结果,将其分为单纯T2DM组(n=107)及T2DM合并NAFLD组(n=377),采用NAFLD肝纤维化评分(NFS)将T2DM合并NAFLD组分为3个亚组:排除纤维化亚组(T2DM+F0组,n=136)、不确定亚组(T2DM+F1组,n=146)及纤维化亚组(T2DM+F2组,n=95),收集纳入人群病史资料及实验室指标。计数资料组间比较采用χ2检验,计量资料两组间比较应用t检验或Mann-Whitney U检验,多组比较使用单因素方差分析或Kruskal-Wallis H检验,logistic回归分析探究促进肝纤维化进展的危险因素,采用受试者工作特征曲线(ROC)分析血清C肽在预测及诊断肝纤维化进展过程的临床价值。 结果 相比于单纯T2DM组,T2DM合并NAFLD组患者C肽水平明显升高(Z=-6.040,P < 0.001),T2DM+F2组不仅C肽明显高于T2DM+F1组和T2DM+F0组[2.89(1.84~3.77) vs 1.97(1.12~2.65) vs 1.87(1.25~2.68),H=36.023,P < 0.001],高空腹C肽率也显著升高(56.84% vs 23.29% vs 24.27%,χ2=37.583,P < 0.001)。logistic回归分析表明,C肽(OR=1.435,95%CI:1.227~1.678,P < 0.001)是T2DM合并NAFLD患者肝维化的危险因素,ROC曲线也显示C肽对预测该类患者肝纤维化具有重要意义,曲线下面积为0.814,最佳截断值为2.405 ng/ml,灵敏度为64.2%,特异度为89.7%,约登指数为0.539。 结论 C肽是T2DM合并NAFLD患者肝纤维化进展的独立危险因素。 Abstract:Objective To investigate the effect of serum C-peptide level on the progression of liver fibrosis in patients with type 2 diabetes mellitus (T2DM) and nonalcoholic fatty liver disease (NAFLD). Methods A total of 484 patients with T2DM who were admitted to Department of Geriatrics, The Second Hospital of Lanzhou University, from December 2018 to July 2020 were enrolled, and according to the results of abdominal ultrasound examination, they were divided into simple T2DM group with 107 patients and T2DM+NAFLD group with 377 patients. According to NAFLD fibrosis score, the patients with T2DM and NAFLD were divided into fibrosis exclusion subgroup (T2DM+F0) with 136 patients, uncertain subgroup (T2DM+F1) with 146 patients, and fibrosis subgroup (T2DM+F2) with 95 patients. Medical history data and laboratory markers were collected. The chi-square test was used for comparison of categorical data; the t-test or the Mann-Whitney U test was used for comparison of continuous data, and a one-way analysis of variance or the Kruskal-Wallis H test was used for comparison between multiple groups; a logistic regression analysis was used to explore the risk factors for the progression of liver fibrosis; the receiver operating characteristic (ROC) curve was used to analyze the clinical value of serum C-peptide in predicting and diagnosing the progression of liver fibrosis. Results Compared with the simple T2DM group, the T2DM+NAFLD group had a significant increase in C-peptide level (Z=-6.040, P < 0.001); compared with the T2DM+F1 and T2DM+F0, the T2DM+F2 had significantly higher C-peptide level [2.89 (1.84-3.77) vs 1.97 (1.12-2.65)/1.87 (1.25-2.68), H=36.023, P < 0.001) and rate of fasting C-peptide (56.84% vs 23.29%/24.27%, χ2=37.583, P < 0.001). The logistic regression analysis showed that C-peptide (OR=1.435, 95% confidence interval: 1.227~1.678, P < 0.001) was a risk factor for liver fibrosis in patients with T2DM and NAFLD, and the ROC curve analysis also showed that C-peptide had great significance in predicting liver fibrosis in such patients, with an area under the ROC curve of 0.814, a sensitivity of 64.2%, a specificity of 89.7%, and a Youden index of 0.539 at the optimal cut-off value of 2.405 ng/ml. Conclusion C-peptide is an independent risk factor for the progression of liver fibrosis in patients with T2DM and NAFLD. -
Key words:
- Diabetes Mellitus, Type 2 /
- Non-alcoholic Fatty Liver Disease /
- C-Peptide
-
随着饮食结构改变,我国非酒精性脂肪性肝病(NAFLD)患病率增长迅速,尤其在2型糖尿病(type 2 diabetes mellitus,T2DM)患者中NAFLD患病率高达75%[1],T2DM是促进NAFLD和肝纤维化的最强预测因子[2-3]。随着NAFLD肝纤维化发展,肝癌、肝全因死亡率及糖尿病微血管并发症风险升高[4-5]。早期识别T2DM患者发生NAFLD及NAFLD肝纤维化的危险因素是延缓肝纤维化发展、减轻糖尿病慢性并发症的先决条件。T2DM人群NAFLD发生及严重肝损伤可能与胰岛功能异常有关,C肽是用于评估内源性胰岛素分泌及β细胞功能储备的最佳指标,但目前关于C肽对NAFLD发生发展作用观点仍存在争议。本研究旨在探讨T2DM合并NAFLD患者肝纤维化与血清C肽水平的关系。
1. 资料与方法
1.1 研究对象
连续性选取2018年12月—2020年7月就诊于兰州大学第二医院特需老年病科的484例T2DM患者。纳入标准:(1)T2DM诊断以《中国2型糖尿病防治指南(2017年版)》[6]为标准;(2)NAFLD的诊断以《非酒精性脂肪性肝病防治指南(2018年更新版)》[7]。排除标准:(1)1型糖尿病、特殊类型糖尿病及T2DM伴有急性并发症、妊娠及哺乳期妇女、风湿免疫性疾病患者;(2)嗜酒患者,乙醇量男>140 g/周,女>70 g/周;(3)合并有病毒性肝炎、药物性肝损伤、胆汁淤积性肝损伤、肝豆状核变性及药物依赖患者;(4)近期服用影响C肽分泌、胰岛素水平及血脂水平药物患者。
1.2 资料采集
采集其病史资料,如性别、年龄、T2DM病程,测量体质量、身高,计算BMI,抽取空腹8 h以上静脉血,测空腹血糖(FPG)、空腹胰岛素(FINS)、空腹C肽(FCP)、糖化血红蛋白(HbA1c)、AST、ALT、AST/ALT、TG、CHO、HDL、LDL、Alb、血尿酸(UA)、PLT,测量静息30 min后的收缩压、舒张压;计算胰β细胞功能指数HOMA-β[8],HOMA-β=20×FINS/(FPG-3.5);计算NAFLD纤维化评分(NFS)[9], NFS=-1.675+0.037×年龄(岁)+0.094×BMI(kg/m2)+1.13×糖耐量异常/糖尿病(是=1,否=0)+0.99×AST/ALT-0.013×PLT(109/L)-0.66× Alb(g/dl)。
1.3 分组方法
根据腹部彩超结果,纳入研究对象被分为单纯T2DM组(n=107)和T2DM合并NAFLD组(n=377);将T2DM合并NAFLD组依据NFS[9]评分分为3个亚组,即:NFS < -1.455为排除肝纤维化亚组(T2DM+F0,n=136);-1.455≤NFS < 0.676为不确定亚组(T2DM+F1,n=146);NFS≥0.676为纤维化亚组(T2DM+F2,n=95)。
1.4 伦理学审查
本研究方案经由兰州大学第二医院伦理委员会批准,批号:2021A-155。患者均知情同意。
1.5 统计学方法
数据分析采用SPSS 25.0软件,正态分布计量资料以x±s表示,两组比较应用t检验,多组比较使用单因素方差分析,进一步两两比较采用Duncan's multiple range test;非正态分布资料以M(P25~P75)表示,两组间比较采用Mann-Whitney U检验,多组间比较采用Kruskal-Wallis H检验,进一步两两比较采用Nemenyi检验;计数资料组间比较采用χ2检验,进一步两两比较采用Bonferroni法多重比较。logistic回归分析探讨影响T2DM合并NAFLD人群肝纤维化进展的独立危险因素,采用受试者工作特征曲线(ROC曲线)评价FCP对T2DM合并NAFLD人群肝纤维化进展的诊断价值。以P < 0.05为差异有统计学意义。
2. 结果
2.1 单纯T2DM组与T2DM合并NAFLD组资料比较
相比于单纯T2DM组,合并NAFLD组患者的FCP、HOMA-β、FINS、FPG、BMI、AST、ALT、HbA1c、CHO、UA、TG、收缩压、舒张压水平均升高,PLT降低,差异均具有统计学意义(P值均 < 0.05)(表 1)。
表 1 单纯T2DM组与T2DM合并NAFLD组资料比较指标 单纯T2DM组(n=107) 合并NAFLD组(n=377) 统计值 P值 男/女(例) 63/44 182/195 χ2=3.748 0.053 年龄(岁) 48.37±7.63 49.33±10.72 t=-0.859 0.391 T2DM病程(年) 3.00(0.94~9.00) 4.00(0.50~9.25) Z=-0.437 0.662 BMI(kg/m2) 24.52±2.95 26.97±3.67 t=-6.338 < 0.001 AST(U/L) 20.00(16.00~25.00) 23.00(18.00~31.00) Z=-3.092 < 0.001 ALT(U/L) 25.00(17.00~37.25) 30.50(21.00~48.00) Z=-3.190 0.001 AST/ALT 0.78(0.61~1.00) 0.77(0.63~0.93) Z=-0.247 0.805 FPG(mmol/L) 9.50±3.99 10.86±4.62 t=-2.778 0.006 Alb(g/L) 45.51±5.02 45.60±4.70 t=-0.175 0.861 PLT(×109/L) 220.44±81.24 196.53±72.08 t=2.936 0.003 HbA1c(%) 8.47±2.02 9.18±2.22 t=-2.976 0.003 FCP(ng/ml) 1.26(0.80~1.96) 2.10(1.29~2.94) Z=-6.040 < 0.001 FINS(mU/L) 7.82(4.46~13.77) 14.80(9.41~20.75) Z=-7.026 < 0.001 HOMA-β 29.79(13.04~70.20) 44.12(23.63~85.58) Z=-3.076 0.002 UA(μmol/L) 313.88±94.87 345.30±92.95 t=-3.072 0.002 CHO(mmol/L) 4.48±0.88 4.69±1.37 t=-2.189 0.029 TG(mmol/L) 1.80(1.37~2.87) 2.27(1.53~3.45) Z=-2.656 0.008 HDL(mmol/L) 1.07±0.28 1.07±0.30 t=-0.092 0.926 LDL(mmol/L) 2.77±0.77 2.78±1.12 t=-0.161 0.873 收缩压(mm Hg) 129.95±21.58 134.55±21.11 t=-1.980 0.048 舒张压(mm Hg) 80.88±12.09 84.79±11.98 t=-2.971 0.003 校正性别,以T2DM是否合并NAFLD为因变量,多因素logistic回归分析显示,FCP、FINS、HbA1c、FPG、UA为T2DM发生NAFLD的独立危险因素(P值均 < 0.05)(表 2)。
表 2 T2DM患者发生NAFLD影响因素的logistics回归分析因素 B值 SE 统计值 OR(95%CI) P值 FCP 0.623 0.019 27.505 1.865(1.478~2.355) < 0.001 FINS 0.084 0.016 27.141 1.088(1.054~1.123) < 0.001 FPG 0.077 0.028 7.463 1.080(1.022~1.141) 0.006 HbA1c 0.159 0.054 8.548 1.173(1.054~1.305) 0.003 UA 0.004 0.001 9.112 1.004 (1.001~1.006) 0.003 2.2 T2DM合并NAFLD各亚组资料比较
各亚组除与NFS计算公式有关的指标如年龄、BMI、AST/ALT、Alb、PLT等差异显著外,T2DM+F2组与T2DM+F0及T2DM+F1组相比:FCP、FINS、T2DM病程、收缩压均升高,而ALT、CHO则不同程度降低(P值均 < 0.05)(表 3)。
表 3 T2DM合并NAFLD各纤维化亚组资料比较指标 T2DM+F0组(n=136) T2DM+F1组(n=146) T2DM+F2组(n=95) 统计值 P值 男/女(例) 67/69 72/74 43/52 χ2=0.462 0.799 年龄(岁) 42.43±9.49 52.47±8.971) 54.38±9.791) F=58.830 < 0.001 T2DM病程(年) 3.00(0.50~7.75) 4.00(0.33~9.00) 6.00(2.00~12.00)1)2) H=11.799 0.003 BMI(kg/m2) 25.91±3.11 26.63±2.91 28.99±4.561)2) F=23.178 < 0.001 AST(U/L) 23.00(18.00~30.00) 23.00(18.00~34.00) 23.00(19.00~29.00) H=0.080 0.961 ALT(U/L) 33.50(23.00~50.00) 30.00(22.00~49.00) 26.00(16.00~40.00)1) H=12.569 0.002 AST/ALT 0.68(0.56~0.83) 0.77(0.63~0.90)1) 0.91(0.72~1.23)1)2) H=50.741 < 0.001 FPG(mmol/L) 10.64±4.22 10.65±4.24 11.50±5.47 F=1.227 0.294 Alb(g/L) 47.94±4.17 45.51±4.01 42.37±4.432) F=49.750 < 0.001 PLT(109/L) 264.74±57.73 175.48±38.251) 127.63±39.781)2) F=258.378 < 0.001 HbA1c(%) 8.95±2.12 9.46±2.15 9.06±2.42 F=2.078 0.127 FCP(ng/ml) 1.87(1.25~2.68) 1.97(1.12~2.65) 2.89(1.84~3.77)1)2) H=36.023 < 0.001 高FCP率(%) 24.27 23.29 56.841)2) χ2=37.583 < 0.001 FINS(mU/L) 12.71(8.21~17.09) 15.20(9.12~19.45) 22.15(12.14~29.58)1)2) H=38.885 < 0.001 HOMA-β 38.62(19.71~78.32) 45.74(23.16~81.92) 53.31(25.78~114.32) H=5.028 0.081 UA(μmol/L) 353.01±87.52 333.38±96.05 352.58±94.71 F=1.970 0.141 CHO(mmol/L) 4.72±1.07 4.86±1.67 4.37±1.781) F=3.792 0.023 TG(mmol/L) 2.24(1.50~3.15) 2.30(1.58~4.22) 2.21(1.74~3.51) H=1.932 0.381 HDL(mmol/L) 1.06±0.29 1.09±0.31 1.08±0.30 F=1.043 0.353 LDL(mmol/L) 2.87±0.90 2.82±1.25 2.60±1.17 F=1.879 0.154 收缩压(mm Hg) 131.26±18.40 134.85±18.64 138.82±26.961) F=3.663 0.027 舒张压(mm Hg) 86.18±11.20 83.86±11.37 84.21±13.79 F=1.479 0.229 注:与T2DM+F0组相比,1)P < 0.05;与T2DM+F1组相比,2)P < 0.05。 通过有序logistics回归分析表明,FCP、FINS、收缩压均是促进T2DM合并NAFLD人群肝纤维化进展的独立危险因素(P值均 < 0.05)(表 4)。
表 4 T2DM合并NAFLD肝纤维化进展影响因素的logistic回归分析因素 B值 SE 统计值 OR(95%CI) P值 FCP 0.361 0.080 20.143 1.435(1.227~1.678) < 0.001 FINS 0.059 0.010 32.095 1.061(1.040~1.082) < 0.001 收缩压 0.013 0.050 8.200 1.013(0.919~1.117) 0.004 2.3 血清FCP水平对T2DM合并NAFLD肝纤维化的预测价值
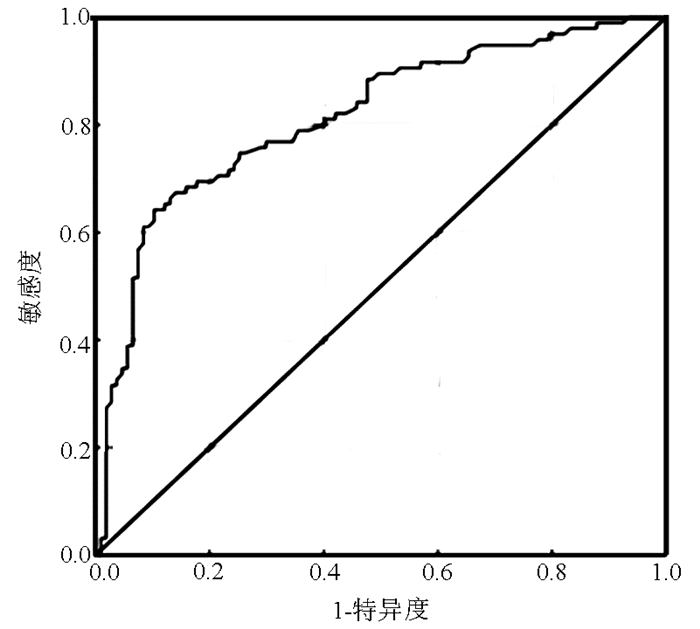
血清FCP可较好的预测T2DM合并NAFLD患者肝纤维化的发生风险,ROC曲线下面积0.814,当FCP=2.405 ng/ml时,灵敏度64.2%,特异度89.7%,约登指数为0.539(图 1)。可见FCP不仅是促进T2DM合并NAFLD发生肝纤维化的独立危险因素,而且对是否存在肝纤维化具有一定诊断价值。
3. 讨论
NAFLD是以肝细胞内脂肪过度堆积为病理特征的疾病,包含单纯性脂肪肝、非酒精性脂肪性肝炎(NASH) 和肝硬化[7],在T2DM人群中不仅患病率高,而且表现出更严重肝损伤及纤维化[1, 10]。C肽是胰岛素原分子裂解时与胰岛素等摩尔释放的肽类激素[11],在循环中半衰期约30 min,而胰岛素半衰期仅4 min,所以C肽免疫分析可用以测定体内胰岛素产生,评估残留β细胞活性[12]。此前关于C肽对NAFLD及肝纤维化的影响结论不统一。部分学者[13-15]认为C肽对延缓T2DM并发症具有有益作用,然而也有研究[14]表明C肽与肝脂肪变性的炎症进展呈正相关,而与纤维化进展呈负相关。本研究通过对比107例单纯T2DM及377例T2DM合并NAFLD临床资料发现,合并NAFLD患者的FCP、FINS及HOMA-β较单纯T2DM组高,调整混杂因素回归分析发现FCP、FINS是T2DM患者发生NAFLD的是独立危险因素。依据NFS对纤维化程度分层后,再次发现FCP是推进肝纤维化进展的影响因素,这与既往部分学者研究成果相符合[16-18],表明内源性胰岛素过度分泌、胰岛β细胞功能异常是肝纤维化的危险因素,而病理解剖肝硬化患者的胰腺肥大也是这一观点的有力佐证。此外,该研究通过ROC曲线分析发现,FCP可较好评估肝纤维化风险,曲线下面积为0.814,且当FCP=2.405 ng/ml时,预测肝纤维化的特异度高达89.7%。
作为肽类激素,一方面,C肽可以直接激活细胞外信号调节酶,介导NF-κB-JNK神经酰胺途径,增加糖原异生蛋白的转录,趋化炎性细胞,调节促炎性细胞因子的产生、核分裂和细胞凋亡,加速肝细胞裂解[19-20],促进肝纤维化发展。另一方面,C肽水平反应了内源性胰岛素分泌,早期胰岛素分泌过量,高胰岛素血症会刺激肝细胞分泌额外的基质,加速肝纤维化发展[21],表明C肽不仅可以直接导致肝损伤,也反映出内源性高胰岛素血症在肝损伤中的作用。在临床工作中,对于T2DM合并NAFLD患者,应在评估肝功能及胰岛功能基础上选择合理降糖药物,而不是单纯增加胰岛素剂量降糖,在使用胰岛素过程中应规避低血糖风险、监测肝功能,避免循环中胰岛素过量加重肝损伤。此外,C肽水平升高,胰岛素分泌过量,可能与外周组织胰岛素敏感度降低代偿反应有关,改善胰岛素敏感度可能是未来治疗靶向,目前已有胰岛素增敏剂罗格列酮改善NASH、缓解肝纤维化病理特征[22-23]、二甲双胍延长NASH肝硬化患者生存期,降低T2DM患者肝癌的发病率[24-26]等研究成果。未来尚需要更多临床及动物实验以探究更优化的降糖及改善肝功能治疗方法。
综上,C肽是推进T2DM合并NAFLD患者肝纤维化的因素,也是反应胰岛功能异常造成肝损伤严重程度的指标,对于存在高C肽水平的T2DM患者,应尽早筛查NAFLD,并进行肝纤维化及肝功能分期,以改善肝脏和心血管疾病结局为目标,遵循个体化原则,合理选择治疗方案,以降低肝硬化、肝癌及糖尿病等慢性并发症的发生风险,提升远期生活质量。
-
表 1 单纯T2DM组与T2DM合并NAFLD组资料比较
指标 单纯T2DM组(n=107) 合并NAFLD组(n=377) 统计值 P值 男/女(例) 63/44 182/195 χ2=3.748 0.053 年龄(岁) 48.37±7.63 49.33±10.72 t=-0.859 0.391 T2DM病程(年) 3.00(0.94~9.00) 4.00(0.50~9.25) Z=-0.437 0.662 BMI(kg/m2) 24.52±2.95 26.97±3.67 t=-6.338 < 0.001 AST(U/L) 20.00(16.00~25.00) 23.00(18.00~31.00) Z=-3.092 < 0.001 ALT(U/L) 25.00(17.00~37.25) 30.50(21.00~48.00) Z=-3.190 0.001 AST/ALT 0.78(0.61~1.00) 0.77(0.63~0.93) Z=-0.247 0.805 FPG(mmol/L) 9.50±3.99 10.86±4.62 t=-2.778 0.006 Alb(g/L) 45.51±5.02 45.60±4.70 t=-0.175 0.861 PLT(×109/L) 220.44±81.24 196.53±72.08 t=2.936 0.003 HbA1c(%) 8.47±2.02 9.18±2.22 t=-2.976 0.003 FCP(ng/ml) 1.26(0.80~1.96) 2.10(1.29~2.94) Z=-6.040 < 0.001 FINS(mU/L) 7.82(4.46~13.77) 14.80(9.41~20.75) Z=-7.026 < 0.001 HOMA-β 29.79(13.04~70.20) 44.12(23.63~85.58) Z=-3.076 0.002 UA(μmol/L) 313.88±94.87 345.30±92.95 t=-3.072 0.002 CHO(mmol/L) 4.48±0.88 4.69±1.37 t=-2.189 0.029 TG(mmol/L) 1.80(1.37~2.87) 2.27(1.53~3.45) Z=-2.656 0.008 HDL(mmol/L) 1.07±0.28 1.07±0.30 t=-0.092 0.926 LDL(mmol/L) 2.77±0.77 2.78±1.12 t=-0.161 0.873 收缩压(mm Hg) 129.95±21.58 134.55±21.11 t=-1.980 0.048 舒张压(mm Hg) 80.88±12.09 84.79±11.98 t=-2.971 0.003 表 2 T2DM患者发生NAFLD影响因素的logistics回归分析
因素 B值 SE 统计值 OR(95%CI) P值 FCP 0.623 0.019 27.505 1.865(1.478~2.355) < 0.001 FINS 0.084 0.016 27.141 1.088(1.054~1.123) < 0.001 FPG 0.077 0.028 7.463 1.080(1.022~1.141) 0.006 HbA1c 0.159 0.054 8.548 1.173(1.054~1.305) 0.003 UA 0.004 0.001 9.112 1.004 (1.001~1.006) 0.003 表 3 T2DM合并NAFLD各纤维化亚组资料比较
指标 T2DM+F0组(n=136) T2DM+F1组(n=146) T2DM+F2组(n=95) 统计值 P值 男/女(例) 67/69 72/74 43/52 χ2=0.462 0.799 年龄(岁) 42.43±9.49 52.47±8.971) 54.38±9.791) F=58.830 < 0.001 T2DM病程(年) 3.00(0.50~7.75) 4.00(0.33~9.00) 6.00(2.00~12.00)1)2) H=11.799 0.003 BMI(kg/m2) 25.91±3.11 26.63±2.91 28.99±4.561)2) F=23.178 < 0.001 AST(U/L) 23.00(18.00~30.00) 23.00(18.00~34.00) 23.00(19.00~29.00) H=0.080 0.961 ALT(U/L) 33.50(23.00~50.00) 30.00(22.00~49.00) 26.00(16.00~40.00)1) H=12.569 0.002 AST/ALT 0.68(0.56~0.83) 0.77(0.63~0.90)1) 0.91(0.72~1.23)1)2) H=50.741 < 0.001 FPG(mmol/L) 10.64±4.22 10.65±4.24 11.50±5.47 F=1.227 0.294 Alb(g/L) 47.94±4.17 45.51±4.01 42.37±4.432) F=49.750 < 0.001 PLT(109/L) 264.74±57.73 175.48±38.251) 127.63±39.781)2) F=258.378 < 0.001 HbA1c(%) 8.95±2.12 9.46±2.15 9.06±2.42 F=2.078 0.127 FCP(ng/ml) 1.87(1.25~2.68) 1.97(1.12~2.65) 2.89(1.84~3.77)1)2) H=36.023 < 0.001 高FCP率(%) 24.27 23.29 56.841)2) χ2=37.583 < 0.001 FINS(mU/L) 12.71(8.21~17.09) 15.20(9.12~19.45) 22.15(12.14~29.58)1)2) H=38.885 < 0.001 HOMA-β 38.62(19.71~78.32) 45.74(23.16~81.92) 53.31(25.78~114.32) H=5.028 0.081 UA(μmol/L) 353.01±87.52 333.38±96.05 352.58±94.71 F=1.970 0.141 CHO(mmol/L) 4.72±1.07 4.86±1.67 4.37±1.781) F=3.792 0.023 TG(mmol/L) 2.24(1.50~3.15) 2.30(1.58~4.22) 2.21(1.74~3.51) H=1.932 0.381 HDL(mmol/L) 1.06±0.29 1.09±0.31 1.08±0.30 F=1.043 0.353 LDL(mmol/L) 2.87±0.90 2.82±1.25 2.60±1.17 F=1.879 0.154 收缩压(mm Hg) 131.26±18.40 134.85±18.64 138.82±26.961) F=3.663 0.027 舒张压(mm Hg) 86.18±11.20 83.86±11.37 84.21±13.79 F=1.479 0.229 注:与T2DM+F0组相比,1)P < 0.05;与T2DM+F1组相比,2)P < 0.05。 表 4 T2DM合并NAFLD肝纤维化进展影响因素的logistic回归分析
因素 B值 SE 统计值 OR(95%CI) P值 FCP 0.361 0.080 20.143 1.435(1.227~1.678) < 0.001 FINS 0.059 0.010 32.095 1.061(1.040~1.082) < 0.001 收缩压 0.013 0.050 8.200 1.013(0.919~1.117) 0.004 -
[1] YOUNOSSI ZM, KOENIG AB, ABDELATIF D, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes[J]. Hepatology, 2016, 64(1): 73-84. DOI: 10.1002/hep.28431. [2] ARRESE M, BARRERA F, TRIANTAFILO N, et al. Concurrent nonalcoholic fatty liver disease and type 2 diabetes: Diagnostic and therapeutic considerations[J]. Expert Rev Gastroenterol Hepatol, 2019, 13(9): 849-866. DOI: 10.1080/17474124.2019.1649981. [3] BRIL F, CUSI K. Management of Nonalcoholic fatty liver disease in patients with type 2 diabetes: A call to action[J]. Diabetes Care, 2017, 40(3): 419-430. DOI: 10.2337/dc16-1787. [4] TARGHER G, LONARDO A, BYRNE CD. Nonalcoholic fatty liver disease and chronic vascular complications of diabetes mellitus[J]. Nat Rev Endocrinol, 2018, 14(2): 99-114. DOI: 10.1038/nrendo.2017.173. [5] ZHANG YC, LI W. Association between diabetes and nonalcoholic fatty liver disease-related hepatocellular carcinoma[J]. J Clin Hepatol, 2020, 36(10): 2329-2332. DOI: 10.3969/j.issn.1001-5256.2020.10.037.张永超, 李威. 糖尿病和非酒精性脂肪性肝病相关肝细胞癌的相互关系[J]. 临床肝胆病杂志, 2020, 36(10): 2329-2332. DOI: 10.3969/j.issn.1001-5256.2020.10.037. [6] Chinese Diabetes Society, Chinese Medical Association. Guidelines for the prevention and control of type 2 diabetes in China (2017 Edition)[J]. Chin J Diabetes, 2018, 10(1): 4-67. DOI: 10.3760/cma.j.issn.1674-5809.2018.01.003.中华医学会糖尿病学分会. 中国2型糖尿病防治指南(2017年版)[J]. 中华糖尿病杂志, 2018, 10(1): 4-67. DOI: 10.3760/cma.j.issn.1674-5809.2018.01.003. [7] National Workshop on Fatty Liver and Alcoholic Liver Disease Chinese Society of Hepatology Chinese Medical Association; Fatty Liver Expert Committee Chinese Medical Doctor Association. Guidelines of prevention and treatment for nonalcoholic fatty liver disease: A 2018 update[J]. J Clin Hepatol, 2018, 34(5): 947-957. DOI: 10.3969/j.issn.1001-5256.2018.05.007.中华医学会肝病学分会脂肪肝和酒精性肝病学组, 中国医师协会脂肪性肝病专家委员会. 非酒精性脂肪性肝病防治指南(2018更新版)[J]. 临床肝胆病杂志, 2018, 34(5): 947-957. DOI: 10.3969/j.issn.1001-5256.2018.05.007. [8] DING Y, FU Q, QIN Y, et al. Analysis of islet beta cell function assessed by C-peptide changing with the disease duration in patients with type 2 diabetes mellitus[J]. Chin J Diabetes, 2020, 12(7): 491-495. DOI: 10.3760/cma.j.cn115791-20200403-00185.丁宇, 付麒, 秦瑶, 等. 2型糖尿病患者C肽评估胰岛β细胞功能随病程变化的分析[J]. 中华糖尿病杂志, 2020, 12(7): 491-495. DOI: 10.3760/cma.j.cn115791-20200403-00185. [9] ANGULO P, HUI JM, MARCHESINI G, et al. The NAFLD fibrosis score: A noninvasive system that identifies liver fibrosis in patients with NAFLD[J]. Hepatology, 2007, 45(4): 846-854. DOI: 10.1002/hep.21496. [10] PAN XZ, QI Y, YANG Y. Research advances in liver dysfunction in patients with type 2 diabetes[J]. J Clin Hepatol, 2020, 36(10): 2370-2374. DOI: 10.3969/j.issn.1001-5256.2020.10.046.潘秀珍, 戚筠, 杨杨. 2型糖尿病患者合并肝功能异常的研究进展[J]. 临床肝胆病杂志, 2020, 36(10): 2370-2374. DOI: 10.3969/j.issn.1001-5256.2020.10.046. [11] STEINER DF, CUNNINGHAM D, SPIGELMAN L, et al. Insulin biosynthesis: Evidence for a precursor[J]. Science, 1967, 157(3789): 697-700. DOI: 10.1126/science.157.3789.697. [12] ROTH J, WHITFORD I, DANKNER R, et al. How the immunoassay transformed C-peptide from a duckling into a swan[J]. Diabetologia, 2012, 55(4): 865-869. DOI: 10.1007/s00125-011-2421-0. [13] NORDQUIST L, JOHANSSON M. Proinsulin C-peptide: Friend or foe in the development of diabetes-associated complications?[J]. Vasc Health Risk Manag, 2008, 4(6): 1283-1288. DOI: 10.2147/vhrm.s3955. [14] WANG N, WANG Y, ZHANG W, et al. C-peptide is associated with NAFLD inflammatory and fibrotic progression in type 2 diabetes[J]. Diabetes Metab Res Rev, 2020, 36(2): e3210. DOI: 10.1002/dmrr.3210. [15] PINGER CW, ENTWISTLE KE, BELL TM, et al. C-Peptide replacement therapy in type 1 diabetes: Are we in the trough of disillusionment?[J]. Mol Biosyst, 2017, 13(8): 1432-1437. DOI: 10.1039/c7mb00199a. [16] MANSOUR A, MOHAJERI-TEHRANI MR, SAMADI M, et al. Risk factors for non-alcoholic fatty liver disease-associated hepatic fibrosis in type 2 diabetes patients[J]. Acta Diabetol, 2019, 56(11): 1199-1207. DOI: 10.1007/s00592-019-01374-x. [17] AKUTA N, KAWAMURA Y, FUJIYAMA S, et al. Predictors of insulin secretion in Japanese patients with histopathologically-confirmed non-alcoholic fatty liver disease[J]. Intern Med, 2020, 59(3): 329-338. DOI: 10.2169/internalmedicine.3555-19. [18] ATSAWARUNGRUANGKIT A, CHENBHANICH J, DICKSTEIN G. C-peptide as a key risk factor for non-alcoholic fatty liver disease in the United States population[J]. World J Gastroenterol, 2018, 24(32): 3663-3670. DOI: 10.3748/wjg.v24.i32.3663. [19] TILG H, MOSCHEN AR. Inflammatory mechanisms in the regulation of insulin resistance[J]. Mol Med, 2008, 14(3-4): 222-231. DOI: 10.2119/2007-00119.Tilg. [20] SHPAKOV AO, GRANSTREM OK. C-peptide physiological effects[J]. Ross Fiziol Zh Im I M Sechenova, 2013, 99(2): 196-211. [21] HUI JM, SUD A, FARRELL GC, et al. Insulin resistance is associated with chronic hepatitis C virus infection and fibrosis progression [corrected][J]. Gastroenterology, 2003, 125(6): 1695-1704. DOI: 10.1053/j.gastro.2003.08.032. [22] RASCHI E, MAZZOTTI A, POLUZZI E, et al. Pharmacotherapy of type 2 diabetes in patients with chronic liver disease: Focus on nonalcoholic fatty liver disease[J]. Expert Opin Pharmacother, 2018, 19(17): 1903-1914. DOI: 10.1080/14656566.2018.1531126. [23] MUSSO G, CASSADER M, PASCHETTA E, et al. Thiazolidinediones and advanced liver fibrosis in nonalcoholic steatohepatitis: A Meta-analysis[J]. JAMA Intern Med, 2017, 177(5): 633-640. DOI: 10.1001/jamainternmed.2016.9607. [24] CHEN HP, SHIEH JJ, CHANG CC, et al. Metformin decreases hepatocellular carcinoma risk in a dose-dependent manner: population-based and in vitro studies[J]. Gut, 2013, 62(4): 606-615. DOI: 10.1136/gutjnl-2011-301708. [25] TSENG CH. Metformin and risk of hepatocellular carcinoma in patients with type 2 diabetes[J]. Liver Int, 2018, 38(11): 2018-2027. DOI: 10.1111/liv.13872. [26] DING QH, SHI DH. Advances on the molecular mechanism of metformin's antitumor effect[J]. Chin J Clin Pharmacol Ther, 2019, 24(3): 350-354. DOI: 10.12092/j.issn.1009-2501.2019.03.019.丁秋花, 史道华. 二甲双胍抗肿瘤作用的分子机制研究进展[J]. 中国临床药理学与治疗学, 2019, 24(3): 350-354. DOI: 10.12092/j.issn.1009-2501.2019.03.019. 期刊类型引用(11)
1. 刘春影,许青,王飞,高磊. 二维剪切波弹性成像对2型糖尿病合并非酒精性脂肪肝病患者肝纤维化的诊断价值. 医疗卫生装备. 2024(09): 73-77 .  百度学术
百度学术2. 谢添,王嘉欣,黄哲,李卓琪,张川. 初诊T2DM合并NAFLD患者肝纤维化与25(OH)D的相关性研究. 中国实验诊断学. 2023(01): 10-13 .  百度学术
百度学术3. 金波,蒋谷芬,朱诗林,曾碧君,何花,高娟,雷晓红. 基于蛋白质组学探讨刮痧干预冠心病痰浊瘀阻证的作用机制. 中医药导报. 2023(02): 111-116 .  百度学术
百度学术4. 钱亮. 血清C肽与糖化血红蛋白联合检验诊断糖尿病的效果研究. 系统医学. 2023(01): 90-93 .  百度学术
百度学术5. 陈张哲,葛丹,司慧峰,王钰哲,凌宏威. 2型糖尿病合并非酒精性脂肪性肝病、进展性肝纤维化的危险因素及其预测效能. 山东医药. 2023(12): 28-33 .  百度学术
百度学术6. 金利萍. 糖尿病合并NAFLD患者肝纤维化程度与尿微量白蛋白的相关性. 新疆医学. 2023(09): 1080-1082 .  百度学术
百度学术7. 夏光萍,唐琴林,周敏,张彤,赵钢. 2型糖尿病合并非酒精性脂肪性肝病患者危险因素的Meta分析. 现代预防医学. 2022(09): 1698-1705+1728 .  百度学术
百度学术8. 吴坚玲,朱扩中,许鑫. 空腹C肽联合胰岛素抵抗指数评估2型糖尿病患者非酒精性脂肪肝肝纤维化的价值. 全科医学临床与教育. 2022(10): 873-876+882 .  百度学术
百度学术9. 谢文瑞,陈咪咪. 2型糖尿病患者血清CA19-9、CEA质量浓度与HbA1c、血糖、C肽、脂类等因素的相关性研究. 贵州医药. 2022(10): 1515-1516+1519 .  百度学术
百度学术10. 王宝君,郭翔宇,胡燕,孙宏峰. 2型糖尿病患者交感皮肤反应、神经传导速度与空腹血清C肽、FGF21、补体因子B水平相关性研究. 临床和实验医学杂志. 2022(20): 2167-2170 .  百度学术
百度学术11. 杜宣,樊华英,李慧娟,施毕旻. 浅谈微信平台辅助CBL教学法在内分泌科实习生教学中的应用. 科技风. 2021(34): 156-158 .  百度学术
百度学术其他类型引用(1)
-




 PDF下载 ( 1916 KB)
PDF下载 ( 1916 KB)

 下载:
下载:

 下载:
下载:
 百度学术
百度学术