儿童肝炎伴发粒细胞减少的临床特征及其进展为再生障碍性贫血的危险因素分析
DOI: 10.3969/j.issn.1001-5256.2021.07.025
Clinical features of children with hepatitis and granulocytopenia and risk factors for progression to aplastic anemia
-
摘要:
目的 总结儿童肝炎伴发粒细胞减少的发生率和临床特征,分析其进展为再生障碍性贫血(AA)的危险因素。 方法 回顾性选取2014年7月—2020年3月解放军总医院第五医学中心肝病医学部肝病科因肝功异常住院的≤18岁患儿2944例,统计病程中新发血象中性粒细胞减少(<1.5×109/L)患儿的临床特点及病情转归,分析其进展为AA的危险因素。正态分布的计量资料2组间比较采用t检验;非正态分布的计量资料2组间比较采用Wilcoxon秩和检验。计数资料2组间比较采用χ2检验。危险因素采用logistic回归分析。 结果 2944例以肝功能异常入院的儿童中38例为肝炎伴发粒细胞减少,发生率为1.3%。68.4%(26/38)为药物性肝损伤、2.6%(1/38)为自身免疫性肝炎、28.9%(11/38)为不明原因肝损伤。血液异常表现为粒细胞减少症21.1%(8/38),粒细胞减少+三系下降47.4%(18/38),粒细胞缺乏症7.9%(3/38),粒细胞缺乏+三系下降23.7%(9/38)。骨髓细胞学检查,14例(36.8%)诊断为肝炎相关再生障碍性贫血(HAAA),24例(63.2%)为非HAAA。HAAA组与非HAAA组的CD4+(8.5% vs 17%,P=0.008)、CD4+/CD8+(0.17 vs 0.47,P=0.015)比较差异均有统计学意义。进一步多因素logistic回归分析发现CD4+下降为HAAA发生的危险因素(OR=0.009, 95%CI: 0~0.838,P<0.05)。38例患儿均常规保肝、降酶、退黄治疗。14例HAAA患儿中6例免疫抑制剂治疗,7例异基因骨髓移植,血象逐渐恢复,1例因重型AA死亡;24例非HAAA患儿中23例经治疗血象逐渐恢复正常,1例因急性肝衰竭死亡。 结论 儿童肝炎伴发粒细胞减少需警惕AA,尤以药物性或不明原因多见,其中CD4+下降可作为AA的预测因子。 Abstract:Objective To investigate the incidence rate and clinical features of children with hepatitis and granulocytopenia and the risk factors for progression to aplastic anemia (AA). Methods A retrospective analysis was performed for 2944 children, aged ≤18 years, who were hospitalized due to abnormal liver function in Pediatric Liver Diseases Treatment and Research Center, The Fifth Medical Center of Chinese PLA General Hospital, from July 2014 to March 2020. Clinical features and prognosis were analyzed for children with new-onset neutropenia (< 1.5×109/L) during the course of the disease, and the risk factors for progression to AA were analyzed. The t-test was used for comparison of normally distributed continuous data between groups, and the Wilcoxon rank-sum test was used for comparison of non-normally distributed continuous data between groups; the chi-square test was used for comparison of categorical data between groups. A logistic regression analysis was used to investigate risk factors. Results Among the 2944 children admitted due to abnormal liver function, 38 had hepatitis with granulocytopenia, with an incidence rate of 1.3%. Among these 38 children, 68.4% (26/38) had drug-induced liver injury, 2.6% (1/38) had autoimmune hepatitis, and 28.9% (11/38) had unexplained liver injury; as for blood abnormalities, 21.1% (8/38) had granulocytopenia, 47.4% (18/38) had granulocytopenia and reductions in three lines of blood cells, 7.9% (3/38) had agranulocytosis, and 23.7% (9/38) had agranulocytosis and reductions in three lines of blood cells. Bone marrow cytology showed that among the 38 children, 14 (36.8%) were diagnosed with hepatitis-associated AA (HAAA) and 24 (63.2%) were diagnosed with non-HAAA. In order to analyze the risk factors for HAAA, there were significant differences in CD4+ (8.5% vs 17%, P=0.008) and CD4+/CD8+ ratio (0.17 vs 0.47, P=0.015) between the HAAA group and the non-HAAA group. Further multivariate logistic regression analysis showed that the reduction in CD4+ was a risk factor for HAAA (β=-4.757, P < 0.05). All 38 children were given routine liver-protecting, transaminase-lowering, and jaundice clearance treatment. Among the 14 children with HAAA, 6 had gradual recovery of hemogram after immunosuppressant therapy, 7 had gradual recovery of hemogram after allogeneic hematopoietic stem cell transplantation, and 1 died due to severe AA; among the 24 children with non-HAAA, 23 had hemogram gradually returning to normal after treatment, and 1 died of acute liver failure. Conclusion AA should be taken seriously for children with hepatitis and granulocytopenia, especially for those with drug-induced or unexplained liver injury, and the reduction in CD4+ may be used as a predictive factor for AA. -
Key words:
- Hepatitis /
- Agranulocytosis /
- Neutropenia /
- Anemia, Aplastic /
- Children /
- Risk Factors
-
自身免疫性胰腺炎(autoimmune pancreatitis,AIP)是一种自身免疫介导的慢性胰腺炎,目前发病机制尚未完全阐明,依据病理特征表现,可分为两种亚型:1型为淋巴浆细胞硬化性胰腺炎,被认为是IgG4相关性疾病(IgG4-related disease,IgG4-RD)的胰腺表现;2型为特发性导管中心性慢性胰腺炎[1]。IgG4-RD可累及全身多个组织器官,累及胆管时表现为IgG4相关硬化性胆管炎(IgG4-related sclerosing cholangitis,IgG4-SC)[2],多数1型AIP患者可同时累及胆管出现IgG4-SC的表现,研究[3-5]显示,合并IgG4-SC的1型AIP患者在临床表现及预后方面较单纯AIP患者更差。为此,本研究总结了AIP合并IgG4-SC患者的临床资料,并与单纯AIP患者进行比较,以加深对该病的理解与认识。
1. 资料与方法
1.1 研究对象
选取2015年6月—2020年1月本院收治的40例1型AIP患者为研究对象,相关病历资料由郑州大学第一附属医院病案科数据库中获取。1型AIP的确诊依据国际统一诊断标准[1]:胰腺实质影像学改变,胰管影像学改变,血清IgG4水平,其他器官受累情况,胰腺组织学改变,以及糖皮质激素的治疗反应。IgG4-SC的确诊依据2012年IgG4-SC日本标准[6],其中单纯出现胆总管末端受累不诊断为IgG4-SC。其他器官受累参照2011年IgG4-RD综合诊断标准[7]。依据上述诊断标准将AIP患者分为合并IgG4-SC患者和单纯AIP患者。
1.2 研究方法
详细采集病史,包括:(1)年龄、性别、过敏史、临床表现、并发症、既往史等一般资料;(2)血常规、肝肾功能、血糖、IgG及其亚类、自身抗体、肿瘤标志物等实验室检查;(3)CT、MRI、磁共振胰胆管成像(MRCP)、经内镜逆行胰胆管造影(ERCP)等影像学检查;(4)组织病理学检查。使用IgG4-RD反应指数(respinder index,RI)评估器官的疾病活动度[8]。采用电话或查询病史资料的方式进行随访研究,随访时间截至2020年6月。治疗应答标准为AIP的临床症状及胰腺和/或胰腺外的影像学异常减轻或消失,复发定义为胰腺或胰腺外器官临床症状及影像学异常出现,伴或不伴血清IgG4浓度升高。
1.3 伦理学审查
本研究通过郑州大学第一附属医院伦理审查委员会审核,批号:2020-KY-473。
1.4 统计学方法
采用SPSS 24.0软件进行统计分析。正态分布的计量资料以x±s表示,两组间比较采用t检验,非正态分布的计量资料以M(P25~P75)表示,两组间比较采用Mann-Whitney U检验。计数资料两组间比较采用Fisher确切检验。采用Kaplan-Meier法计算复发率并绘制曲线,采用log-rank检验进行比较。P < 0.05为差异有统计学意义。
2. 结果
2.1 一般资料
共计纳入1型AIP确诊患者38例,疑似病例2例。其中单纯AIP组29例,AIP合并IgG4-SC组11例。两组患者在年龄、性别、病程、过敏史、合并糖尿病和接受激素治疗上差异均无统计学意义(P值均>0.05)(表 1)。单个器官受累7例(17.5%),2个器官受累者7例(17.5%),3个及以上器官受累者26例(65.0%)。最常累及的器官依次为淋巴结28例(70%)、胆管11例(27.5%)、肺部10例(25.0%)、涎腺6例(15.0%)、肾脏5例(12.5%)、泪腺或眼眶3例(7.5%)、腹膜后纤维化2例(5.0%)。AIP合并IgG4-SC组器官受累数目及初始治疗前RI值均高于单纯AIP组(P值均 < 0.05)(表 1)。
表 1 两组AIP患者的临床资料比较项目 全部患者(n=40) 单纯AIP组(n=29) AIP合并IgG4-SC组(n=11) 统计值 P值 年龄(岁) 56.28±11.17 55.59±10.68 57.82±12.78 t=-0.533 0.597 男/女(例) 36/4 26/3 10/1 >0.05 病程(月) 1.0(0.5~2.8) 1.0(0.5~2.5) 2.0(1.0~3.0) Z=-1.095 0.280 过敏史[例(%)] 6(15.0) 5(17.2) 1(9.1) >0.05 糖尿病[例(%)] 16(40.0) 12(41.3) 4(36.4) >0.05 器官受累数目(个) 3.0(2.0~4.0) 3.0(1.5~3.5) 3.0(3.0~4.0) Z=-2.172 0.035 RI 12.0(9.0~15.0) 12.0(9.0~13.5) 12.0(12.0~15.0) Z=-2.157 0.032 接受激素治疗患者[例(%)] 34(85.0) 23(79.3) 11(100.0) 0.162 2.2 临床表现
40例AIP患者中常见的临床表现包括梗阻性黄疸28例(70%)、体质量下降16例(40%)、轻度上腹痛18例(45%),其次有纳差、乏力、腹泻等表现,16例(40%)伴有糖尿病。
2.3 实验室检查
AIP合并IgG4-SC组患者血清IgG水平明显高于单纯AIP患者(P < 0.05)。其他实验室检查指标如血常规(Hb、WBC、PLT、嗜酸性粒细胞计数)、肝功能(ALT、AST、ALP、GGT、TBil、球蛋白)、肿瘤标志物CA19-9、淀粉酶、脂肪酶的差异均无统计学意义(P值均>0.05)。40例血清IgG4均阳性(≥1.35 g/L),34例(85%)血清IgG4升高>2倍正常值上限(ULN)(≥2.7 g/L),两组患者血清IgG4>2×ULN的比例无明显差异(P>0.05)(表 2)。
表 2 两组患者实验室指标比较项目 全部患者(n=40)> 单纯AIP组(n=29) AIP合并IgG4-SC组(n=11) 统计值 P值 Hb(g/L) 125.3±13.5 127.8±13.4 118.7±11.7 t=1.985 0.054 WBC(×109/L) 6.0±1.7 6.2±1.8 5.4±1.5 t=1.157 0.254 PLT(×109/L) 195.7±64.7 201.0±72.0 181.8±38.8 t=1.077 0.289 嗜酸性粒细胞计数(×109/L) 0.25(0.13~0.45) 0.24(0.13~0.47) 0.14(0.10~0.40) Z=-0.879 0.385 ALT(U/L) 137.0(42.3~265.0) 168.0(39.5~334.5) 121.0(72.0~187.0) Z=-0.591 0.570 AST(U/L) 86.5(39.5~173.6) 87.0(27.5~205.5) 86.0(57.0~146.0) Z=-0.454 0.660 GGT(U/L) 342.0(85.5~787.5) 341.0(42.0~892.0) 527.0(146.0~642.0) Z=-0.227 0.830 ALP(U/L) 258.0(125.8~475.5) 251.0(111.0~495.0) 391.0(178.0~469.0) Z=-0.984 0.334 TBil(μmol/L) 54.1(17.4~109.3) 53.4(10.6~118.0) 54.7(32.9~107.0) Z=-0.197 0.850 球蛋白(g/L) 32.0(27.4~38.0) 30.2(26.6~38.2) 34.0(30.0~36.5) Z=-0.924 0.369 CA19-9(g/L) 32.1(17.6~79.4) 28.0(9.3~56.6) 64.2(21.2~81.5) Z=-1.459 0.151 IgG(g/L) 15.5(14.4~20.7) 14.8(13.3~15.7) 21.0(15.8~23.7) Z=-2.711 0.004 IgG4≥2×ULN[例(%)] 34(85.0) 23(79.3) 11(100.0) 0.162 淀粉酶(U/L) 45.0(18.3~71.0) 49.0(22.0~74.0) 31.0(16.0~61.0) Z=-1.496 0.368 脂肪酶(U/L) 33.5(13.9~94.4) 46.0(14.0~101.4) 15.5(9.1~30.2) Z=-0.915 0.144 2.4 影像学检查
所有患者均行CT检查,其中22例(55%)表现为胰腺弥漫性肿大增粗呈腊肠样外观,局灶性肿大者18例(45%),其中累及胰头者9例,累及胰体或胰尾部6例,同时累及胰头及胰体或胰尾的多灶型3例。单纯AIP患者所有影像学类型均可见,而AIP合并IgG4-SC患者的胰腺肿大以弥漫型多见,其次为胰头型及多灶型,未见单独胰体或胰尾部受累。40例CT平扫检查均提示胰腺密度均匀,无胰腺结石或钙化,增强期胰腺延迟强化。7例(17.5%)可见胰周增厚的包膜环绕,形成“包膜样”外观。30例(75%)行MRI/MRCP检查,7例(17.5%)行ERCP检查。31例(77.5%)患者出现胆管弥漫性或局灶性狭窄伴胆管壁增厚,仅末端胆管受累者20例(50%),11例(27.5%)患者出现胆管其他部位受累,其中肝内胆管4例,肝门部胆管5例,肝外及胰腺外7例,同时累及胆总管下段9例。
2.5 治疗及随访
34例(85%)患者接受糖皮质激素初始治疗,诱导缓解期选用泼尼松或甲泼尼松20~50 mg/d维持治疗2~4周后,每1~2周减量5 mg,2~3个月后以小剂量激素维持治疗3个月以上,1例患者因病情较重接受激素联合免疫抑制剂(吗替麦考酚酯)治疗。其余6例因病情较轻或拒绝使用激素,仅予以对症治疗,均出现不同程度的自行缓解。所有经激素治疗的患者均反应较好,胰腺异常影像表现及症状减轻或消失。40例患者中,4例(10%)误诊为胰胆管肿瘤性疾病,其中1例误诊为肝内胆管恶性肿瘤行肝占位切除术,3例怀疑为胰腺恶性肿瘤,1例行胰十二指肠切除术,2例行外科胆肠吻合术并进行胰头肿物活检,术后病理提示胰腺慢性炎症,结合临床表现诊断AIP,接受激素治疗。单纯AIP组与AIP合并IgG4-SC组在是否接受激素治疗上未见明显差异(P>0.05)(表 1)。
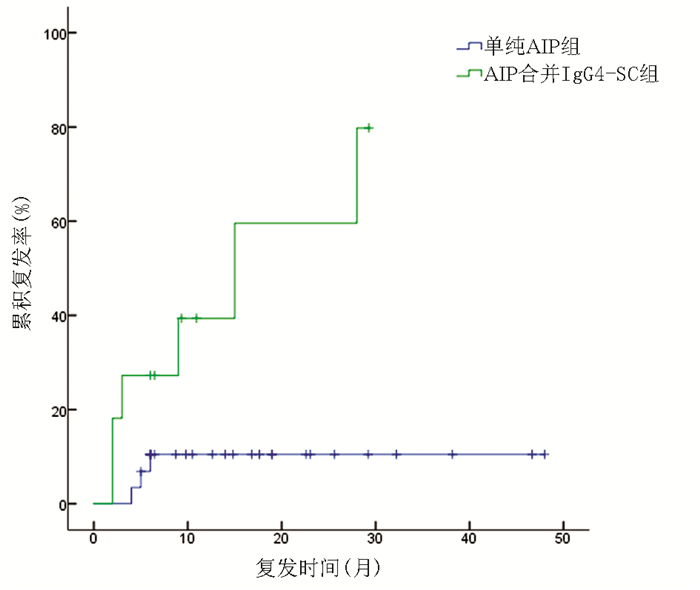
40例患者均获得随访,中位随访时间为15.8(6.5~31.3)个月,9例患者复发,总体复发率为22.5%,其中6例为AIP合并IgG4-SC患者,3例为单纯AIP患者。复发患者重新予以激素或加用免疫抑制剂治疗,均获得缓解,2例在随访过程中出现再次复发,予以小剂量激素长期维持治疗,未再复发。与单纯AIP组相比,AIP合并IgG4-SC组复发率更高,差异有统计学意义(χ2=8.155,P=0.004)(图 1)。
3. 讨论
1型AIP是一种少见的特殊类型慢性胰腺炎,为IgG4相关性疾病的胰腺表现。临床表现缺乏特异性,易被误诊为胰胆管恶性肿瘤,进行不必要的手术治疗。目前AIP的诊断采用临床表现、实验室检查、影像学表现及病理学多方面进行综合评估[9]。IgG4-SC是IgG4-RD患者胆道受累的表现形式,与AIP关系密切,约90%以上患者伴有AIP[10]。国外研究[11]表明,未合并IgG4-SC的AIP患者预后要明显优于合并IgG4-SC的AIP患者。因此,早期识别AIP合并IgG4-SC患者的临床特征对改善AIP患者预后尤为重要。
AIP好发于中老年男性,男女性别比为2.94∶ 1,好发年龄为60~69岁,但可累及各个年龄段[12]。本组患者平均发病年龄56.28岁,男性占比90%。最常见的临床症状为梗阻性黄疸,多因胰腺炎症波及胆管或压迫胆管所致,其次为上腹部不适、体质量下降、纳差等表现。AIP患者可出现胰腺分泌功能受损而表现为糖尿病,发生率为42%~78%[13],本组患者中糖尿病发生率为40%。AIP作为一种全身性疾病的局部表现,常伴有胰腺外器官受累,单独胰腺受累少见[14]。本研究中中位受累器官数目为3(2~4)个,中位IgG4-RD反应指数为12(9~15),最常累及的器官包括淋巴结、胆管、肺部、涎腺、肾脏等,与文献报道相似[15]。与单纯AIP组相比,AIP合并IgG4-SC组在一般情况上未见明显差异,但累计器官数量及初始治疗前RI值更高。
本研究发现单纯AIP组与AIP合并IgG4-SC组患者在血常规、肝功能、肿瘤标志物CA19-9、AMY、LIP方面的差异均无统计学意义,AIP合并IgG4-SC组患者血清IgG水平明显高于单纯AIP患者,与国外研究结果相似[5]。血清IgG4检测是AIP国际诊断共识唯一纳入的血清学指标,相关Meta分析[16]表明,IgG4诊断AIP的灵敏度为74%,特异度为93%。本研究中所有患者血清IgG4均阳性,85%的患者血清IgG4≥2×ULN,提示血清IgG4检测对AIP患者有较高的诊断性能;其中AIP合并IgG4-SC组血清IgG4均≥2×ULN,升高率为100%,单纯AIP组为79.3%,两者分布并无明显差异。本文未能对IgG4进行定量检测,结合AIP合并IgG4-SC组血清IgG更高,AIP合并IgG4-SC可能代表更高的炎症活动度,需要尽早给予治疗。
IgG4-SC可累及肝内外胆管,以肝外胆管尤其是胆总管下段多见。研究[17]表明,约64%的IgG4-SC患者仅表现为胆总管下段受累。目前对仅有胆总管下段受累的AIP患者是否应纳入IgG4-SC范畴尚有争议。此现象可能是肿大的胰腺压迫所致,因为在仅有胰体或胰尾受累的患者中很少观察到末端胆管狭窄,且AIP国际诊断共识也认为仅有末端胆管受累不应作为胰腺外器官受累的依据。然而在部分手术切除的标本中也可发现胆管壁IgG4+浆细胞的浸润,提示这一现象是胰腺炎症波及胆管的结果[18]。本研究患者中,77.5%出现胆管局灶或弥漫性狭窄,50%仅累及末端胆管,27.5%出现胆管其他部位受累。由于临床工作中难以鉴别胆管狭窄原因,因此仅表现为末端胆管狭窄的患者并未纳入本研究定义的IgG4-SC中。
影像学检查是AIP诊断过程中的重要手段,典型表现为胰腺弥漫性肿大呈“腊肠样”改变,伴有延迟强化及胰管不规则狭窄,30%~40%患者可出现特异性的胰周“假包膜”征象,为胰腺炎症波及胰周所致,部分患者表现为局灶性或多灶性改变,胆管受累时表现为弥漫或节段性胆管狭窄、管壁增厚[19-20]。胰腺肿大的累及部位不同时其临床表现存在差异,Lv等[15]发现,弥漫性及胰头部肿大时胆管受累相比胰腺体尾部肿大更为多见。本研究中AIP合并IgG4-SC组均表现为弥漫性肿大或胰头累及,而胰体或胰尾部累及仅见于单纯AIP组,这一结果与文献[15]报道相符。
治疗方面,85%接受了糖皮质激素治疗,均获得有效缓解,两组患者在治疗方法的选择上无明显差异。20%~60%的AIP患者在激素诱导缓解后出现复发,可发生在激素减量、停用或维持治疗时[11]。本研究中复发率为22.5%,单因素分析显示,在中位随访时间15.8(6.5~31.3)个月中,合并IgG4-SC是AIP患者复发的危险因素。一项国际多中心研究[21]表明,合并IgG4-SC是AIP复发的高危因素,然而仅有末端胆管受累却与复发无明显相关。相关Meta分析[22]也表明,近段胆管受累的AIP患者复发率更高,这可能代表机体内存在更广泛的炎症反应,相对于未累及胆管的AIP患者其疾病活动度更高,从而更易复发。
综上所述,本研究比较了单纯AIP及AIP合并IgG4-SC的临床表现以及复发情况,相对于单纯AIP组,AIP合并IgG4-SC组血清IgG水平及基础RI值更高、受累器官更多,且在随访过程中更易复发。AIP合并IgG4-SC可能预示着更高的疾病活动度,提示此类患者在诊疗过程中应接受更严格的临床评估,在治疗后也应接受更严格的临床随访,警惕疾病再次复发。
-
表 1 38例肝炎患儿发生血象异常的类型及分布特点
项目 粒细胞减少症(n=8) 粒细胞缺乏症(n=3) 粒细胞减少+ 三系下降(n=18) 粒细胞缺乏+ 三系下降(n=9) 总计 病因(例) 药物 6 1 14 5 26 不明原因 2 2 3 4 11 自身免疫性肝炎 0 0 1 0 1 发生时间(d) 19.0(6.5~55.5) 105.0(27~140) 42.0(28~73) 30.0(27.5~63.5) 发病时期(例) 极期 2 0 6 2 10 恢复期 6 3 12 7 28 HAAA(例) 0 0 5 9 14 骨穿结果(例) 增生减低 1 1 7 8 17 增生活跃 6 2 9 1 18 未做 1 0 2 0 3 表 2 38例肝炎患儿粒细胞减少时生化、血象及免疫学指标与肝炎发病时的比较
指标 肝炎发病时 发生粒细胞减少时 统计值 P值 ALT(U/L) 1 141.00(644.58~1 482.13) 206.00(65.25~592.75) Z=4.805 <0.001 AST(U/L) 1 005.00(714.63~1 489.28) 185.00(72.00~735.50) Z=4.473 <0.001 TBil(μmol/L) 199.35(115.85~270.48) 66.75(26.75~205.33) Z=3.719 <0.001 DBil(μmol/L) 148.85(76.75~211.88) 52.50(18.30~170.40) Z=2.587 0.010 PTA(%) 65.17±17.02 93.3±26.98 t=6.635 <0.001 WBC(×109/L) 5.10±2.85 3.03±1.61 t=5.802 <0.001 NEUT(×109/L) 3.19±2.52 1.69±1.37 t=5.047 <0.001 RBC(×1012/L) 4.46±0.57 3.88±0.72 t=5.377 <0.001 Hb(g/L) 126.66±16.15 114.24±18.54 t=5.591 <0.001 PLT(×109/L) 204.00±113.23 128.37±113.16 t=4.490 <0.001 T淋巴细胞(%) 69.89±14.33 68.29±17.26 t=0.951 0.348 CD4+(%) 18.50±14.08 19.39±15.22 t=1.384 0.175 CD8+(%) 44.16±18.04 43.05±19.04 t=0.773 0.445 CD4+/CD8+ 0.28(0.15~0.73) 0.35(0.15~0.80) Z=0.918 0.359 表 3 38例肝炎伴发粒细胞减少患儿的治疗及预后
项目 粒细胞减少症 粒细胞缺乏症 粒细胞减少+三系下降 粒细胞缺乏+三系下降 总计 常规治疗(例) 5 1 12 3 21 激素和/或丙种球蛋白治疗(例) 3 2 6 6 17 HAAA治疗(例) ATG序贯CsA 0 0 1 0 1 CsA 0 0 3 2 5 allo-HSCT 0 0 2 5 7 转归(例) 恢复 7 3 18 8 36 死亡 1 0 0 1 2 表 4 儿童肝炎伴发粒细胞减少发生AA的单因素分析
因素 HAAA组(n=14) 非HAAA组(n=24) 统计值 P值 男性[例(%)] 11(78.6) 13(54.2) χ2=2.263 1.132 年龄(岁) 9.8(5.7~11.2) 10.8(9.5~13.5) Z=1.181 0.238 BMI(kg/m2) 18.80±2.01 16.96±3.11 t=1.965 0.057 病因(药物)[例(%)] 7(50.0) 19(79.2) χ2=3.481 0.062 皮疹[例(%)] 2(14.3) 6(25.0) χ2=0.684 0.365 感染[例(%)] 8(57.1) 12(50.0) χ2=0.181 0.671 激素[例(%)] 7(50.0) 10(41.7) χ2=0.248 0.618 ALT(U/L) 1 218.5(730.0~1 668.0) 796.0(422.0~1 471.0) Z=1.210 0.226 AST(U/L) 1 082.9(337.0~1 328.0) 971.0(669.0~1 590.0) Z=0.393 0.694 TBil(μmol/L) 263.8(156.0~308.0) 241.6(163.6~354.3) Z=0.030 0.976 WBC(×109/L) 5.22(2.50~6.70) 4.67(2.88~6.15) Z=0.303 0.762 NEUT(×109/L) 3.17(1.51~4.51) 2.34(1.48~3.82) Z=0.832 0.405 T淋巴细胞百分比(%) 67.5(53.5~80.5) 73.0(63.0~81.0) Z=1.014 0.310 CD4+(%) 8.5(6.5~14.5) 17.0(10.0~43.0) Z=2.638 0.008 CD8+(%) 47.5(35.5~60.0) 33.0(25.0~60.0) Z=1.499 0.134 CD4+/CD8+ 0.17(0.15~0.35) 0.47(0.18~1.44) Z=2.422 0.015 -
[1] QI PJ, ZHENG J, MA J, et al. Clinical features and treatment outcome of hepatitis associated aplastic anemia in 43 children[J]. Chin J Appl Clin Pediatr, 2017, 32(3): 216-219. DOI: 10.3760/cma.j.issn.2095-428X.2017.03.013.漆佩静, 郑杰, 马洁, 等. 儿童肝炎相关再生障碍性贫血43例临床特征及治疗转归分析[J]. 中华实用儿科临床杂志, 2017, 32(3): 216-219. DOI: 10.3760/cma.j.issn.2095-428X.2017.03.013. [2] Chinese Society of Infectious Diseases and Parasitology, Chinese Society of Hepatology, Chinese Medical Association. Prevention and treatment of viral hepatitis[J]. Chin J Infect Dis, 2001, 19(1): 56-62. DOI: 10.3760/j.issn:1000-6680.2001.01.027.中华医学会传染病与寄生虫病学分会、肝病学分会. 病毒性肝炎防治方案[J]. 中华传染病杂志, 2001, 19(1): 56-62. DOI: 10.3760/j.issn:1000-6680.2001.01.027. [3] Drug-induced Liver Disease Study Group, Chinese Society of Hepatology, Chinese Medical Association. Guidelines for the management of drug-induced liver injury[J]. J Clin Hepatol, 2015, 31(11): 1752-1768. DOI: 10.3760/cma.j.issn.1007-3418.2015.11.004.中华医学会肝病学分会药物性肝病学组. 药物性肝损伤诊治指南(2015年版)[J]. 临床肝胆病杂志, 2015, 31(11): 1752-1768. DOI: 10.3760/cma.j.issn.1007-3418.2015.11.004. [4] Chinese Society of Hepatology, Chinese Medical Association; Chinese Society of Gastroenterology, Chinese Medical Association; Chinese Society of Infectious Diseases, Chinese Medical Association. Consensus on the diagnosis and management of autoimmune hepatitis (2015)[J]. J Clin Hepatol, 2016, 32(1): 9-22. DOI: 10.3969/j.issn.100l-5256.2016.01.002.中华医学会肝病学分会, 中华医学会消化病学分会, 中华医学会感染病学分会. 自身免疫性肝炎诊断和治疗共识(2015)[J]. 临床肝胆病杂志, 2016, 32(1): 9-22. DOI: 10.3969/j.issn.100l-5256.2016.01.002. [5] BARONE A, LUCARELLI A, ONOFRILLO D, et al. Diagnosis and management of acquired aplastic anemia in childhood. Guidelines from the Marrow Failure Study Group of the Pediatric Haemato-Oncology Italian Association (AIEOP)[J]. Blood Cells Mol Dis, 2015, 55(1): 40-47. DOI: 10.1016/j.bcmd.2015.03.007. [6] Study Group of Hematology, Chinese Pediatric Society, Chinese Medical Association; Editorial Board of Chinese Journal of Pediatrics. Recommendation for the diagnosis and treatment of acquired aplastic anemia in children[J]. Chin J Pediatr, 2014, 52(2): 103-106. DOI: 10.3760/cma.j.issn.0578-1310.2014.02.006.中华医学会儿科学分会血液学组, 《中华儿科杂志》编辑委员会. 儿童获得性再生障碍性贫血诊疗建议[J]. 中华儿科杂志, 2014, 52(2): 103-106. DOI: 10.3760/cma.j.issn.0578-1310.2014.02.006. [7] RAUFF B, IDREES M, SHAH SA, et al. Hepatitis associated aplastic anemia: A review[J]. Virol J, 2011, 8: 87. DOI: 10.1186/1743-422X-8-87. [8] LOCASCIULLI A, BACIGALUPO A, BRUNO B, et al. Hepatitis-associated aplastic anaemia: Epidemiology and treatment results obtained in Europe. A report of The EBMT aplastic anaemia working party[J]. Br J Haematol, 2010, 149(6): 890-895. DOI: 10.1111/j.1365-2141.2010.08194.x. [9] WANG HQ, TU MF, FU R, et al. The clinical and immune characteristics of patients with hepatitis-associated aplastic anemia in China[J]. PLoS One, 2014, 9(5): e98142. DOI: 10.1371/journal. Pope.0098142. [10] QIAO XH, XIE XT, SHI W, et al. Clinic features of hepatitis associated aplastic anemia for children by evidence-based medical analysis[J]. Chin J Appl Clin Pediatr, 2013, 28(15): 1155-1158. DOI: 10.3760/cma.j.issn.2095-428X. 2013.15.011.乔晓红, 谢晓恬, 石苇, 等. 儿童肝炎相关再生障碍性贫血循证医学分析[J]. 中华实用儿科临床杂志, 2013, 28(15): 1155-1158. DOI: 10.3760/cma.j.issn.2095-428X. 2013.15.011. [11] MOLLESTON JP, FONTANA RJ, LOPEZ MJ, et al. Characteristics of idiosyncratic drug-induced liver injury in children: Results from the DILIN prospective study[J]. J Pediatr Gastroenterol Nutr, 2011, 53(2): 182-189. DOI: 10.1097/MPG.0b013e31821d6cfd. [12] GAFAR F, ARIFIN H, JURNALIS YD, et al. Antituberculosis drug-induced liver injury in children: Incidence and risk factors during the two-month intensive phase of therapy[J]. Pediatr Infect Dis, 2019, 38: 50-53. DOI: 10.1097/INF.0000000000002192. [13] DANAN G, BENICHOU E. Causality assessment of adverse reactions to drugs-I. A novel method based on the conclusions of international consensus meetings: Application to drug-induced liver injuries[J]. Clin Epidemiol, 1993, 46: 1323-1330. DOI: 10.1016/0895-4356(93)90101-6. [14] CHEN Y, LIN N, SUN M. Clinical research of childhood hepatitis-associated aplastic anemia[J]. Chin Pediatr Emerg Med, 2015, 22(9): 603-606. DOI: 10.3760/cma.j.issn.1673-4912.2015.09.003.陈莹, 林楠, 孙梅. 儿童肝炎相关性再生障碍性贫血临床特征研究[J]. 中国小儿急救医学, 2015, 22(9): 603-606. DOI: 10.3760/cma.j.issn.1673-4912.2015.09.003. [15] MU J, CHEN F, HE Q. Hepatitis-associated aplastic anaemia in children: A case report[J]. J Clin Hepatol, 2019, 35(8): 1797-1799. DOI: 10.3969/j.issn.1001-5256.2019.08.029.穆静, 陈芳, 何强. 儿童肝炎相关再生障碍性贫血1例报告[J]. 临床肝胆病杂志, 2019, 35(8): 1797 -1799. DOI: 10.3969/j.issn.1001-5256.2019.08.029. [16] RAUFF B, IDREES M, SHAH SA, et al. Hepatitis associated aplastic anemia: A review[J]. Virol J, 2011, 8: 87. DOI: 10.1186/1743-422X-8-87. [17] WANG H, TU M, FU R, et al. The clinical and immune characteristics of patients with hepatitis-associated aplastic anemia in China[J]. PLoS One, 2014, 9(5): e98142. DOI: 10.1371/journal.pone.0098142. [18] BABUSHOK DV, GRIGNON AL, LI Y, et al. Disrupted lymphocyte homeostasis in hepatitis-associated acquired aplastic anemia is associated with short telomeres[J]. Am J Hematol, 2016, 91(2): 243-247. DOI: 10.1002/ajh.24256. [19] ALSAKKAL M, AL-KHATEEB M, ALHALABY M, et al. Hepatitis-associated aplastic anemia: A report of 3 cases associated with HAV[J]. J Pediatr Hematol Oncol, 2019, 41(3): e164, e166. DOI: 10.1097/MPH.0000000000001199. [20] ZHANG XM, LUO RM. Progress in diagnosis and treatment of hepatitis associated aplastic in children[J]. Infect Dis Info, 2019, 32(2): 171-174. DOI: 10.3969/j.issn.1007-8134.2019.02.019.张晓妹, 罗荣牡. 儿童肝炎相关再生障碍性贫血的诊治进展[J]. 传染病信息, 2019, 32(2): 171-174. DOI: 10.3969/j.issn.1007-8134.2019.02.019. [21] IKEDA T, MORIMOTO A, NAKAMURA S, et al. A marked decrease in CD4-positive lymphocytes at the onset of hepatitis in a patient with hepatitis-associated aplastic anemia[J]. J Pediatr Hematol Oncol, 2012, 34(5): 375-377. DOI: 10.1097/MPH.0b013e31822bf699. [22] McKENZIE RB, BERQUIST WE, NADEAU KC, et al. Novel protocol including liver biopsy to identify and treat CD8+ T-cell predominant acute hepatitis and liver failure[J]. Pediatr Transplant, 2014, 18(5): 503-509. DOI: 10.1111/petr.12296. [23] GONÇALVES V, CALADO R, PALARÉ MJ, et al. Hepatitis-associated aplastic anaemia: A poor prognosis[J]. BMJ Case Rep, 2013, 2013: bcr2012007968. DOI: 10.1136/bcr-2012-007968. [24] TU MF, SHAO ZH, LIU H, et al. The clinical features of hepatitis associated aplastic anemia[J]. Chin J Hemotal, 2005, 26(4): 239-242. DOI: 10.3760/j:issn:0253-2727.2005.04.012.涂梅峰, 邵宗鸿, 刘鸿, 等. 肝炎相关再生障碍性贫血的临床特征[J]. 中华血液学杂志, 2005, 26(4): 239-242. DOI: 10.3760/j:issn:0253-2727.2005.04.012. 期刊类型引用(5)
1. 吴振虎,姚雷,崔碧,李海斌,丁建峰. 增强CT及多参数MRI在局灶自身免疫性胰腺炎和胰腺癌诊断中的应用. 中国CT和MRI杂志. 2024(04): 100-102 .  百度学术
百度学术2. 董力宁,闫威,张洁,杨大为,刘朋,徐辉,杨正汉,王振常,靳二虎. IgG4相关硬化性胆管炎初诊患者CT和MRI表现与血清IgG4水平升高相关性研究. CT理论与应用研究. 2023(02): 231-239 .  百度学术
百度学术3. 彭程,贺舜民,涂广平,余栎,孙吉春,汪东文,李志强,余枭. 自身免疫性胰腺炎的诊治分析:附2例报告. 中国普通外科杂志. 2023(03): 416-423 .  百度学术
百度学术4. 杨显文,麻芝英. 以黄疸为首发表现不伴AIP的IgG4相关硬化性胆管炎1例. 中国医药导报. 2022(24): 175-178 .  百度学术
百度学术5. 李天云,董军强,解非,赵向乾,赵娓娓. 脾静脉改变在自身免疫性胰腺炎CT诊断中的价值. 临床医学研究与实践. 2021(31): 115-118 .  百度学术
百度学术其他类型引用(2)
-




 PDF下载 ( 1975 KB)
PDF下载 ( 1975 KB)

 下载:
下载:

 下载:
下载:  百度学术
百度学术