非酒精性脂肪性肝病与结直肠腺瘤、结直肠癌发生风险相关性的Meta分析
DOI: 10.3969/j.issn.1001-5256.2021.07.028
Association of nonalcoholic fatty liver disease with the risk of colorectal adenoma and colorectal cancer: A Meta-analysis
-
摘要:
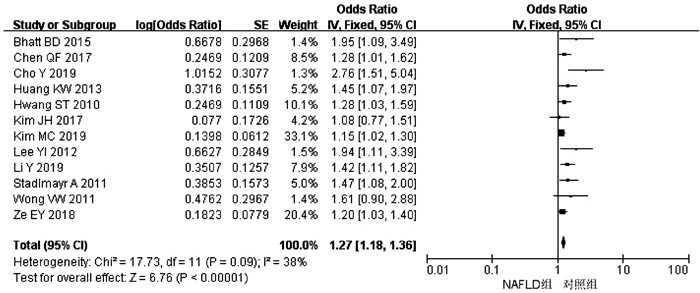
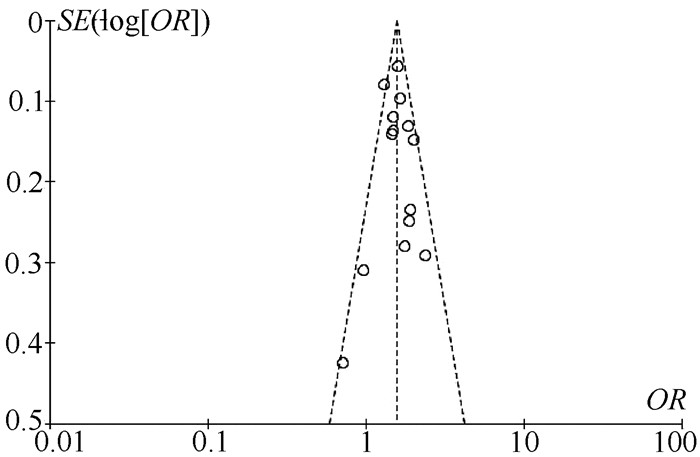
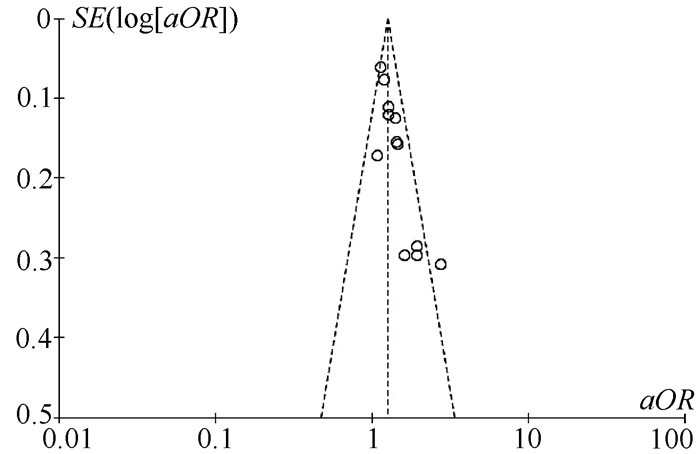
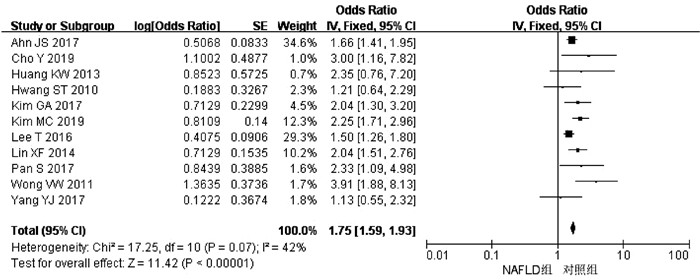
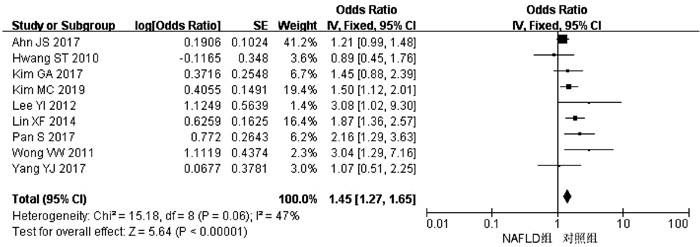
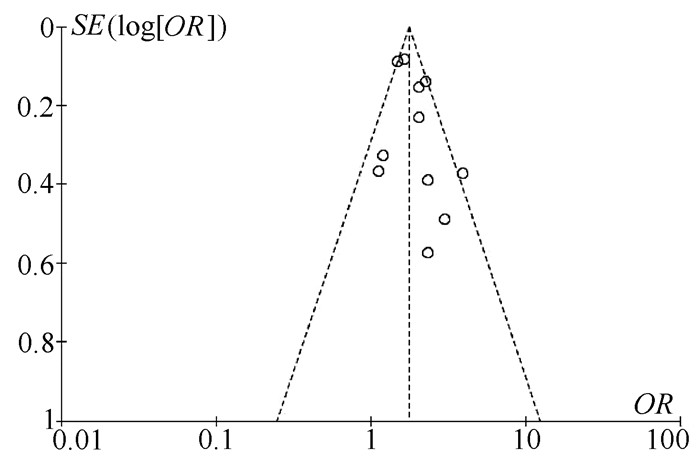
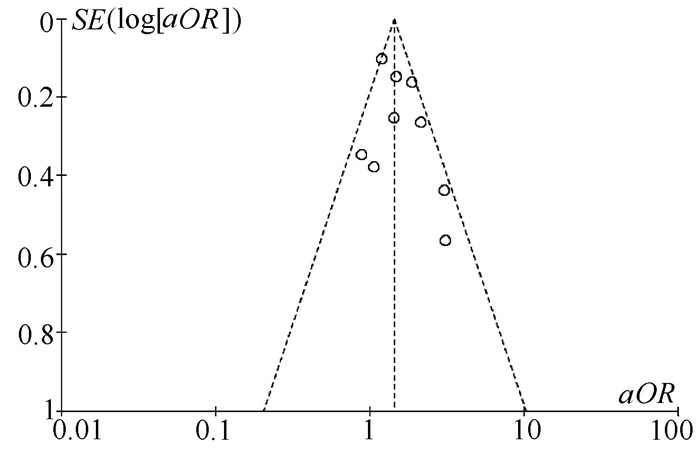
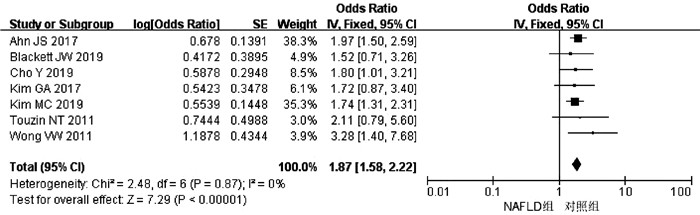
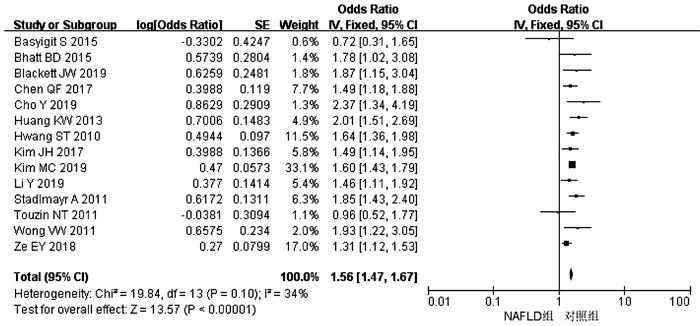
目的 系统评价非酒精性脂肪性肝病(NAFLD)的存在及严重程度与结直肠腺瘤(CRA)和结直肠癌(CRC)发生风险的关联性。 方法 在PubMed、Embase、Web of Science、The Cochrane Library、中国知网、万方、维普数据库检索2019年12月前公开发表的有关NAFLD与CRA和CRC发生风险关系的中英文文献。对检索结果根据纳入和排除标准进行二次筛选、质量评价及相关数据的提取,采用RevMan 5.3软件进行Meta分析。 结果 共纳入3项纵向队列研究和18项横断面研究,包括130 271例研究对象。Meta分析结果显示:NAFLD患者发生CRA(aOR=1.27,95%CI:1.18~1.36,P<0.000 01)以及进展期CRA/CRC(aOR=1.45,95%CI:1.27~1.65,P<0.000 01)的风险增加。NAFLD的严重程度会影响这种相关性:NASH和/或进展期肝纤维化的患者发生CRA/CRC的风险明显增加(aOR=1.93,95%CI:1.61~2.31,P<0.000 01)。 结论 NAFLD的存在与CRA和CRC发生风险的增加相关,而且NAFLD严重程度越高,CRA和CRC的发生风险越高。 -
关键词:
- 非酒精性脂肪性肝病 /
- 结直肠肿瘤 /
- Meta分析(主题)
Abstract:Objective To systematically evaluate the association of the presence and severity of nonalcoholic fatty liver disease (NAFLD) with the risk of colorectal adenoma (CRA) and colorectal cancer (CRC). Methods PubMed, Embase, Web of Science, The Cochrane Library, CNKI, Wanfang Data, and VIP databases were searched for Chinese and English articles on the association of NAFLD with CRA and CRC published up to December 2019. Secondary screening, quality assessment, and data extraction were performed according to inclusion and exclusion criteria, and RevMan 5.3 software was used to perform the meta-analysis. Results A total of 3 longitudinal cohort studies and 18 cross-sectional studies were included, with 130 271 subjects in total. The meta-analysis showed that NAFLD patients had an increased risk of CRA (adjusted odds ratio [aOR]=1.27, 95% confidence interval [CI]: 1.18-1.36, P < 0.000 01) and advanced CRA/CRC (aOR=1.45, 95%CI: 1.27-1.65, P < 0.000 01). The severity of NAFLD affected such association, and patients with nonalcoholic steatohepatitis and/or advanced liver fibrosis had a significant increase in the risk of CRA/CRC (aOR=1.93, 95%CI: 1.61-2.31, P < 0.000 01). Conclusion The presence of NAFLD is associated with an increased risk of CRA and CRC, and the higher the severity of NAFLD, the higher the risk of CRA and CRC. -
表 1 NAFLD与CRA发生风险之间的关系
作者和年份 国家 试验设计 NAFLD/ 总样本量 NAFLD和CRA诊断 OR(95%CI) aOR(95%CI) 调整混杂因素 质量评分 Cho Y 2019[7] 韩国 横断面研究 379/476 肝活检和结肠镜 2.37(1.34~4.20) 2.76(1.51~5.06) 年龄、性别、DM、HTN 9 Basyigit S 2015[8] 土耳其 横断面研究 65/127 超声和结肠镜 0.72(0.31~1.65) - - 8 Hwang ST 2010[9] 韩国 横断面研究 945/2917 超声和结肠镜 1.64(1.36~1.98) 1.28(1.03~1.60) 年龄、性别、吸烟史、MS、HTN、DM 9 Stadlmayr A 2011[10] 奥利地 横断面研究 632/1211 超声和结肠镜 1.85(1.43~2.40) 1.47(1.08~2.00) 年龄、性别、BMI、葡萄糖耐受不良 8 Touzin NT 2011[11] 美国 横断面研究 94/233 肝活检和结肠镜 0.96(0.52~1.77) - - 8 Wong VW 2011[12] 中国 横断面研究 199/380 肝活检、MRI和结肠镜 1.93(1.22~3.06) 1.61(0.90~2.90) 年龄、性别、吸烟史、CRC家族史、BMI、DM、HTN 9 Lee YI 2012[13] 韩国 纵向队列研究 831/5517 超声和结肠镜 3.46(2.09~5.72) 1.94(1.11~3.40) 年龄、BMI、BP、FG、TC、TG、HDL 8 Huang KW 2013[14] 中国 横断面研究 945/1522 超声和结肠镜 2.01(1.51~2.69) 1.45(1.07~1.98) 年龄、性别、BMI、葡萄糖耐受不良 9 Lin XF 2014[15] 中国 横断面研究 263/2315 超声和结肠镜 0.64(0.49~0.83) - - 9 Bhatt BD 2015[16] 美国 横断面研究 68/591 肝活检和结肠镜 1.78(1.02~3.08) 1.95(1.09~3.48) 年龄、饮酒史、CTP分级 8 Chen QF 2017[17] 中国 横断面研究 779/3686 超声和结肠镜 1.49(1.18~1.87) 1.28(1.01~1.64) 年龄、性别、吸烟史、饮酒史、MS 9 Kim JH 2017[18] 韩国 横断面研究 309/1007 超声和结肠镜 1.49(1.14~1.94) 1.08(0.77~1.51) 年龄、性别、吸烟史、饮酒史、BMI、运动 9 Ze EY 2018[19] 韩国 横断面研究 1512/2976 超声和结肠镜 1.31(1.12~1.53) 1.20(1.03~1.41) 年龄、性别、BMI、MS、脂肪肝指数 9 Blackett JW 2019[20] 美国 横断面研究 123/369 肝活检和结肠镜 1.87(1.15~3.03) - - 9 Kim MC 2019[21] 韩国 横断面研究 2395/6332 超声和结肠镜 1.60(1.43~1.79) 1.15(1.02~1.30) 年龄、性别、肥胖、吸烟史、HTN、DM、HLP、MS 9 Li Y 2019[22] 中国 横断面研究 502/1089 超声和结肠镜 1.46(1.11~1.92) 1.42(1.11~2.04) 年龄、性别、BMI、TG、TC、HDL、LDL、AST、ALT、FG、UA、CAP 9 注:MS,代谢综合征;HTN,高血压;BP,血压;DM,糖尿病;HLP:高脂血症;FG,空腹血糖;UA,尿酸;CAP,受控衰减参数。 表 2 NAFLD与进展期CRA/CRC发生风险之间的关系
作者和年份 国家 试验设计 NAFLD/ 总样本量 NAFLD和CRA/CRC诊断 OR(95%CI) aOR(95%CI) 调整混杂因素 质量评分 Cho Y 2019[7] 韩国 横断面研究 379/476 肝活检和结肠镜 3.00(1.16~7.82) - - 9 Basyigit S 2015[8] 土耳其 横断面研究 65/127 超声和结肠镜 0.15(0.04~0.55) - - 8 Hwang ST 2010[9] 韩国 横断面研究 945/2917 超声和结肠镜 1.21(0.64~2.29) 0.89(0.45~1.75) 年龄、性别、吸烟史 9 Wong VW 2011[12] 中国 横断面研究 199/380 肝活检、MRI和结肠镜 3.91(1.88~8.11) 3.04(1.29~7.20) 年龄、性别、吸烟史、CRC家族史、BMI、DM、HTN 9 Lee YI 2012[13] 韩国 纵向队列研究 831/5517 超声和结肠镜 8.71(3.10~24.48) 3.08(1.02~9.34) 年龄、BMI、BP、FG、TC、TG、HDL 8 Huang KW 2013[14] 中国 横断面研究 620/1522 超声和结肠镜 2.35(0.76~7.20) - - 7 Lin XF 2014[15] 中国 横断面研究 263/2315 超声和结肠镜 2.04(1.51~2.76) 1.87(1.36~2.57) TG、UA、PLT 9 Kim MC 2019[21] 韩国 横断面研究 2395/6332 超声和结肠镜 2.25(1.71~2.95) 1.50(1.12~2.01) 年龄、性别、肥胖、吸烟史、HTN、DM、HLP、MS 9 Ahn JS 2016[23] 韩国 横断面研究 831/26 540 超声和结肠镜 1.66(1.41~1.96) 1.21(0.99~1.47) 年龄、性别、吸烟史、饮酒史、BMI、CRC家族史、使用阿司匹林、FG、使用降糖药、TC、TG、使用降脂药、SBP、使用降压药 9 Lee T 2016[24] 韩国 横断面研究 14 655/44 220 超声和结肠镜 1.50(1.26~1.80) - - 9 Yang YJ 2017[25] 韩国 纵向队列研究 441/1023 超声和结肠镜 1.13(0.55~2.32) 1.07(0.51~2.26) 年龄、BMI、HTN、DM、使用阿司匹林或NSAIDs、使用降脂药、低风险和高指数结肠镜检查结果 9 Pan S 2017[26] 中国 横断面研究 573/1793 超声和结肠镜 2.33(1.09~4.98) 2.16(1.29~3.22) 年龄、MS 9 Kim GA 2017[27] 韩国 纵向队列研究 8721/25 947 超声和结肠镜 2.04(1.30~3.19) 1.45(0.88~2.38) 年龄、性别、吸烟史、DM、HTN、GGT、HDL、LDL、TG 9 注:SBP,收缩压。 表 3 NAFLD的严重程度与CRA/CRC发生风险之间的关系
作者和年份 国家 试验设计 NAFLD/ 总样本量 NAFLD和CRA/CRC诊断 OR(95%CI) aOR(95%CI) 调整混杂因素 质量评分 Cho Y 2019[7] 韩国 横断面研究 379/476 肝活检和结肠镜 NASH:1.80 (1.01~3.22) NASH:2.08 (1.12~3.86) 年龄、性别、DM、HTN 9 Touzin NT 2011[11] 美国 横断面研究 94/233 肝活检和结肠镜 NASH:2.11 (0.79~5.60) - - 8 Wong VW 2011[12] 中国 横断面研究 199/380 肝活检、MRI和结肠镜 NASH:3.28 (1.40~7.65) NASH:5.34 (1.92~14.84) 年龄、性别、吸烟史、CRC家族史、BMI、DM、HTN 9 Blackett JW 2019[20] 美国 横断面研究 123/369 肝活检和结肠镜 NASH:1.52 (0.71~3.26) NASH:2.47 (0.67~9.10) 年龄、性别、HLP、肥胖、DM、结肠镜检查指征 9 Kim MC 2019[21] 韩国 横断面研究 2395/6332 超声和结肠镜 FIB-4:1.74 (1.31~2.34) FIB-4:1.78 (1.32~2.40) 性别、肥胖、吸烟史、HTN、DM、HLP、MS 9 Ahn JS 2016[23] 韩国 横断面研究 831/26 540 超声和结肠镜 FIB-4:1.97 (1.50~2.58) FIB-4:1.91 (1.45~2.52) 年龄、性别、吸烟史、饮酒史、BMI、CRC家族史、使用阿司匹林、FG、使用降糖药、TC、TG、使用降脂药、SBP、使用降压药 9 Kim GA 2017[27] 韩国 纵向队列研究 8721/25947 超声和结肠镜 FIB-4:1.72 (0.87~3.39) FIB-4:1.64 (0.81~3.30) 性别、吸烟史、DM、HTN、GGT、HDL、LDL、TG 9 -
[1] BRAY F, FERLAY J, SOERJOMATARAM I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2018, 68(6): 394-424. DOI: 10.3322/caac.21492. [2] LESLIE A, CAREY FA, PRATT NR, et al. The colorectal adenoma-carcinoma sequence[J]. Br J Surg, 2002, 89(7): 845-860. DOI: 10.1046/j.1365-2168.2002.02120.x. [3] AHMED RL, SCHMITZ KH, ANDERSON KE, et al. The metabolic syndrome and risk of incident colorectal cancer[J]. Cancer, 2006, 107(1): 28-36. DOI: 10.1002/cncr.21950. [4] TAVANI A, BRAVI F, BOSETTI C, et al. Diabetes mellitus and subsite-specific colorectal cancer risks in the Iowa Women's Health Study[J]. Cancer Epidemiol Biomarkers Prev, 2005, 14(9): 2277. DOI: 10.1158/1055-9965.EPI-05-0331. [5] BARDOU M, BARKUN AN, MARTEL M. Obesity and colorectal cancer[J]. Gut, 2013, 62(6): 933-947. DOI: 10.1136/gutjnl-2013-304701. [6] LONARDO A, NASCIMBENI F, MANTOVANI A, et al. Hypertension, diabetes, atherosclerosis and NASH: Cause or consequence?[J]. J Hepatol, 2018, 68(2): 335-352. DOI: 10.1016/j.jhep.2017.09.021. [7] CHO Y, LIM SK, JOO SK, et al. Nonalcoholic steatohepatitis is associated with a higher risk of advanced colorectal neoplasm[J]. Liver Int, 2019, 39(9): 1722-1731. DOI: 10.1111/liv.14163. [8] BASYIGIT S, UZMAN M, KEFELI A, et al. Absence of non-alcoholic fatty liver disease in the presence of insulin resistance is a strong predictor for colorectal carcinoma[J]. Int J Clin Exp Med, 2015, 8(10): 18601-18610. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4694373/ [9] HWANG ST, CHO YK, PARK JH, et al. Relationship of non-alcoholic fatty liver disease to colorectal adenomatous polyps[J]. J Gastroenterol Hepatol, 2010, 25(3): 562-567. DOI: 10.1111/j.1440-1746.2009.06117.x. [10] STADLMAYR A, AIGNER E, STEGER B, et al. Nonalcoholic fatty liver disease: An independent risk factor for colorectal neoplasia[J]. J Intern Med, 2011, 270(1): 41-49. DOI: 10.1111/j.1365-2796.2011.02377.x. [11] TOUZIN NT, BUSH KN, WILLIAMS CD, et al. Prevalence of colonic adenomas in patients with nonalcoholic fatty liver disease[J]. Therap Adv Gastroenterol, 2011, 4(3): 169-176. DOI: 10.1177/1756283X11402118. [12] WONG VW, WONG GL, TSANG SW, et al. High prevalence of colorectal neoplasm in patients with non-alcoholic steatohepatitis[J]. Gut, 2011, 60(6): 829-836. DOI: 10.1136/gut.2011.237974. [13] LEE YI, LIM YS, PARK HS. Colorectal neoplasms in relation to non-alcoholic fatty liver disease in Korean women: A retrospective cohort study[J]. J Gastroenterol Hepatol, 2012, 27(1): 91-95. DOI: 10.1111/j.1440-1746.2011.06816.x. [14] HUANG KW, LEU HB, WANG YJ, et al. Patients with nonalcoholic fatty liver disease have higher risk of colorectal adenoma after negative baseline colonoscopy[J]. Colorectal Dis, 2013, 15(7): 830-835. DOI: 10.1111/codi.12172. [15] LIN XF, SHI KQ, YOU J, et al. Increased risk of colorectal malignant neoplasm in patients with nonalcoholic fatty liver disease: A large study[J]. Mol Biol Rep, 2014, 41(5): 2989-2997. DOI: 10.1007/s11033-014-3157-y. [16] BHATT BD, LUKOSE T, SIEGEL AB, et al. Increased risk of colorectal polyps in patients with non-alcoholic fatty liver disease undergoing liver transplant evaluation[J]. J Gastrointest Oncol, 2015, 6(5): 459-468. DOI: 10.3978/j.issn.2078-6891.2015.050. [17] CHEN QF, ZHOU XD, SUN YJ, et al. Sex-influenced association of non-alcoholic fatty liver disease with colorectal adenomatous and hyperplastic polyps[J]. World J Gastroenterol, 2017, 23(28): 5206-5215. DOI: 10.3748/wjg.v23.i28.5206. [18] KIM JH, CHO KI, KIM YA, et al. Elevated neutrophil-to-lymphocyte ratio in metabolic syndrome is associated with increased risk of colorectal adenoma[J]. Metab Syndr Relat Disord, 2017, 15(8): 393-399. DOI: 10.1089/met.2017.0041. [19] ZE EY, KIM BJ, JUN DH, et al. The fatty liver index: A simple and accurate predictor of colorectal adenoma in an average-risk population[J]. Dis Colon Rectum, 2018, 61(1): 36-42. DOI: 10.1097/DCR.0000000000000973. [20] BLACKETT JW, VERNA EC, LEBWOHL B. Increased prevalence of colorectal adenomas in patients with nonalcoholic fatty liver disease: A cross-sectional study[J]. Dig Dis, 2020, 38(3): 222-230. DOI: 10.1159/000502684. [21] KIM MC, PARK JG, JANG BI, et al. Liver fibrosis is associated with risk for colorectal adenoma in patients with nonalcoholic fatty liver disease[J]. Medicine (Baltimore), 2019, 98(6): e14139. DOI: 10.1097/MD.0000000000014139. [22] LI Y, LIU S, GAO Y, et al. Association between NAFLD and risk of colorectal adenoma in Chinese han population[J]. J Clin Transl Hepatol, 2019, 7(2): 99-105. DOI: 10.14218/JCTH.2019.00010. [23] AHN JS, SINN DH, MIN YW, et al. Non-alcoholic fatty liver diseases and risk of colorectal neoplasia[J]. Aliment Pharmacol Ther, 2017, 45(2): 345-353. DOI: 10.1111/apt.13866. [24] LEE T, YUN KE, CHANG Y, et al. Risk of colorectal neoplasia according to fatty liver severity and presence of gall bladder polyps[J]. Dig Dis Sci, 2016, 61(1): 317-324. DOI: 10.1007/s10620-015-3873-8. [25] YANG YJ, BANG CS, SHIN SP, et al. Clinical impact of non-alcoholic fatty liver disease on the occurrence of colorectal neoplasm: Propensity score matching analysis[J]. PLoS One, 2017, 12(8): e0182014. DOI: 10.1371/journal.pone.0182014. [26] PAN S, HONG W, WU W, et al. The relationship of nonalcoholic fatty liver disease and metabolic syndrome for colonoscopy colorectal neoplasm[J]. Medicine (Baltimore), 2017, 96(2): e5809. DOI: 10.1097/MD.0000000000005809. [27] KIM GA, LEE HC, CHOE J, et al. Association between non-alcoholic fatty liver disease and cancer incidence rate[J]. J Hepatol, 2017. DOI: 10.1016/j.jhep.2017.09.012. [28] KANG HW, KIM D, KIM HJ, et al. Visceral obesity and insulin resistance as risk factors for colorectal adenoma: A cross-sectional, case-control study[J]. Am J Gastroenterol, 2010, 105(1): 178-187. DOI: 10.1038/ajg.2009.541. [29] CUSI K. Role of insulin resistance and lipotoxicity in non-alcoholic steatohepatitis[J]. Clin Liver Dis, 2009, 13(4): 545-563. DOI: 10.1016/j.cld.2009.07.009. [30] WEI EK, GIOVANNUCCI E, FUCHS CS, et al. Low plasma adiponectin levels and risk of colorectal cancer in men: a prospective study[J]. J Natl Cancer Inst, 2005, 97(22): 1688-1694. DOI: 10.1093/jnci/dji376. [31] BUZZETTI E, PINZANI M, TSOCHATZIS EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD)[J]. Metabolism, 2016, 65(8): 1038-1048. DOI: 10.1016/j.metabol.2015.12.012. [32] HYOGO H, YAMAGISHI S, IWAMOTO K, et al. Elevated levels of serum advanced glycation end products in patients with non-alcoholic steatohepatitis[J]. J Gastroenterol Hepatol, 2007, 22(7): 1112-1119. DOI: 10.1111/j.1440-1746.2007.04943.x. [33] ABIRU S, MIGITA K, MAEDA Y, et al. Serum cytokine and soluble cytokine receptor levels in patients with non-alcoholic steatohepatitis[J]. Liver Int, 2006, 26(1): 39-45. DOI: 10.1111/j.1478-3231.2005.01191.x. -



 PDF下载 ( 3069 KB)
PDF下载 ( 3069 KB)

 下载:
下载: