雌激素缺乏对绝经后女性非酒精性脂肪性肝病的影响
DOI: 10.3969/j.issn.1001-5256.2021.07.049
利益冲突声明:所有作者均声明不存在利益冲突。
作者贡献声明:赵晨露负责撰写论文; 赵文霞负责指导撰写文章, 修改论文。
Influence of estrogen deficiency on metabolic associated fatty liver disease in postmenopausal women
-
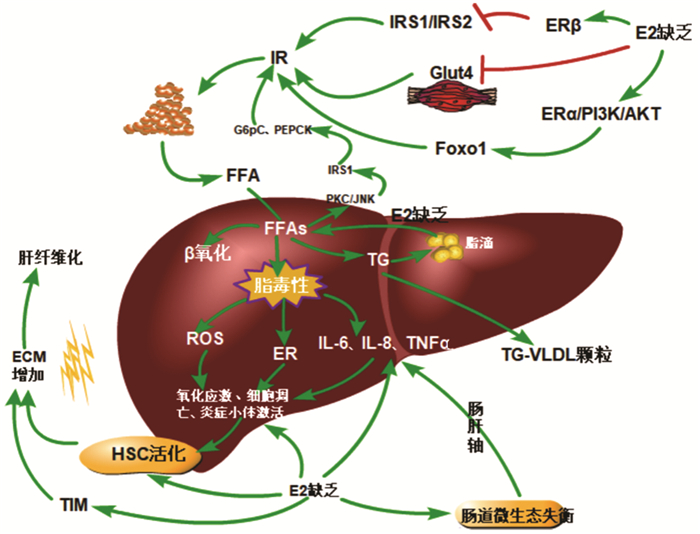
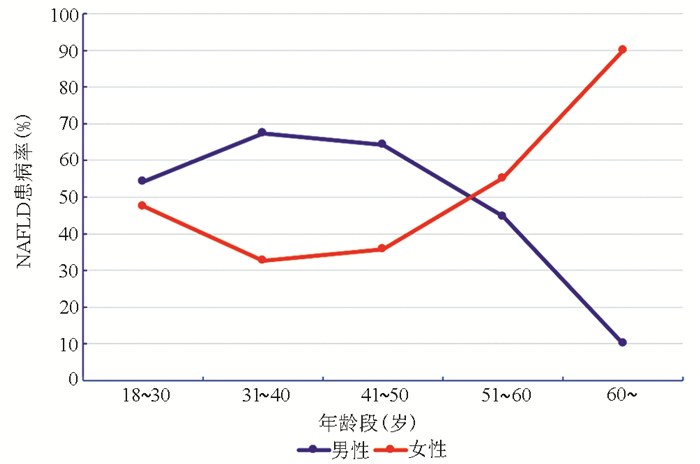
摘要: 流行病学数据显示, 绝经后女性非酒精性脂肪性肝病(NAFLD)的发病率显著高于绝经前女性和同龄期男性。针对这一临床现象, 重点从胰岛素抵抗、肝脂质代谢、肝纤维化和肠道菌群四个方面总结了雌激素缺乏对绝经后女性NAFLD潜在的影响机制, 为绝经后女性NAFLD的早期临床防治提供参考。Abstract: Epidemiological data have shown that postmenopausal women have a significantly higher incidence rate of metabolic associated fatty liver disease (MAFLD) than premenopausal women and men of the same age. Aiming at this phenomenon, this article summarizes the potential influence mechanism of estrogen deficiency on MAFLD in postmenopausal women from the four aspects of insulin resistance, liver lipid metabolism, liver fibrosis, and intestinal flora, so as to provide a reference for the early clinical prevention and treatment of MAFLD in postmenopausal women.
-
Key words:
- Estrogen /
- Non-alcoholic Liver Disease /
- Postmenopause
-
[1] ESLAM M, SANYAL AJ, GEORGE J, et al. MAFLD: A consensus-driven proposed nomenclature for metabolic associated fatty liver disease[J]. Gastroenterology, 2020, 158(7): 1999-2014.e1. DOI: 10.1053/j.gastro.2019.11.312. [2] XUE R, FAN JG. Brief introduction of an international expert consensus statement: A new definition of metabolic associated fatty liver disease[J]. J Clin Hepatol, 2020, 36(6): 34-37. DOI: 10.3969/j.issn.1001-5256.2020.06.007.薛芮, 范建高. 代谢相关脂肪性肝病新定义的国际专家共识简介[J]. 临床肝胆病杂志, 2020, 36(6): 34-37. DOI: 10.3969/j.issn.1001-5256.2020.06.007. [3] National Workshop on Fatty Liver and Alcoholic Liver Disease, Chinese Society of Hepatology, Chinese Medical Association; Fatty Liver Expert Committee, Chinese Medical Doctor Association. Guidelines of prevention and treatment for nonalcoholic fatty liver disease: A 2018 update[J]. J Clin Hepatol, 2018, 34(5): 947-957. DOI: 10.3969/j.issn.1001-5256.2018.05.007.中华医学会肝病学分会脂肪肝和酒精性肝病学组, 中国医师协会脂肪性肝病专家委员会. 非酒精性脂肪性肝病防治指南(2018年更新版)[J]. 临床肝胆病杂志, 2018, 34(5): 947-957. DOI: 10.3969/j.issn.1001-5256.2018.05.007. [4] DISTEFANO JK. NAFLD and NASH in postmenopausal women: Implications for diagnosis and treatment[J]. Endocrinology, 2020, 161(10): bqaa134. DOI: 10.1210/endocr/bqaa134. [5] RYU S, SUH BS, CHANG Y, et al. Menopausal stages and non-alcoholic fatty liver disease in middle-aged women[J]. Eur J Obstet Gynecol Reprod Biol, 2015, 190: 65-70. DOI: 10.1016/j.ejogrb.2015.04.017. [6] LONARDO A, NASCIMBENI F, BALLESTRI S, et al. Sex differences in nonalcoholic fatty liver disease: State of the art and identification of gaps[J]. Hepatology, 2019, 70(4): 1457-1469. DOI: 10.1002/hep.30626. [7] QIU S, VAZQUEZ JT, BOULGER E, et al. Hepatic estrogen receptor α is critical for regulation of gluconeogenesis and lipid metabolism in males[J]. Sci Rep, 2017, 7(1): 1661. DOI: 10.1038/s41598-017-01937-4. [8] HART-UNGER S, ARAO Y, HAMILTON KJ, et al. Hormone signaling and fatty liver in females: Analysis of estrogen receptor α mutant mice[J]. Int J Obes (Lond), 2017, 41(6): 945-954. DOI: 10.1038/ijo.2017.50. [9] FRIEDMAN SL, NEUSCHWANDER-TETRI BA, RINELLA M, et al. Mechanisms of NAFLD development and therapeutic strategies[J]. Nat Med, 2018, 24(7): 908-922. DOI: 10.1038/s41591-018-0104-9. [10] LIAO XJ, ZHANG LN, LI X, et al. Relationship between sex hormone levels and insulin resistance and liver enzyme abnormalities in postmenopausal women with NAFLD[J]. Shanxi Med J, 2019, 48(14): 1658-1659. DOI: 10.3969/j.issn.0253-9926.2019.14.004.廖雪姣, 张丽娜, 李轩, 等. 绝经后女性非酒精性脂肪性肝病患者性激素水平与胰岛素抵抗肝酶学异常的关系[J]. 山西医药杂志, 2019, 48(14): 1658-1659. DOI: 10.3969/j.issn.0253-9926.2019.14.004. [11] HU J, NI L, HUO LY, et al. Association between estrogen level and the risk of cardiovascular disease in perimenopausal type 2 diabetic patients[J]. Zhejiang Med J, 2020, 42(9): 913-916. DOI: 10.12056/j.issn.1006-2785.2020.42.9.2019-1785.胡家, 倪林, 霍丽霞, 等. 围绝经期2型糖尿病患者雌激素与心血管危险因素的相关性研究[J]. 浙江医学, 2020, 42(9): 913-916. DOI: 10.12056/j.issn.1006-2785.2020.42.9.2019-1785. [12] DELLA TORRE S. Non-alcoholic fatty liver disease as a canonical example of metabolic inflammatory-based liver disease showing a sex-specific prevalence: Relevance of estrogen signaling[J]. Front Endocrinol (Lausanne), 2020, 11: 572490. DOI: 10.3389/fendo.2020.572490. [13] QUIRÓS COGNUCK S, REIS WL, SILVA M, et al. Sex differences in body composition, metabolism-related hormones, and energy homeostasis during aging in Wistar rats[J]. Physiol Rep, 2020, 8(20): e14597. DOI: 10.14814/phy2.14597. [14] MAUVAIS-JARVIS F, LE MAY C, TIANO JP, et al. The role of estrogens in pancreatic islet physiopathology[J]. Adv Exp Med Biol, 2017, 1043: 385-399. DOI: 10.1007/978-3-319-70178-3_18. [15] YAN H, YANG W, ZHOU F, et al. Estrogen improves insulin sensitivity and suppresses gluconeogenesis via the transcription factor foxo1[J]. Diabetes, 2019, 68(2): 291-304. DOI: 10.2337/db18-0638. [16] SUKANYA V, PANDIYAN V, VIJAYARANI K, et al. A study on insulin levels and the expression of glut 4 in streptozotocin (STZ) induced diabetic rats treated with mustard oil diet[J]. Indian J Clin Biochem, 2020, 35(4): 488-496. DOI: 10.1007/s12291-019-00852-x. [17] YEO YH, LAI YC. Redox regulation of metabolic syndrome: Recent developments in skeletal muscle insulin resistance and non-alcoholic fatty liver disease (NAFLD)[J]. Curr Opin Physiol, 2019, 9: 79-86. DOI: 10.1016/j.cophys.2019.05.003. [18] PALMISANO BT, ZHU L, ECKEL RH, et al. Sex differences in lipid and lipoprotein metabolism[J]. Mol Metab, 2018, 15: 45-55. DOI: 10.1016/j.molmet.2018.05.008. [19] PALMISANO BT, ZHU L, STAFFORD JM. Role of estrogens in the regulation of liver lipid metabolism[J]. Adv Exp Med Biol, 2017, 1043: 227-256. DOI: 10.1007/978-3-319-70178-3_12. [20] PAQUETTE A, WANG D, JANKOWSKI M, et al. Effects of ovariectomy on PPAR alpha, SREBP-1c, and SCD-1 gene expression in the rat liver[J]. Menopause, 2008, 15(6): 1169-1175. DOI: 10.1097/gme.0b013e31817b8159. [21] BUNIAM J, CHUKIJRUNGROAT N, KHAMPHAYA T, et al. Estrogen and voluntary exercise attenuate cardiometabolic syndrome and hepatic steatosis in ovariectomized rats fed a high-fat high-fructose diet[J]. Am J Physiol Endocrinol Metab, 2019, 316(5): e908, e921. DOI: 10.1152/ajpendo.00466.2018. [22] LI FJ, WEI SN, WANG LY, et al. Estrogen reduces lipid deposition in liver cells by inhibiting perilipin 2[J]. J Cardiovascular Pulmonary Dis, 2018, 37(7): 687-691. DOI: 10.3969/j.issn.1007-5062.2018.07.022.李凤娟, 魏苏宁, 王绿娅, 等. 雌激素抑制脂滴包被蛋白Perilipin2减少肝细胞脂质沉积[J]. 心肺血管病杂志, 2018, 37(7): 687-691. DOI: 10.3969/j.issn.1007-5062.2018.07.022. [23] ZHANG ZC, LIU Y, XIAO LL, et al. Upregulation of miR-125b by estrogen protects against non-alcoholic fatty liver in female mice[J]. J Hepatol, 2015, 63(6): 1466-1475. DOI: 10.1016/j.jhep.2015.07.037. [24] RAZMJOU S, ABDULNOUR J, BASTARD JP, et al. Body composition, cardiometabolic risk factors, physical activity, and inflammatory markers in premenopausal women after a 10-year follow-up: A MONET study[J]. Menopause, 2018, 25(1): 89-97. DOI: 10.1097/GME.0000000000000951. [25] DONATO GB, FUCHS SC, OPPERMANN K, et al. Association between menopause status and central adiposity measured at different cutoffs of waist circumference and waist-to-hip ratio[J]. Menopause, 2006, 13(2): 280-285. DOI: 10.1097/01.gme.0000177907.32634.ae. [26] SUTTON-TYRRELL K, WILDMAN RP, MATTHEWS KA, et al. Sex-hormone-binding globulin and the free androgen index are related to cardiovascular risk factors in multiethnic premenopausal and perimenopausal women enrolled in the Study of Women Across the Nation (SWAN)[J]. Circulation, 2005, 111(10): 1242-1249. DOI: 10.1161/01.CIR.0000157697.54255.CE. [27] CHANG ML, YANG Z, YANG SS. Roles of adipokines in digestive diseases: Markers of inflammation, metabolic alteration and disease progression[J]. Int J Mol Sci, 2020, 21(21): 8308. DOI: 10.3390/ijms21218308. [28] HART-UNGER S, ARAO Y, HAMILTON KJ, et al. Hormone signaling and fatty liver in females: Analysis of estrogen receptor α mutant mice[J]. Int J Obes (Lond), 2017, 41(6): 945-954. DOI: 10.1038/ijo.2017.50. [29] YASUDA M, SHIMIZU I, SHIBA M, et al. Suppressive effects of estradiol on dimethylnitrosamine-induced fibrosis of the liver in rats[J]. Hepatology, 1999, 29(3): 719-727. DOI: 10.1002/hep.510290307. [30] TUROLA E, PETTA S, VANNI E, et al. Ovarian senescence increases liver fibrosis in humans and zebrafish with steatosis[J]. Dis Model Mech, 2015, 8(9): 1037-1046. DOI: 10.1242/dmm.019950. [31] CHOI E, KIM W, JOO SK, et al. Expression patterns of STAT3, ERK and estrogen-receptor α are associated with development and histologic severity of hepatic steatosis: A retrospective study[J]. Diagn Pathol, 2018, 13(1): 23. DOI: 10.1186/s13000-018-0698-8. [32] LEE YH, SON JY, KIM KS, et al. Estrogen deficiency potentiates thioacetamide-induced hepatic fibrosis in sprague-dawley rats[J]. Int J Mol Sci, 2019, 20(15): 3709. DOI: 10.3390/ijms20153709. [33] KO SH, KIM HS. Menopause-associated lipid metaboic disorders and foods beneficial for postmenopausal women[J]. Nutrients, 2020, 12(1): 202. DOI: 10.3390/nu12010202. [34] KLAIR JS, YANG JD, ABDELMALEK MF, et al. A longer duration of estrogen deficiency increases fibrosis risk among postmenopausal women with nonalcoholic fatty liver disease[J]. Hepatology, 2016, 64(1): 85-91. DOI: 10.1002/hep.28514. [35] WU ZY, XU YW, ZHANG LM. Analysis of the correlation between liver fibrosis and duration of menopause and estrogen deficiency in NAFLD women[J]. Chin Hepatol, 2019, 24(10): 1182-1184. DOI: 10.3969/j.issn.1008-1704.2019.10.031.吴宗妍, 徐优文, 张立梅. NAFLD女性患者肝纤维化与绝经时间和雌激素缺乏时间的相关性分析[J]. 肝脏, 2019, 24(10): 1182-1184. DOI: 10.3969/j.issn.1008-1704.2019.10.031. [36] PARK SH, PARK YE, LEE J, et al. Lack of association between early menopause and non-alcoholic fatty liver disease in postmenopausal women[J]. Climacteric, 2020, 23(2): 173-177. DOI: 10.1080/13697137.2019.1650018. [37] QUAN M, XING HC. progress on intestinal flora and chronic liver diseases[J/CD]. Chin J Liver Dis(Electronic Edition), 2019, 11(3): 26-30. DOI: 10.3969/j.issn.1674-7380.2019.03.005.全敏, 邢卉春. 肠道菌群与慢性肝病相关研究进展[J/CD]. 中国肝脏病杂志(电子版), 2019, 11(3): 26-30. DOI: 10.3969/j.issn.1674-7380.2019.03.005. [38] LEUNG C, RIVERA L, FURNESS JB, et al. The role of the gut microbiota in NAFLD[J]. Nat Rev Gastroenterol Hepatol, 2016, 13(7): 412-425. DOI: 10.1038/nrgastro.2016.85. [39] QIAO B, ZHOU Y, MA WJ, et al. Intestinal microflora imbalance in non-alcoholic fatty liver disease[J/CD]. Chin J Liver Dis(Electronic Edition), 2020, 12(4): 29-33. DOI: 10.3969/j.issn.1674-7380.2020.04.005.乔兵, 周永, 马文洁, 等. 肠道菌群失调在非酒精性脂肪性肝病中研究进展[J/CD]. 中国肝脏病杂志(电子版), 2020, 12(4): 29-33. DOI: 10.3969/j.issn.1674-7380.2020.04.005. [40] BAFFY G. Potential mechanisms linking gut microbiota and portal hypertension[J]. Liver Int, 2019, 39(4): 598-609. DOI: 10.1111/liv.13986. [41] BAKER JM, AL-NAKKASH L, HERBST-KRALOVETZ MM. Estrogen-gut microbiome axis: Physiological and clinical implications[J]. Maturitas, 2017, 103: 45-53. DOI: 10.1016/j.maturitas.2017.06.025. [42] SANTOS-MARCOS JA, RANGEL-ZUÑIGA OA, JIMENEZ-LUCENA R, et al. Influence of gender and menopausal status on gut microbiota[J]. Maturitas, 2018, 116: 43-53. DOI: 10.1016/j.maturitas.2018.07.008. [43] ZHAO H, CHEN J, LI X, et al. Compositional and functional features of the female premenopausal and postmenopausal gut microbiota[J]. FEBS Lett, 2019, 593(18): 2655-2664. DOI: 10.1002/1873-3468.13527. [44] LI H, LIU WX. progress on the correlation between intestinal flora and obesity[J]. Medical Recapitulate, 2020, 17: 3386-3393. DOI: 10.3969/j.issn.1006-2084.2020.17.012.李虹, 刘维新. 肠道菌群与肥胖相关性的研究进展[J]. 医学综述, 2020, 17: 3386-3393. DOI: 10.3969/j.issn.1006-2084.2020.17.012. [45] MU J, TAN F, ZHOU X, et al. Lactobacillus fermentum CQPC06 in naturally fermented pickles prevents non-alcoholic fatty liver disease by stabilizing the gut-liver axis in mice[J]. Food Funct, 2020, 11(10): 8707-8723. DOI: 10.1039/d0fo01823f. [46] SHIN JH, PARK YH, SIM M, et al. Serum level of sex steroid hormone is associated with diversity and profiles of human gut microbiome[J]. Res Microbiol, 2019, 170(4-5): 192-201. DOI: 10.1016/j.resmic.2019.03.003. [47] GIESSEN J, WOUDE CJ, PEPPELENBOSCH MP, et al. A direct effect of sex hormones on epithelial barrier function in inflammatory bowel disease models[J]. Cells, 2019, 8(3): 261. DOI: 10.3390/cells8030261. -



 PDF下载 ( 2193 KB)
PDF下载 ( 2193 KB)

 下载:
下载: