慢性乙型肝炎免疫耐受期患者显著肝损伤的列线图模型及其预测价值分析
DOI: 10.3969/j.issn.1001-5256.2021.07.010
Value of a nomogram model in predicting significant liver injury in patients with immune-tolerant phase chronic hepatitis B
-
摘要:
目的 分析慢性HBV感染免疫耐受期(IT-CHB)患者显著肝损伤的高危因素并建立列线图预测模型。 方法 回顾性分析2002年8月—2017年12月在解放军总医院第五医学中心接受肝活检的382例慢性HBV感染者的资料,按照肝组织是否存在显著肝损伤分为2组,显著肝损伤组(≥G2或S2,n=82)和非显著肝损伤组(n=300)。正态分布的计量数据2组间比较采用独立样本t检验;非正态分布数据2组间比较采用Mann- Whitney U检验; 多组比较采用Kruskal-Wallis H检验;计数资料2组间比较采用χ2检验。相关性分析采用Spearman秩相关。采用单/多因素logistic回归方法筛选高危因素,并建立列线图模型,用C-指数、ROC曲线、校准曲线以及Bootstrap法来评价列线图的区分度及校准度。 结果 2组年龄、HBV DNA载量、ALT、AST、PLT比较差异均有统计学意义(t=-7.071,Z值别为-4.924、-3.693、-6.945、-0.585、-5.723,P值均<0.001)。Logistic回归分析显示年龄(OR=1.074,95%CI: 1.043~1.107,P<0.001),HBV DNA载量(OR=0.442,95%CI: 0.314~0.624,P<0.001),AST(OR=1.096,95%CI: 1.051~1.142,P<0.001),PLT(OR=0.992,95%CI: 0.986~0.998,P=0.006)是显著肝损伤的高危因素。基于以上因素建立列线图模型,预测显著肝损伤的C-指数为0.845,并且有拟合度高的校正曲线,其ROC曲线下面积(AUC)为0.845(95%CI: 0.795~0.895),显著优于APRI(AUC=0.781,95%CI: 0.723~0.840)以及FIB-4 (AUC=0.802,95%CI: 0.746~0.859)。 结论 免疫耐受期具有显著肝损伤的患者比例并不少见,基于年龄、HBV DNA、AST、PLT构建的列线图模型具有良好的预测准确性,可用于个体化预测IT-CHB患者的显著肝损伤,减少肝活检,为抗病毒的精准治疗提供参考。 Abstract:Objective To investigate the high-risk factors for significant liver injury in patients with immune-tolerant phase chronic hepatitis B (IT-CHB), and to establish a nomogram predictive model. Methods A retrospective analysis was performed for the data of 382 patients with chronic HBV infection who underwent liver biopsy in The Fifth Medical Center of Chinese PLA General Hospital from August 2002 to December 2017, and according to the presence or absence of significant liver injury, the patients were divided into significant liver injury group (≥G2/S2) with 82 patients and non-significant liver injury group with 300 patients. The independent samples t-test was used for comparison of normally distributed continuous data between groups, and the Mann-Whitney U test was used for comparison of non-normally distributed continuous data between groups; the Kruskal-Wallis H test was used for comparison between multiple groups; the chi-square test was used for comparison of categorical data between groups. The Spearman rank correlation test was used to investigate correlation. Univariate and multivariate logistic regression analyses were used to screen out high-risk factors and establish a nomogram model. Concordance index (C-index), the receiver operating characteristic (ROC) curve, calibration curve, and the bootstrap method were used to evaluate the discrimination and calibration abilities of the nomogram. Results There were significant differences between the two groups in age, HBV DNA load, alanine aminotransferase, aspartate aminotransferase (AST), and platelet count (PLT) (t=-7.071, Z=-4.924, -3.693, -6.945, -0.585 and -5.723, all P < 0.001). The logistic regression analysis showed that age (odds ratio [OR]=1.074, 95% confidence interval [CI]: 1.043-1.107, P < 0.001), HBV DNA load (OR=0.442, 95%CI: 0.314-0.624, P < 0.001), AST(OR=1.096, 95%CI: 1.051-1.142, P < 0.001), and PLT(OR=0.992, 95%CI: 0.986-0.998, P=0.006) were high-risk factors for significant liver injury. The nomogram model established based on the above factors had a C-index of 0.845 in predicting significant liver injury and had a well-fitted calibration curve, with an area under the ROC curve (AUC) of 0.845 (95%CI: 0.795-0.895), which was significantly better than aspartate aminotransferase-to-platelet ratio index (AUC=0.781, 95%CI: 0.723-0.840) and fibrosis-4(AUC=0.802, 95%CI: 0.746-0.859). Conclusion There is a high proportion of IT-CHB patients with significant liver injury. The nomogram model established based on age, HBV DNA, AST, and PLT has a good predictive accuracy and can be used to predict significant liver injury in IT-CHB patients individually, reduce the need for liver biopsy, and provide a reference for precise antiviral treatment. -
Key words:
- Hepatitis B, Chronic /
- Immune Tolerance /
- Nomograms
-
慢性HBV感染的自然史划分为4个期,即免疫耐受期、免疫清除期、免疫控制期和再活动期[1]。目前,对于处于免疫清除期以及再活动期的慢性乙型肝炎(CHB)患者,各大指南均推荐抗病毒治疗,对于免疫耐受期则不推荐抗病毒治疗,建议长期随访[1-4]。然而,有研究[5-11]表明,10%~49%免疫耐受期CHB (Immune-tolerant CHB,IT-CHB) 患者经肝组织病理学检查证实存在明显的肝脏炎症和/或纤维化,若不积极治疗,发展至肝硬化及肝癌的风险增加。IT-CHB患者是否抗病毒治疗尚存在争议[12-18],而评估肝组织学显著肝脏炎症及纤维化对于抗病毒治疗具有重要意义,肝活检仍然是金标准,但其有创性及不易重复等缺点限制了临床应用。本研究通过分析IT-CHB患者显著肝损伤(≥G2/S2)的高危因素,构建无创的个体化列线图预测模型,旨在为指导IT-CHB抗病毒治疗提供参考依据。
1. 资料和方法
1.1 研究对象
回顾性选取2002年8月—2017年12月在解放军总医院第五医学中心住院的IT-CHB患者。免疫耐受期的诊断标准符合2018年版美国肝病学会CHB指南[2]中的定义。纳入标准:(1)年龄>18岁;(2)HBsAg阳性及HBeAg阳性>1年;(3)ALT水平持续正常(男性35 U/L,女性25 U/L)>1年;(4)HBV DNA>1×106 IU/ml;(5)接受肝活检。排除标准:(1)合并其他病毒感染;(2)其他类型肝脏疾病;(3)失代偿期肝硬化;(4)肝癌或其他恶性肿瘤病史;(5)严重的心脏、肾脏或者其他脏器的原发疾病或精神系统疾病。
1.2 肝组织学检查
采用16G活检针进行超声引导下经皮肝活检,要求肝组织长度≥15 mm,至少包括11个汇管区[19]。由2名经验丰富的病理医师进行双盲法阅片,肝组织炎症分级和纤维化分期标准参照《慢性乙型肝炎防治指南(2015年版)》[20]。显著肝损伤(≥G2/S2)定义为肝组织学存在明显的肝脏炎症(≥G2)或纤维化(≥S2)。
1.3 血清学检测
采用贝克曼库尔特AU5421全自动生化仪检测血清ALT、AST、TBil、PLT等。乙型肝炎血清学标志物采用罗氏E170电化学发光法检测。计算APRI指数和FIB-4指数,APRI = (AST/正常值上限×100)/PLT,FIB-4=(年龄×AST)/(PLT×ALT1/2)[21]。
1.4 伦理学审查
本研究通过解放军总医院第五医学中心伦理委员会审批,批号:2020056D。
1.5 统计学方法
采用SPSS 22.0进行统计分析。正态分布的计量数据以x±s表示,2组间比较采用独立样本t检验;非正态分布数据以M(P25~P75)表示,2组间比较采用Mann- Whitney U检验; 多组比较采用Kruskal-Wallis H检验;计数资料2组间比较采用χ2检验。相关性分析采用Spearman秩相关。通过多因素logistic回归模型进入法筛选显著肝损伤的相关因素,采用R语言(3.6.1)的RMS(Regression Modeling Strategies)程序包构建列线图模型,通过Bootstrap重抽样法对模型进行内部验证,用一致性指数(C-指数)、ROC曲线、校准曲线来评价列线图的区分度及校准度。P<0.05为差异有统计学意义。
2. 结果
2.1 一般资料
共纳入382例IT-CHB患者,其中82例(21.5%)存在显著肝损伤。肝组织炎症活动度分级: G0 29例(7.6%)、G1 301例(78.8%)、G2 50例(13.1%)、G3 2例(0.5%);肝组织纤维化分期: S0 57例(14.9%)、S1 251例(65.7%)、S2 39例(10.2%)、S3 23例(6.0%)、S4 12例(3.1%)。按照是否存在显著肝损伤(≥G2/S2)分为2组,2组年龄、HBV DNA载量、ALT、AST、PLT比较差异均有统计学意义(P值均<0.001)(表 1)。
表 1 患者基线的一般资料指标 总体(n=382) 非显著肝损伤组(n=300) 显著肝损伤组(n=82) 统计值 P值 男性[例(%)] 261(68.3) 201(67.0) 60(73.2) χ2=1.133 0.287 年龄(岁) 33.3±10.2 31.5±9.1 39.9±11.2 t=-7.071 <0.001 年龄段[例(%)] χ2=56.472 <0.001 <30岁 161(42.1) 147(49.0) 14(17.1) 30~39岁 130(34.0) 106(35.3) 24(29.3) 40~49岁 64(16.8) 35(11.7) 29(35.4) ≥50岁 27(7.1) 12(4.0) 15(18.3) 乙型肝炎家族史[例(%)] 221(57.9) 173(57.7) 48(58.5) χ2=0.020 0.888 BMI(kg/m2) 23.2±3.53 23.0±3.4 23.7±3.9 t=-1.021 0.308 HBV DNA(log10IU/ml) 8.4(7.8~8.8) 8.4(8.0~8.8) 7.9(6.9~8.5) Z=-4.924 <0.001 ALT(U/L) 23.0(18.0~28.0) 23.0(18.0~28.0) 25.5(21.0~32.0) Z=-3.693 <0.001 AST(U/L) 23.0(19.0~27.0) 21.0(19.0~26.0) 28.0(23.0~34.0) Z=-6.945 <0.001 TBil(μmol/L) 11.1(8.3~15.3) 10.9(8.3~15.3) 11.5(8.6~15.4) Z=-0.585 0.559 PLT(×109/L) 202(164~234) 208(176~239) 161(137~209) Z=-5.723 <0.001 2.2 年龄与肝组织损伤病理学的关系
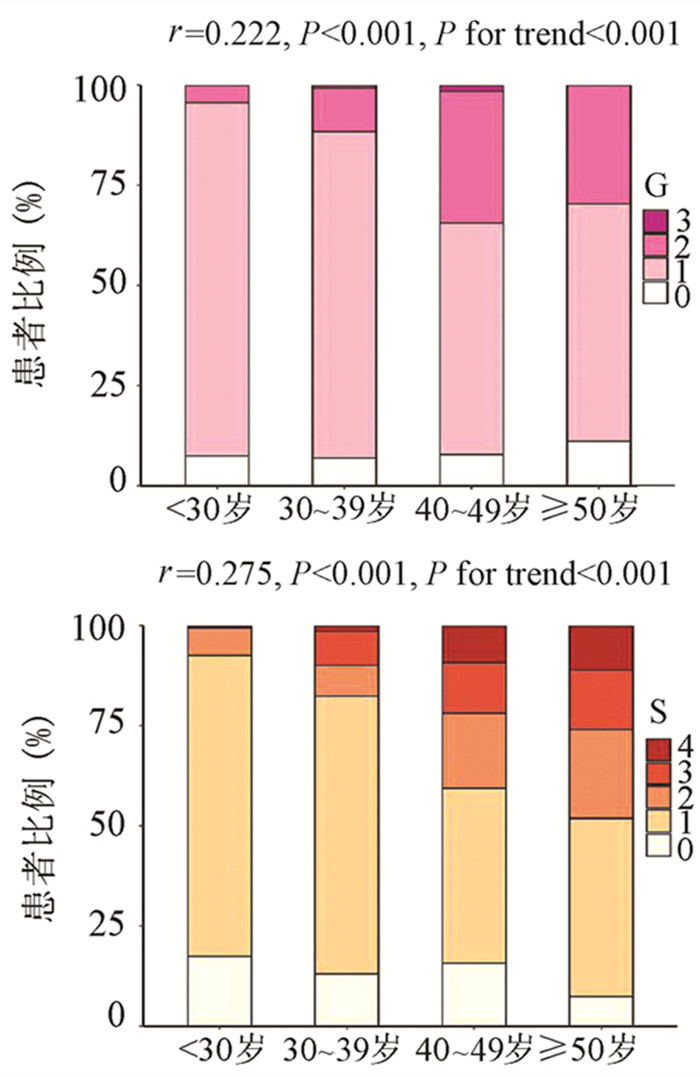
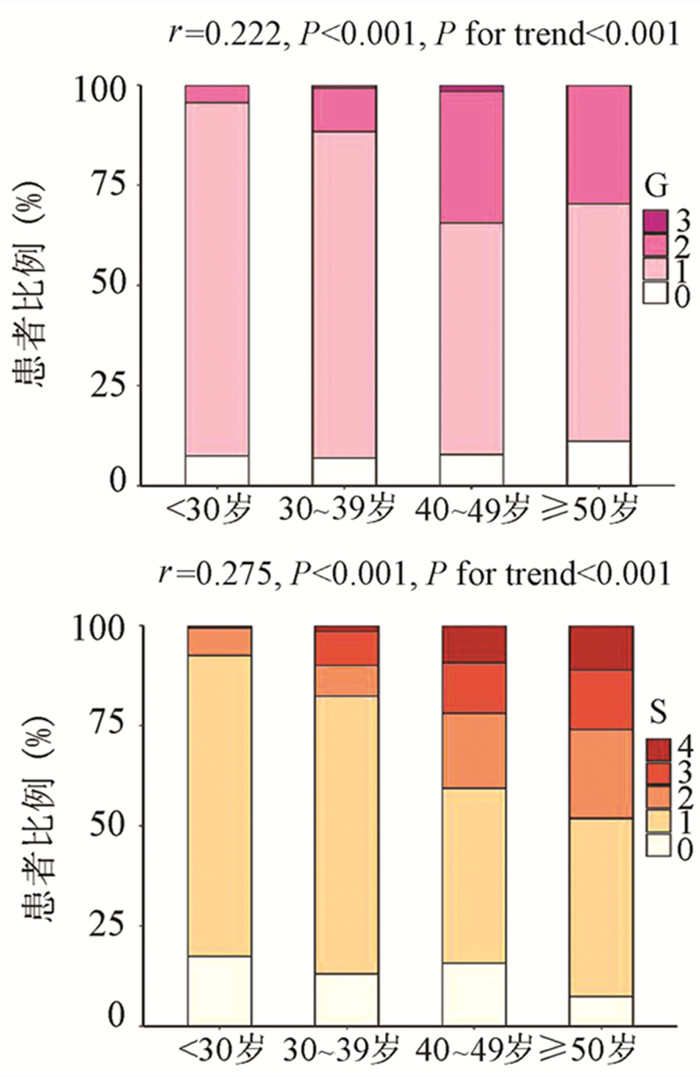
为了评估年龄对IT-CHB显著肝损伤的影响,将患者分为4个年龄段,即<30岁、30~39岁、40~49岁和≥50岁。随年龄的增加,肝组织炎症及纤维化程度逐渐升高,趋势性检验结果表明差异均具有统计学意义(P值均<0.001)。Spearman等级相关分析显示,两者呈正相关(r值分别为0.222、0.275,P值均<0.001)(图 1)。Logistic单因素分析结果显示,较年龄<30岁组,30~39岁组、40~49岁组、年龄≥50岁组出现显著肝损伤的可能性分别为2.4倍(95%CI: 1.175~4.811)、8.7倍(95%CI: 4.165~18.175)、13.1倍(95%CI: 5.146~33.477)(P值均<0.05)。
2.3 HBV DNA与肝组织损伤病理学的关系
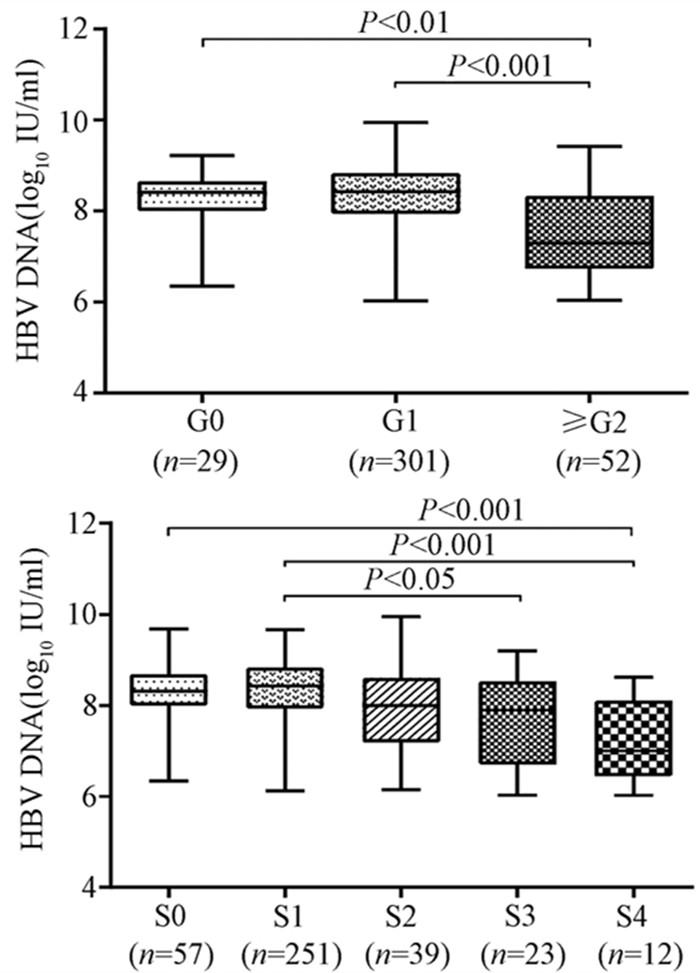
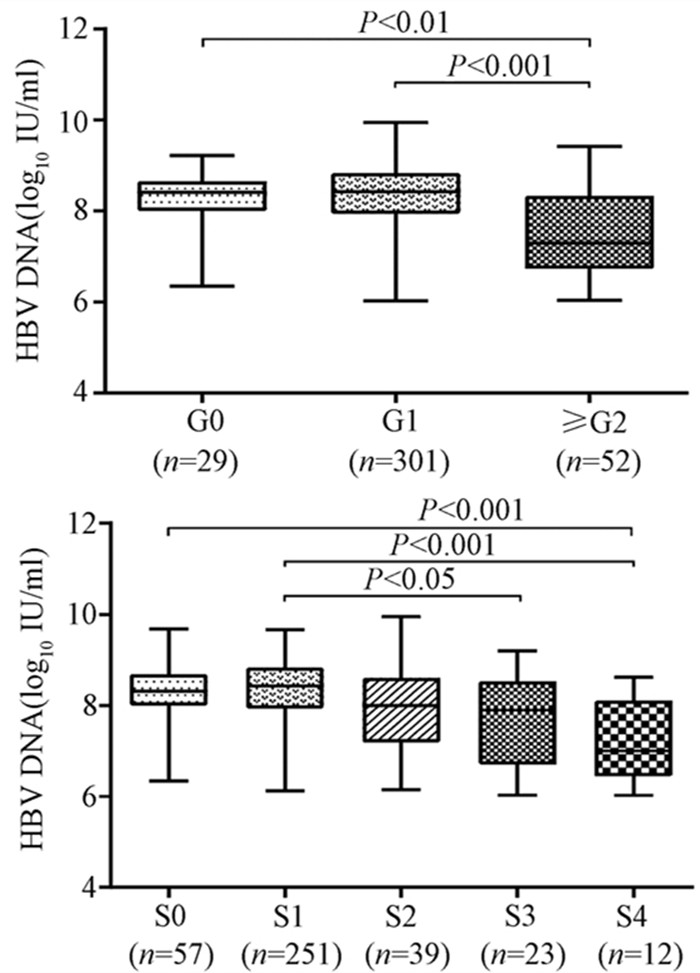
随着肝脏坏死性炎症的加剧,HBV DNA水平呈下降趋势(H=34.161,P<0.001),组间两两比较结果显示:G0组、G1组与≥G2组之间差异有统计学意义(H值分别为80.688、96.903,P值均<0.05),而GO组与G1组无差异(图 2)。伴随肝纤维化的进展,HBV DNA同样表现出下降的趋势(H=26.627,P<0.001),组间两两比较显示,S0与S4、S1与S4、S1与S3之间差异均有统计学意义(H值分别为112.287、125.953、74.354,P值均<0.05)(图 2)。
2.4 显著肝损伤的单因素及多因素分析
为进一步构建无创预测模型,基于无创参数中单因素分析P<0.05的变量作为自变量,以显著肝损伤作为因变量进行logistic回归分析。结果显示,年龄、HBV DNA水平、AST以及PLT是显著肝损伤的独立影响因素(P值均<0.01)(表 2)。
表 2 显著肝损伤的logistic回归分析因素 单因素分析 多因素分析 OR(95%CI) P值 OR(95%CI) P值 年龄 1.084 (1.057~1.113) <0.001 1.074(1.043~1.107) <0.001 HBV DNA 0.437 (0.324~0.589) <0.001 0.442(0.314~0.624) <0.001 ALT 1.076(1.036~1.119) <0.001 1.009(0.959~1.060) 0.736 AST 1.132 (1.089~1.177) <0.001 1.096(1.051~1.142) <0.001 PLT 0.985 (0.98~0.991) <0.001 0.992(0.986~0.998) 0.006 2.5 列线图的制作与检验
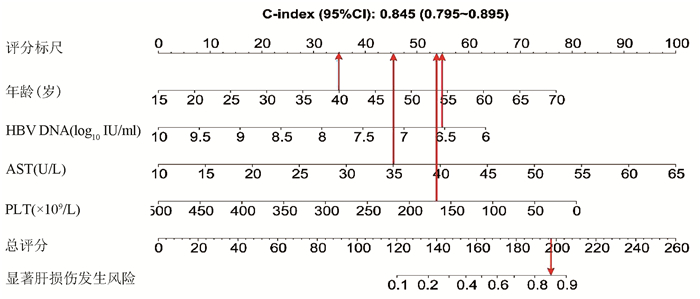
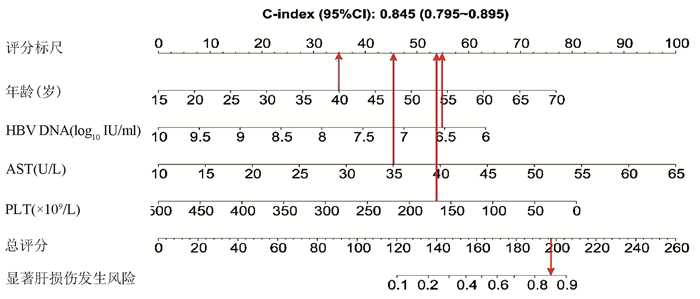
基于logistic回归分析结果,将独立影响因素引入R软件建立预测显著肝损伤的个体化列线图预测模型,并绘制校准曲线和ROC曲线。结果显示,列线图模型预测IT-CHB发生显著肝损伤的C-指数的ROC曲线下面积(AUC)为0.845(95%CI: 0.795~0.895), 明显优于单独使用APRI(AUC=0.781, 95%CI: 0.723~0.840)以及FIB-4(AUC=0.802, 95%CI: 0.746~0.859),差异有统计学意义。校正曲线贴近于理想曲线(对角线),斜率为1.017,Hosmer-Lemeshow拟合优度检验χ2=8.224,P=0.412,提示模型预测值与实际观测值之间的差异无统计学意义,预测模型有良好的校准度。ROC曲线分析显示,列线图的AUC高于APRI、FIB-4,预测IT-CHB患者显著肝损伤的最佳界值为141.4,其敏感度、特异度分别为74.4%、84.7%,差异有统计学意义(P<0.05)(表 3, 图 3)。
表 3 列线图、APRI、FIB-4诊断显著肝损伤的效能比较诊断参数 AUC 95%CI 界值 敏感度(%) 特异度(%) 阳性预测值(%) 阴性预测值(%) Youden指数 列线图 0.845 0.795 ~ 0.895 141.4 74.4 84.7 57.0 92.4 0.59 APRI 0.781 0.723 ~ 0.840 0.338 70.7 77.7 46.4 90.7 0.48 FIB-4 0.802 0.746 ~ 0.859 0.882 78.1 72.3 43.5 92.3 0.50 3. 讨论
全球慢性HBV感染者约2.92亿人,其中约5940万处于免疫耐受期,我国的IT-CHB患者约有1584万例[22]。目前国内外指南对于免疫耐受期的定义尚存在争议[1-4, 23-25],ALT正常上限的标准亦不同, 按照美国肝病学会的标准意味我国IT-CHB患者并非全部处于免疫耐受阶段。单纯用病毒学、ALT水平评估免疫耐受可能存在临床误判,“真正”的免疫耐受需在肝活检基础上进一步确诊,目前多项研究[5-9]表明,10%~49% IT-CHB患者存在明显的肝细胞炎症坏死和肝纤维化病理学改变, 此类患者是否应抗病毒治疗逐渐成为热点问题。
本研究发现IT-CHB患者中21.5%(82/382)存在显著肝损伤,19.4%(74/382)呈显著肝纤维化,其中12例患者(3.1%)处于S4期,提示并不是全部IT-CHB患者均不需要治疗,如何筛选出需要治疗的患者尤为重要。本研究筛选出4个显著肝损伤的高危因素,包括年龄、HBV DNA水平、AST以及PLT,其中AST、PLT作为APRI、FIB-4的参数之一,已被充分证实与肝纤维化程度有关[21, 26]。既往研究[27-28]表明,年龄是CHB患者疾病进展的独立危险因素,尤其年龄>30岁时,HBV相关性肝纤维化、肝硬化、肝癌患者的比例显著增加。Xing等[6]发现年龄是影响肝组织炎症及纤维化的独立预测因子,这一点与本研究结果一致,将IT-CHB患者的年龄分为4个年龄亚组,结果表明,随年龄的增加,肝组织炎症及纤维化程度逐渐升高。关于HBV DNA,我国台湾的大样本研究[29]发现高HBV DNA水平CHB患者进展至肝硬化的风险增加,但其中81.6%(2923/3582) 为HBeAg阴性患者,不属于IT-CHB患者,因此该研究无法准确反映高HBV DNA水平与IT-CHB患者肝纤维化的关系。而本研究发现,IT-CHB患者随着肝脏炎症及纤维化程度的加重,HBV DNA呈下降趋势;并且轻度肝损伤(<G2/S2)的IT- CHB患者中位HBV DNA水平更高(8.4 log10 IU/ml),因此单纯HBV DNA水平并不能准确反映出IT-CHB患者纤维化程度。基于上述分析,本研究建立了无创的列线图模型用于预测IT-CHB患者的显著肝损伤,该模型具有无创的优势,并将多因素分析结果可视化、量化、个体化,具有可重复性,可作为肝活检的有效替代方式。根据该列线图模型,假设某40岁的IT-CHB患者,HBV DNA水平为6.56 log10 IU/ml,AST 35 U/L,PLT 166×109/L,则该患者总得分为188.6分,发生显著肝损伤的概率高达85%,需积极抗病毒治疗。
综上所述,免疫耐受期具有显著肝损伤的患者比例并不少见,基于年龄、HBV DNA、AST、PLT 4个因素构建的列线图模型具有良好的预测准确性,可用于个体化预测IT-CHB患者的显著肝损伤,减少肝活检,为抗病毒的精准治疗提供参考。
-
表 1 患者基线的一般资料
指标 总体(n=382) 非显著肝损伤组(n=300) 显著肝损伤组(n=82) 统计值 P值 男性[例(%)] 261(68.3) 201(67.0) 60(73.2) χ2=1.133 0.287 年龄(岁) 33.3±10.2 31.5±9.1 39.9±11.2 t=-7.071 <0.001 年龄段[例(%)] χ2=56.472 <0.001 <30岁 161(42.1) 147(49.0) 14(17.1) 30~39岁 130(34.0) 106(35.3) 24(29.3) 40~49岁 64(16.8) 35(11.7) 29(35.4) ≥50岁 27(7.1) 12(4.0) 15(18.3) 乙型肝炎家族史[例(%)] 221(57.9) 173(57.7) 48(58.5) χ2=0.020 0.888 BMI(kg/m2) 23.2±3.53 23.0±3.4 23.7±3.9 t=-1.021 0.308 HBV DNA(log10IU/ml) 8.4(7.8~8.8) 8.4(8.0~8.8) 7.9(6.9~8.5) Z=-4.924 <0.001 ALT(U/L) 23.0(18.0~28.0) 23.0(18.0~28.0) 25.5(21.0~32.0) Z=-3.693 <0.001 AST(U/L) 23.0(19.0~27.0) 21.0(19.0~26.0) 28.0(23.0~34.0) Z=-6.945 <0.001 TBil(μmol/L) 11.1(8.3~15.3) 10.9(8.3~15.3) 11.5(8.6~15.4) Z=-0.585 0.559 PLT(×109/L) 202(164~234) 208(176~239) 161(137~209) Z=-5.723 <0.001 表 2 显著肝损伤的logistic回归分析
因素 单因素分析 多因素分析 OR(95%CI) P值 OR(95%CI) P值 年龄 1.084 (1.057~1.113) <0.001 1.074(1.043~1.107) <0.001 HBV DNA 0.437 (0.324~0.589) <0.001 0.442(0.314~0.624) <0.001 ALT 1.076(1.036~1.119) <0.001 1.009(0.959~1.060) 0.736 AST 1.132 (1.089~1.177) <0.001 1.096(1.051~1.142) <0.001 PLT 0.985 (0.98~0.991) <0.001 0.992(0.986~0.998) 0.006 表 3 列线图、APRI、FIB-4诊断显著肝损伤的效能比较
诊断参数 AUC 95%CI 界值 敏感度(%) 特异度(%) 阳性预测值(%) 阴性预测值(%) Youden指数 列线图 0.845 0.795 ~ 0.895 141.4 74.4 84.7 57.0 92.4 0.59 APRI 0.781 0.723 ~ 0.840 0.338 70.7 77.7 46.4 90.7 0.48 FIB-4 0.802 0.746 ~ 0.859 0.882 78.1 72.3 43.5 92.3 0.50 -
[1] Chinese Society of Infectious Diseases, Chinese Medical Association, Chinese Society of Infectious Diseases, Chinese Medical Association. Guidelines for the prevention and treatment of chronic hepatitis B (version 2019)[J]. J Clin Hepatol, 2019, 35(12): 2648-2669. DOI: 10.3969/j.issn.1001-5256.2019.12.007.中华医学会肝病学分会, 中华医学会感染病学分会. 慢性乙型肝炎防治指南(2019年版)[J]. 临床肝胆病杂志, 2019, 35(12): 2648-2669. DOI: 10.3969/j.issn.1001-5256.2019.12.007. [2] TERRAULT NA, LOK A, MCMAHON BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance[J]. Hepatology, 2018, 67(4): 1560-1599. DOI: 10.1002/hep.29800. [3] SARIN SK, KUMAR M, LAU GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: A 2015 update[J]. Hepatol Int, 2016, 10(1): 1-98. DOI: 10.1007/s12072-015-9675-4. [4] European Association for the Study of the Liver. EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection[J]. J Hepatol, 2017, 67(2): 370-398. DOI: 10.1016/j.jhep.2017.03.021. [5] GAO HY, LIU N, LI CX, et al. Clinical features and liver histopathological analysis of patients in the immune tolerance stage of chronic hepatitis B virus infection[J]. Chin Hepatol, 2018, 23(2): 136-139. DOI: 10.14000/j.cnki.issn.1008-1704.2018.02.012.高红艳, 刘娜, 李春霞, 等. 慢性乙型肝炎病毒感染者免疫耐受期的临床特征与肝组织病理学分析[J]. 肝脏, 2018, 23(2): 136-139. DOI: 10.14000/j.cnki.issn.1008-1704.2018.02.012. [6] XING YF, ZHOU DQ, HE JS, et al. Clinical and histopathological features of chronic hepatitis B virus infected patients with high HBV DNA viral load and normal alanine aminotransferase level: A multicentre-based study in China[J]. PLoS One, 2018, 13(9): e0203220. DOI: 10.1371/journal.pone.0203220. [7] KUMAR M, SARIN SK, HISSAR S, et al. Virologic and histologic features of chronic hepatitis B virus-infected asymptomatic patients with persistently normal ALT[J]. Gastroenterology, 2008, 134(5): 1376-1384. DOI: 10.1053/j.gastro.2008.02.075. [8] NGUYEN MH, GARCIA RT, TRINH HN, et al. Histological disease in Asian-Americans with chronic hepatitis B, high hepatitis B virus DNA, and normal alanine aminotransferase levels[J]. Am J Gastroenterol, 2009, 104(9): 2206-2213. DOI: 10.1038/ajg.2009.248. [9] SETO WK, LAI CL, IP PP, et al. A large population histology study showing the lack of association between ALT elevation and significant fibrosis in chronic hepatitis B[J]. PLoS One, 2012, 7(2): e32622. DOI: 10.1371/journal.pone.0032622. [10] KIM HL, KIM GA, PARK JA, et al. Cost-effectiveness of antiviral treatment in adult patients with immune-tolerant phase chronic hepatitis B[J]. Gut, 2020. [Online ahead of print]. DOI: 10.1136/gutjnl-2020-321309. [11] KIM GA, LIM YS, HAN S, et al. High risk of hepatocellular carcinoma and death in patients with immune-tolerant-phase chronic hepatitis B[J]. Gut, 2018, 67(5): 945-952. DOI: 10.1136/gutjnl-2017-314904. [12] GAO HY, LIU N, LI CX, et al. Research advances in influencing factors for natural prognosis of patients with chronic HBV infection in immune tolerance phase[J]. J Clin Hepatol, 2017, 33(8): 1572-1575. DOI: 10.3969/j.issn.1001-5256.2017.08.035.高红艳, 刘娜, 李春霞, 等. 慢性HBV感染者免疫耐受期自然转归相关影响因素的研究进展[J]. 临床肝胆病杂志, 2017, 33(8): 1572-1575. DOI:10. 3969/j.issn.1001-5256.2017.08.035. [13] ZHOU LL, LIU N, LI CX, et al. Research advances in the diagnosis and treatment of patients in the immune tolerance stage of chronic hepatitis B virus infection[J]. J Clin Hepatol, 2019, 35(8): 1824-1827. DOI: 10.3969/j.issn.1001-5256.2019.08.039.周路路, 刘娜, 李春霞, 等. 慢性HBV感染免疫耐受期诊治进展[J]. 临床肝胆病杂志, 2019, 35(8): 1824-1827. DOI: 10.3969/j.issn.1001-5256.2019.08.039. [14] LIU M, ZUO LL, ZHANG RY, et al. Research progress of antiviral therapy in patients with chronic HBV infection in immune tolerance phase[J]. Chin J Infect Dis, 2020, 38(11): 750-752. DOI: 10.3760/cma.j.cn311365-20190422-00138.刘敏, 左丽丽, 张茹薏, 等. 乙型肝炎免疫耐受期患者进行抗病毒治疗的研究进展[J]. 中华传染病杂志, 2020, 38(11): 750-752. DOI: 10.3760/cma.j.cn311365-20190422-00138. [15] ZHUANG H. Should patients in the immune tolerance stage of chronic hepatitis B virus infection be treated?[J]. J Clin Hepatol, 2021, 37(2): 272-277. DOI: 10.3969 /j.issn.1001-5256.2021.02.007.庄辉. 慢性HBV感染免疫耐受期应否治疗?[J]. 临床肝胆病杂志, 2021, 37(2): 272-277. DOI: 10.3969 /j.issn.1001-5256.2021.02.007. [16] LOK AS, MCMAHON BJ, BROWN RS Jr, et al. Antiviral therapy for chronic hepatitis B viral infection in adults: A systematic review and meta-analysis[J]. Hepatology, 2016, 63(1): 284-306. DOI: 10.1002/hep.28280. [17] LEE HW, CHON YE, KIM BK, et al. Negligible HCC risk during stringently defined untreated immune-tolerant phase of chronic hepatitis B[J]. Eur J Intern Med, 2021, 84: 68-73. DOI: 10.1016/j.ejim.2020.10.022. [18] KLAIR JS, VANCURA J, MURALI AR. PRO: Patients with chronic hepatitis B in immune-tolerant phase should be treated[J]. Clin Liver Dis (Hoboken), 2020, 15(1): 21-24. DOI: 10.1002/cld.892. [19] ROCKEY DC, CALDWELL SH, GOODMAN ZD, et al. Liver biopsy[J]. Hepatology, 2009, 49(3): 1017-1044. DOI: 10.1002/hep.22742. [20] Chinese Society of Hepatology and Chinese Society of Infectious Diseases, Chinese Medical Association. The guideline of prevention and treatment for chronic hepatitis B(2015 version)[J]. J Clin Hepatol, 2015, 31(12): 1941-1960. DOI: 10.3969/j.issn.1001-5256.2015.12.002.中华医学会肝病学分会, 中华医学会感染病学分会. 慢性乙型肝炎防治指南(2015年版)[J]. 临床肝胆病杂志, 2015, 31(12): 1941-1960. DOI: 10. 3969 /j. issn. 1001-5256. 2015. 12. 002. [21] ZHUANG H. Correction note on the estimated number of patients in the immune - tolerant phase of hepatitis B virus infection in China and globally[J]. J Clin Hepatol, 2021, 37(4): 785-786. DOI: 10.3969 /j.issn.1001-5256.2021.04.012.庄辉. 全球和我国HBV感染免疫耐受期患者人数估计更正说明[J]. 临床肝胆病杂志, 2021, 37(4): 785-786. DOI: 10.3969/j.issn.1001-5256.2021.04.012 [22] Polaris Observatory Collaborators. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: A modelling study[J]. Lancet Gastroenterol Hepatol, 2018, 3(6): 383-403. DOI: 10.1016/S2468-1253(18)30056-6. [23] BERTOLETTI A, KENNEDY PT. The immune tolerant phase of chronic HBV infection: New perspectives on an old concept[J]. Cell Mol Immunol, 2015, 12(3): 258-263. DOI: 10.1038/cmi.2014.79. [24] LIAW YF, CHU CM. Immune tolerance phase of chronic hepatitis B[J]. Gastroenterology, 2017, 152(5): 1245-1246. DOI: 10.1053/j.gastro.2016.11.057. [25] KENNEDY PTF, BERTOLETTI A, MASON WS. Reply to immune tolerance phase of chronic hepatitis B[J]. Gastroenterology, 2017, 152(5): 1246-1247. DOI: 10.1053/j.gastro.2017.03.002. [26] WANG H, XUE L, YAN R, et al. Comparison of FIB-4 and APRI in Chinese HBV-infected patients with persistently normal ALT and mildly elevated ALT[J]. J Viral Hepat, 2013, 20(4): e3-e10. DOI: 10.1111/jvh.12010. [27] FATTOVICH G, BORTOLOTTI F, DONATO F. Natural history of chronic hepatitis B: Special emphasis on disease progression and prognostic factor[J]. J Hepatol, 2008, 48(2): 335-352. DOI: 10.1016/j.jhep.2007.11.011. [28] RAFFETTI E, FATTOVICH G, DONATO F. Incidence of hepatocellular carcinoma in untreated subjects with chronic hepatitis B: A systematic review and meta-analysis[J]. Liver Int, 2016, 36(9): 1239-1251. DOI: 10.1111/liv.13142. [29] ILOEJE UH, YANG HI, SU J, et al. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load[J]. Gastroenterology, 2006, 130(3): 678-686. DOI: 10.1053/j.gastro.2005.11.016. 期刊类型引用(12)
1. 王可欣,陈椿,贺梦雯,李乐,刘妍,王洪波,王春艳,赵景民,纪冬. 减数分裂内切酶1高表达对肝细胞癌预后的影响. 解放军医学杂志. 2024(06): 643-650 .  百度学术
百度学术2. 刘立,董志坚,常丽仙,徐肇元,李国忠,张丽华,刘春云. HBeAg阳性ALT正常不确定期患者肝组织炎症活动及其影响因素. 中华肝脏病杂志. 2024(04): 325-331 .  百度学术
百度学术3. 王春艳,纪冬,陈艳,周光德,董政,王建军,陈国凤,杨永平. 慢性乙型肝炎患者经恩替卡韦治疗后获得显著组织学应答的影响因素及列线图模型构建. 解放军医学杂志. 2023(02): 143-150 .  百度学术
百度学术4. 李乐,廖昊,思兰兰,陈容娟,王钧,张珊,徐东平,纪冬,刘妍. 新型血清学标志物在不同类型乙型肝炎病毒感染中的检出特征分析. 解放军医学杂志. 2023(02): 163-167 .  百度学术
百度学术5. 张珊,陈松海,刘妍,王春艳,付懿铭,陆荫英,纪冬,陈国凤. 非酒精性脂肪性肝病对乙肝病毒相关肝细胞癌患者生存的影响. 解放军医学杂志. 2023(02): 157-162 .  百度学术
百度学术6. 纪冬,杨永平. 慢性乙型肝炎临床热点问题解析. 解放军医学杂志. 2023(02): 132-137 .  百度学术
百度学术7. 黄圣楷,孙龙. 慢性HBV感染肝脏炎症风险预测列线图模型的建立与验证. 海南医学院学报. 2023(12): 910-915 .  百度学术
百度学术8. 付宝云,熊渝婷,王文畅,邓亚,郭畅,杨武才,王春艳,纪冬,王建军. 丙氨酸转氨酶正常的慢性乙型肝炎患者肝组织学改变的关联因素分析. 解放军医学院学报. 2023(11): 1218-1223 .  百度学术
百度学术9. 王可欣,纪冬. 乙型肝炎病毒与肝细胞癌关系研究进展. 传染病信息. 2022(02): 166-171 .  百度学术
百度学术10. 陈松海,王春艳,郭畅,张珊,邓亚,陆荫英,纪冬. 预测HBV相关肝细胞癌生存的列线图模型的建立. 临床肝胆病杂志. 2022(07): 1566-1571 .  本站查看
本站查看11. 王建军,纪冬,陈艳,董政,杨永平. 慢性乙型肝炎患者恩替卡韦治疗后肝纤维化逆转的影响因素分析. 解放军医学院学报. 2022(07): 719-723 .  百度学术
百度学术12. 李同. 慢性乙型肝炎患者接受核苷类似物抗病毒治疗发生耐药后停药的临床分析. 中国医药指南. 2021(27): 14-16 .  百度学术
百度学术其他类型引用(2)
-




 PDF下载 ( 2739 KB)
PDF下载 ( 2739 KB)

 下载:
下载:



 下载:
下载:
 百度学术
百度学术