高密度脂蛋白胆固醇对HBV相关慢加急性肝衰竭严重程度及预后的预测价值
DOI: 10.3969/j.issn.1001-5256.2021.07.030
Value of high-density lipoprotein cholesterol in evaluating the severity and prognosis of hepatitis B virus-associated acute-on-chronic liver failure
-
摘要:
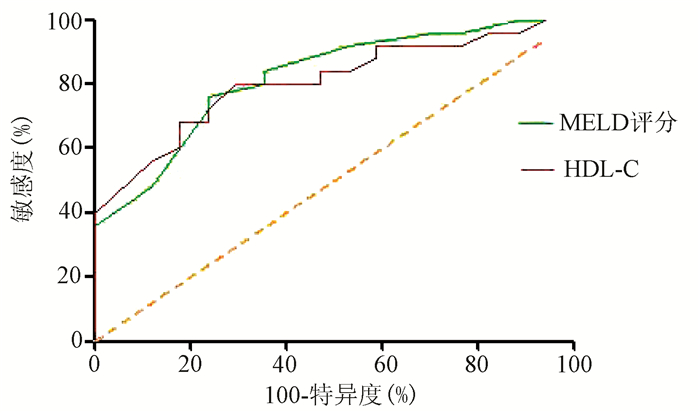
目的 探讨高密度脂蛋白胆固醇(HDL-C)与HBV相关慢加急性肝衰竭(HBV-ACLF)预后的关系及其预测价值。 方法 选取苏州大学附属第一医院感染科2015年1月—2019年1月收治的肝病患者,根据病情发展的不同阶段,分为HBV-ACLF组(n=42),肝硬化组(n=30)和慢性肝炎组(n=25),同时选取同期健康者(n=24)作为对照。收集患者一般临床资料,包括性别、年龄、PT、Alb、TBil、SCr、尿素氮(BUN)、TC、TG、HDL-C、LDL-C、MELD评分。其中HBV-ACLF组进一步分为好转组(n=17)与未好转组(n=25),随访时间3个月。不符合正态分布的连续性变量两组间比较采用Mann-Whitney U检验,多组间比较采用Kruskal-Wallis H检验,进一步组内比较采用Wilcoxon秩和检验;影响患者预后的独立危险因素用二元logistic回归分析,受试者工作特征曲线(ROC曲线)用于分析预测变量的准确性。 结果 肝硬化组、HBV-ACLF组、慢性肝炎组、健康组在PT、Alb、BUN、TBil、TC、TG、HDL-C、LDL-C、MELD评分上差异均有统计学意义(χ2值分别为75.134、44.638、10.253、80.357、55.067、19.858、68.174、52.492、64.359,P值均<0.05)。各组间进一步两两比较发现,HDL-C水平在HBV-ACLF组[0.12(0.08~0.30)mmol/L]明显低于肝硬化组[0.79(0.60~1.01)mmol/L]、慢性肝炎组[1.06(0.88~1.44) mmol/L]、健康组[2.03(1.36~2.98)mmol/L](Z值分别为3.821、5.921、7.228,P值均<0.001)。HBV-ACLF好转组HDL-C水平明显高于未好转组[0.20(0.11~0.49)mmol/L vs 0.10(0.07~0.15)mmol/L,Z =-1.628,P=0.014]。进一步二元logistic回归,发现HDL-C(OR=0.003,95%CI:0~0.548,P=0.029)与MELD评分(OR=1.588,95%CI:1.032~2.443,P=0.035)是影响HBV-ACLF预后的独立危险因素。HDL-C对HBV-ACLF预后预测的ROC曲线下面积(AUC)为0.807,截断值为0.175 mmol/L,敏感度0.706,特异度0.800,95%CI:0.677~0.937,P=0.001;MELD对HBV-ACLF预后预测的AUC为0.822,截断值为26.500,敏感度0.760,特异度0.765,95%CI:0.696~0.928,P<0.001。 结论 HDL-C是HBV-ACLF患者预后的独立危险因素,在HBV-ACLF预后中具有良好的预测价值。 Abstract:Objective To investigate the association of high-density lipoprotein cholesterol (HDL-C) with the prognosis of hepatitis B virus-associated acute-on-chronic liver failure (HBV-ACLF) and its predictive value. Methods The patients with liver disease who were admitted to Department of Infectious Diseases, The First Affiliated Hospital of Soochow University, from January 2015 to January 2019 were enrolled, and according to the stage of disease progression, they were divided into HBV-ACLF group with 42 patients, liver cirrhosis group with 30 patients, and chronic hepatitis group with 25 patients. A total of 24 healthy individuals were enrolled as healthy control group. General clinical data were collected, including sex, age, prothrombin time (PT), albumin (Alb), total bilirubin (TBil), serum creatinine, blood urea nitrogen (BUN), total cholesterol (TC), triglyceride (TG), HDL-C, low-density lipoprotein cholesterol (LDL-C), and Model for End-Stage Liver Disease (MELD) score. The HBV-ACLF group was further divided into improvement group with 17 patients and non-improvement group with 25 patients, and the patients were followed up for 3 months. The Mann-Whitney U test was used for comparison of non-normally distributed continuous variables between two groups; the Kruskal-Wallis H test was used for comparison between multiple groups, and the Wilcoxon rank-sum test was used for comparison within each group. A binary logistic regression analysis was used to investigate independent risk factors for prognosis, and the receiver operating characteristic (ROC) curve was used to evaluate the accuracy of the variables in prediction. Results There were significant differences in PT, Alb, BUN, TBil, TC, TG, HDL-C, LDL-C, and MELD scores between the liver cirrhosis group, the HBV-ACLF group, the chronic hepatitis group, and the healthy control group (χ2=75.134, 44.638, 10.253, 80.357, 55.067, 19.858, 68.174, 52.492, and 64.359, all P < 0.05). Further comparison between two groups showed that the HBV-ACLF group had a significantly lower level of HDL-C than the liver cirrhosis group [0.12 (0.08-0.30) mmol/L vs 0.79 (0.60-1.01) mmol/L, Z=3.821, P < 0.001], the chronic hepatitis group [0.12 (0.08-0.30) mmol/L vs 1.06(0.88-1.44) mmol/L, Z=5.921, P < 0.001], and the healthy control group [0.12 (0.08-0.30) mmol/L vs 2.03 (1.36-2.98) mmol/L, Z=7.228, P < 0.001]. The improvement group had a significantly higher level of HDL-C than the non-improvement group [0.20 (0.11-0.49) mmol/L vs 0.10 (0.07-0.15) mmol/L, Z=-1.628, P=0.014]. The binary logistic regression analysis showed that HDL-C (odds ratio [OR]=0.003, 95% confidence interval [CI]: 0-0.548, P=0.029) and MELD score (OR=1.588, 95%CI: 1.032-2.443, P=0.035) were independent influencing factors for the prognosis of HBV-ACLF. HDL-C had an area under the ROC curve (AUC) of 0.807 in predicting the prognosis of HBV-ACLF, with a sensitivity of 0.706, a specificity 0.800, and a 95% CI of 0.677-0.937 at the optimal cut-off value of 0.175 mmol/L; MELD score had an AUC of 0.822, with a sensitivity of 0.760, a specificity of 0.765, and a 95% CI of 0.696-0.928 at the optimal cut-off value of 26.500. Conclusion HDL-C is an independent risk factor for the prognosis of patients with HBV-ACLF and has a good value in predicting the prognosis of HBV-ACLF. -
Key words:
- Hepatitis B Virus /
- Acute-on-chronic Liver Falure /
- Cholesterol, HDL /
- Prognosis
-
表 1 一般临床资料
指标 肝硬化组(n=30) HBV-ACLF组(n=42) 慢性肝炎组(n=25) 健康组(n=24) χ2值 P值 男/女(例) 21/9 24/18 16/9 12/12 5.230 0.162 年龄(岁) 43(39~56) 47(37~58) 37(31~43) 45(20~53) 4.930 0.178 PT(s) 16.0(14.2~18.3) 25.2(20.0~32.7) 12.0(11.2~13.2) 10.8(10.2~12.0) 75.134 <0.001 Alb(g/L) 29.6(25.8~32.5) 30.9(27.9~33.2) 46.8(42.9~51.1) 39.0(36.0~43.0) 44.638 <0.001 Cr(μmol/L) 66.3(52.1~89.7) 60.5(50.2~72.2) 65.4(55.3~73.0) 68.0(59.0~76.0) 2.717 0.437 BUN(mmol/L) 6.2(4.3~9.7) 3.9(3.0~5.4) 4.6(3.9~5.3) 4.9(4.1~6.4) 10.253 0.017 TBil(μmol/L) 43.0(20.4~54.4) 305.0(230.3~448.1) 14.2(10.6~18.1) 7.8(5.6~12.3) 80.357 <0.001 TC(mmol/L) 3.3(2.7~4.3) 2.6(2.0~3.0) 4.3(3.8~4.8) 4.5(3.9~5.0) 55.067 <0.001 TG(mmol/L) 0.80(0.64~0.96) 1.13(0.84~1.49) 1.41(0.95~1.69) 1.49(1.31~1.62) 19.858 <0.001 HDL-C(mmol/L) 0.79(0.60~1.01) 0.12(0.08~0.30) 1.06(0.88~1.44) 2.03(1.36~2.98) 68.174 <0.001 LDL-C(mmol/L) 1.72(1.28~2.00) 1.21(0.75~1.43) 2.46(1.92~2.84) 2.49(1.94~2.89) 52.492 <0.001 MELD评分 13(12~16) 28(23~30) 6(6~10) - 64.359 <0.001 表 2 HBV-ACLF组不同预后患者临床资料比较
指标 好转组(n=17) 未好转组(n=25) 统计值 P值 男/女(例) 12/5 18/7 χ2=1.874 0.185 年龄(岁) 41(34~47) 52(40~61) Z=-2.424 0.015 PT(s) 21.2(17.4~25.6) 29.8(21.7~33.3) Z=-2.666 0.008 Alb(g/L) 30.0(27.7~33.8) 31.6(27.8~33.2) Z=-0.282 0.778 Cr(μmol/L) 57.1(49.2~70.1) 62.6(51.3~72.4) Z=-0.795 0.427 BUN(mmol/L) 4.0(3.0~5.6) 3.8(3.1~5.5) Z=-0.167 0.868 TBil(μmol/L) 280.0(182.7~480.1) 347.5(240.4~440.8) Z=-0.653 0.513 TC(mmol/L) 2.7(2.2~3.4) 2.5(2.0~2.9) Z=-1.281 0.200 TG(mmol/L) 1.21(0.82~1.68) 1.08(0.83~1.21) Z=-1.487 0.137 HDL-C(mmol/L) 0.20(0.11~0.49) 0.10(0.07~0.15) Z=-2.452 0.014 LDL-C(mmol/L) 1.22(0.93~1.66) 1.00(0.69~1.34) Z=-1.628 0.104 MELD评分 24(22~28) 28(25~30) Z=-2.720 0.007 表 3 HBV-ACLF预后影响因素分析
指标 OR 95%CI P值 年龄 1.071 0.997~1.150 0.059 PT 0.986 0.818~1.189 0.883 HDL-C 0.003 0~0.548 0.029 MELD评分 1.588 1.032~2.443 0.035 -
[1] ANNEMA W, TIETGE UJ. Regulation of reverse cholesterol transport - a comprehensive appraisal of available animal studies[J]. Nutr Metab (Lond), 2012, 9(1): 25. DOI: 10.1186/1743-7075-9-25. [2] ETOGO-ASSE FE, VINCENT RP, HUGHES SA, et al. High density lipoprotein in patients with liver failure; relation to sepsis, adrenal function and outcome of illness[J]. Liver Int, 2012, 32(1): 128-136. DOI: 10.1111/j.1478-3231.2011.02657.x. [3] TSAI MH, PENG YS, CHEN YC, et al. Low serum concentration of apolipoprotein A-I is an indicator of poor prognosis in cirrhotic patients with severe sepsis[J]. J Hepatol, 2009, 50(5): 906-915. DOI: 10.1016/j.jhep.2008.12.024. [4] Liver Failure and Artificial Liver Group, Chinese Society of Infectious Diseases, Chinese Medical Association; Severe Liver Disease and Artificial Liver Group, Chinese Society of Hepatology, Chinese Medical Association. Guideline for diagnosis and treatment of liver failure(2018)[J]. J Clin Hepatol, 2019, 35(1): 38-44. DOI: 10.3969/j.issn.1001-5256.2019.01.007.中华医学会感染病学分会肝衰竭与人工肝学组, 中华医学会肝病学分会重型肝病与人工肝学组. 肝衰竭诊治指南(2018年版)[J]. 临床肝胆病杂志, 2019, 35(1): 38-44. DOI: 10.3969/j.issn.1001-5256.2019.01.007. [5] Chinese Society of Hepatology, Chinese Medical Association. Chinese guidelines on the management of liver cirrhosis[J]. J Clin Hepatol, 2019, 35(11): 2408-2425. DOI: 10.3969/j.issn.1001-5256.2019.11.006.中华医学会肝病学分会. 肝硬化诊治指南[J]. 临床肝胆病杂志, 2019, 35(11): 2408-2425. DOI: 10.3969/j.issn.1001-5256.2019.11.006. [6] Chinese Society of Infectious Diseases, Chinese Medical Association; Chinese Society of Hepatology, Chinese Medical Association. Guidelines for the prevention and treatment of chronic hepatitis B (version 2019)[J]. J Clin Hepatol, 2019, 35(12): 2648-2669. DOI: 10.3969/j.issn.1001-5256.2019.12.007.中华医学会肝病学分会, 中华医学会感染病学分会. 慢性乙型肝炎防治指南(2019年版)[J]. 临床肝胆病杂志, 2019, 35(12): 2648-2669. DOI: 10.3969/j.issn.1001-5256.2019.12.007. [7] van LEEUWEN HJ, HEEZIUS EC, DALLINGA GM, et al. Lipoprotein metabolism in patients with severe sepsis[J]. Crit Care Med, 2003, 31(5): 1359-1366. DOI: 10.1097/01.CCM.0000059724.08290.51. [8] DELGADO-COELLO B, BRIONES-ORTA MA, MACÍAS-SILVA M, et al. Cholesterol: Recapitulation of its active role during liver regeneration[J]. Liver Int, 2011, 31(9): 1271-1284. DOI: 10.1111/j.1478-3231.2011.02542.x. [9] MANKA P, OLLIGES V, BECHMANN LP, et al. Low levels of blood lipids are associated with etiology and lethal outcome in acute liver failure[J]. PLoS One, 2014, 9(7): e102351. DOI: 10.1371/journal.pone.0102351. [10] CHRISTOU L, PAPPAS G, FALAGAS ME. Bacterial infection-related morbidity and mortality in cirrhosis[J]. Am J Gastroenterol, 2007, 102(7): 1510-1517. DOI: 10.1111/j.1572-0241.2007.01286.x. [11] HUANG XP, WANG Y, CHEN L, et al. Elevated serum prostaglandin E2 predicts the risk of infection in hepatitis B virus-related acute-on-chronic liver failure patients[J]. Asian Pac J Trop Med, 2017, 10(9): 916-920. DOI: 10.1016/j.apjtm.2017.08.008. [12] KIM HY, CHANG Y, PARK JY, et al. Characterization of acute-on-chronic liver failure and prediction of mortality in Asian patients with active alcoholism[J]. J Gastroenterol Hepatol, 2016, 31(2): 427-433. DOI: 10.1111/jgh.13084. [13] OKADA N, SANADA Y, URAHASHI T, et al. Endotoxin metabolism reflects hepatic functional reserve in end-stage liver disease[J]. Transplant Proc, 2018, 50(5): 1360-1364. DOI: 10.1016/j.transproceed.2018.01.052. [14] WIEST R, LAWSON M, GEUKING M. Pathological bacterial translocation in liver cirrhosis[J]. J Hepatol, 2014, 60(1): 197-209. DOI: 10.1016/j.jhep.2013.07.044. [15] SANADA Y, MIZUTA K, URAHASHI T, et al. Impact of hepatic clearance of endotoxin using endotoxin activity assay[J]. Hepatol Int, 2012, 6(4): 778-782. DOI: 10.1007/s12072-011-9289-4. [16] CLÀRIA J, STAUBER RE, COENRAAD MJ, et al. Systemic inflammation in decompensated cirrhosis: Characterization and role in acute-on-chronic liver failure[J]. Hepatology, 2016, 64(4): 1249-1264. DOI: 10.1002/hep.28740. [17] TRIEB M, RAINER F, STADLBAUER V, et al. HDL-related biomarkers are robust predictors of survival in patients with chronic liver failure[J]. J Hepatol, 2020, 73(1): 113-120. DOI: 10.1016/j.jhep.2020.01.026. [18] LEVINE DM, PARKER TS, DONNELLY TM, et al. In vivo protection against endotoxin by plasma high density lipoprotein[J]. Proc Natl Acad Sci U S A, 1993, 90(24): 12040-12044. DOI: 10.1073/pnas.90.24.12040. [19] FESSLER MB, PARKS JS. Intracellular lipid flux and membrane microdomains as organizing principles in inflammatory cell signaling[J]. J Immunol, 2011, 187(4): 1529-1535. DOI: 10.4049/jimmunol.1100253. -



 PDF下载 ( 2128 KB)
PDF下载 ( 2128 KB)

 下载:
下载:


