Influence of interleukin-6 on the expression and function of programmed death-1 in CD8+ T cells from patients with hepatocellular carcinoma
-
摘要:
目的 观察血浆IL-6及程序性死亡受体(PD-1)在肝细胞癌(HCC)患者外周血CD8+T淋巴细胞中的表达,评估IL-6对HCC患者CD8+T淋巴细胞中PD-1表达和功能的影响。 方法 纳入2019年1月—2019年9月期间在陕西省人民医院或空军军医大学第二附属医院(第四军医大学唐都医院)就诊的HCC患者44例(HCC组),同时纳入年龄和性别匹配的健康对照者19例(HC组),采集外周血,分离血浆和外周血单个核细胞,分选CD8+T淋巴细胞,ELISA法检测血浆IL-6水平,流式细胞术检测PD-1在CD8+T淋巴细胞中的表达水平。使用IL-6中和抗体刺激分选的CD8+T淋巴细胞24 h,CCK-8法检测细胞增殖,ELISA法检测培养上清IFNγ和TNFα水平,实时定量PCR法检测穿孔素、颗粒酶B和颗粒溶素mRNA相对表达量,Western blot法检测STAT3和Src磷酸化水平。计量资料两组间比较采用t检验或配对t检验;计数资料组间比较采用χ2检验。 结果 HCC组患者血浆IL-6水平较HC组显著升高[(99.67±20.92)pg/mL vs (81.05±16.76)pg/mL,t=3.427,P=0.001 1]。虽然CD3+CD8+T淋巴细胞比例在HCC组和HC组患者之间的差异无统计学意义(P>0.05),但PD-1+CD8+细胞比例在HCC组患者中显著升高(3.79%±1.36% vs 2.20%±0.47%,t=5.335,P<0.000 1)。使用IL-6中和抗体抑制HCC组患者CD8+T淋巴细胞的IL-6虽不影响细胞增殖,但可降低PD-1表达(2.67%±0.91% vs 3.33%±1.12%,t=2.177,P=0.035),增加IFNγ分泌[(13.50±3.82)pg/mL vs (10.82±1.37)pg/mL,t=3.170,P=0.002 8],穿孔素和颗粒酶B mRNA相对表达量亦显著升高(t值分别为6.161、14.140,P值均<0.000 1),同时伴有磷酸化STAT3水平降低(P<0.000 1)。 结论 抗人IL-6中和抗体可能通过增加穿孔素和颗粒酶B水平、增强细胞因子分泌以及抑制PD-1表达增强HCC患者CD8+T淋巴细胞的功能。 -
关键词:
- 癌,肝细胞 /
- CD8阳性T淋巴细胞 /
- 程序性细胞死亡受体1 /
- 白细胞介素6
Abstract:Objective To investigate the influence of interleukin-6(IL-6) on the expression and function of programmed death-1(PD-1) in patients with hepatocellular carcinoma (HCC) by measuring the plasma level of IL-6 and the expression of PD-1 in peripheral blood CD8+ T cells from HCC patients. Methods A total of 44 HCC patients who attended Shaanxi Provincial People's Hospital or The Second Affiliated Hospital of Air Force Medical University & Tangdu Hospital of Fourth Military Medical University from January to September 2019 were enrolled as HCC group, and 19 healthy controls, matched for age and sex, were enrolled as HC group. Peripheral blood was collected, and plasma and peripheral blood mononuclear cells were isolated to separate CD8+ T cells. ELISA was used to measure the plasma level of IL-6, and flow cytometry was used to measure the expression level of PD-1 in CD8+ T cells. The separated CD8+ T cells were stimulated with anti-IL-6 neutralizing antibody for 24 hours; CCK-8 assay was used to measure cell proliferation, ELISA was used to measure the levels of interferon-γ (IFNγ) and tumor necrosis factor-α (TNFα) in supernatant, real-time PCR was used to measure the relative mRNA expression levels of perforin, granzyme B, and granulysin, and Western blot was used to measure the phosphorylation levels of STAT3 and Src. The t-test or the paired t-test was used for comparison of continuous data between two groups, and the chi-square test was used for comparison of categorical data between groups. Results Compared with the HC group, the HCC group had a significant increase in the plasma level of IL-6 (99.67±20.92 pg/mL vs 81.05±16.76 pg/mL, t=3.427, P=0.001 1). There was no significant difference in the percentage of CD3+CD8+ T cells between the HCC group and the HC group, while there was a significant increase in the percentage of PD-1+CD8+ cells in HCC patients (3.79%±1.36% vs 2.20%±0.47%, t=5.335, P < 0.000 1). In the patients with HCC, although anti-IL-6 neutralizing antibody for inhibiting IL-6 in CD8+ T cells did not affect cell proliferation, it downregulated the expression of PD-1 (2.67%±0.91% vs 3.33%±1.12%, t=2.177, P=0.035) and increased the secretion of IFNγ (13.50±3.82 pg/mL vs 10.82±1.37 pg/mL, t=3.170, P=0.002 8), and there were also significant increases in the relative mRNA expression levels of perforin and granzyme B (t=6.161 and 14.140, both P < 0.000 1) and a significant reduction in the level of phosphorylated STAT3 (P < 0.000 1). Conclusion Anti-IL-6 neutralizing antibody can enhance the function of CD8+ T cells in HCC patients possibly by increasing the levels of perforin and granzyme B, improving the secretion of cytokines, and inhibiting the expression of PD-1. -
恶性肿瘤患者外周血和肿瘤微环境中的CD8+T淋巴细胞由于长期接受肿瘤抗原刺激造成免疫检查点分子表达水平升高,细胞应答和效应功能明显减弱,造成CD8+T淋巴细胞功能衰竭,不能清除肿瘤细胞,导致疾病进展和肿瘤转移[1-2]。针对程序性死亡受体-1(programmed death-1, PD-1)、程序性死亡配体(programmed death-ligand 1, PD-L1) 等免疫检查点分子的抑制剂已应用于多种恶性肿瘤的临床治疗中,取得了良好的临床疗效[3-4]。但调控免疫细胞中PD-1表达的机制尚未完全阐明。阻断IL-6信号通路可通过抑制PD-1/PD-L1信号通路增强机体抗肿瘤活性[5],IL-6介导的信号通路参与了肝细胞癌(HCC)的发病[6]。但IL-6是否能够通过调控HCC患者PD-1表达从而影响CD8+T淋巴细胞功能尚不清楚。因此,本研究观察PD-1在HCC患者外周血CD8+T淋巴细胞中的表达,并利用体外细胞培养系统研究IL-6对HCC患者CD8+T淋巴细胞PD-1表达的影响,初步探讨HCC患者CD8+T淋巴细胞的功能状态及其在HCC发病中的机制。
1. 资料与方法
1.1 研究对象
本研究严格按照《赫尔辛基宣言》原则进行,招募于2019年1月—2019年9月期间在陕西省人民医院或空军军医大学第二附属医院(第四军医大学唐都医院)就诊的HCC患者(HCC组)。纳入标准:(1)年龄18~60岁;(2)诊断符合《原发性肝癌诊疗规范(2019年版)》[7]的要求;(3)入组前未接受过手术、介入、放疗、免疫及靶向等治疗。排除标准:(1)合并重要脏器(心脏、肝脏、肾脏、神经系统)功能障碍;(2) 合并其他恶性肿瘤;(3)合并其他精神障碍;(4)合并获得性免疫缺陷综合征;(5)合并自身免疫性疾病。同时,招募19例年龄和性别匹配的健康对照者(HC组)。
1.2 方法
1.2.1 实验试剂和材料
Ficoll淋巴细胞分离液购自美国Sigma公司;IL-6、IFNγ和TNFα ELISA试剂盒购自武汉华美生物公司;抗人IL-6中和抗体购自美国InvivoGen公司;抗人CD3-PerCP、抗人CD8-APC Cy7、抗人PD-1(CD279)-FITC购自美国BD公司;CD8+T淋巴细胞分选试剂盒购自德国美天旎公司;CCK-8试剂盒购自武汉碧云天公司;Trizol试剂购自美国Invitrogen公司;反转录试剂盒和TB Green实时定量PCR试剂盒购自TaKaRa公司;兔抗信号传导及转录激活蛋白3(signal transducer and activator of transcription 3, STAT3)、兔抗STAT3 (phospho Y705)、兔抗Src、兔抗Src(phospho Y418)、兔抗GAPDH、山羊抗兔IgG均购自美国Abcam公司。FACS Calibur流式细胞仪为美国BD公司产品;ABI 7500型荧光定量PCR仪为美国Applied Biosystem公司产品。
1.2.2 外周血单个核细胞(PBMC)和血浆分离
清晨、空腹采集所有入组志愿者EDTA抗凝外周血10 mL,4 ℃、1000 r/min离心10 min后收集上层血浆冻存备用,下层血细胞使用Ficoll淋巴细胞分离液,采用密度梯度离心法分离PBMC,以107个/mL的浓度冻存备用。
1.2.3 流式细胞检测CD8+T淋巴细胞表面PD-1的表达
取106个PBMC,使用抗人CD3-PerCP、抗人CD8-APC Cy7、抗人PD-1-FITC对其进行染色,使用FACS Calibur流式细胞仪对染色后的细胞进行检测,使用FlowJo V10软件进行分析。
1.2.4 CD8+T淋巴细胞分选和培养
取107个PBMC,使用CD8+T淋巴细胞分选试剂盒按说明书要求分选CD8+T淋巴细胞,采用流式细胞术对分选后的细胞进行纯度鉴定,纯化比例>95%。参考既往文献[8]报道中的操作,使用抗人IL-6中和抗体(终浓度为20 ng/mL)刺激分选的CD8+T淋巴细胞24 h,收集细胞和上清进行后续实验。
1.2.5 细胞增殖试验
将分选的CD8+T淋巴细胞接种于96孔板,每个志愿者的CD8+T淋巴细胞设立4个复孔,每孔中加入2×105个纯化的CD8+T淋巴细胞,其中2个孔加入终浓度为20 ng/mL的抗人IL-6中和抗体培养(为实验组),另外2个孔加入等量的培养液(为对照组),培养12 h后收集上清和细胞。使用CCK-8细胞增殖检测试剂盒按说明书要求对细胞增殖检测。以OD450 nm表示细胞增殖水平。
1.2.6 ELISA检测细胞因子含量
对血浆中IL-6、培养上清中IFNγ和TNFα使用ELISA试剂盒进行检测。
1.2.7 实时定量PCR
对抗人IL-6中和抗体处理后的CD8+T淋巴细胞提取总RNA,按TaKaRa反转录试剂盒要求逆转录cDNA,采用TB Green实时定量PCR法对CD8+T淋巴细胞中穿孔素、颗粒酶B和颗粒溶素mRNA的相对表达量进行检测。引物序列见表 1。
表 1 实时定量PCR引物序列基因名称 上游引物(5′-3′) 下游引物(5′-3′) 穿孔素 CGCCTACCTCAGGCTTATCTC CCTCGACAGTCAGGCAGTC 颗粒酶B TGGGGGACCCAGAGATTAAAA TTTCGTCCATAGGAGACAATGC 颗粒溶素 ACTGAAGATGGTGGATAAGCC GCCCTGGGTAACTCTAGACTG GAPDH GCACCGTCAAGGCTGAGAAC TGGTGAAGACGCCAGTGG 1.2.8 Western blot
取抗人IL-6中和抗体处理后的CD8+T淋巴细胞,加入含有Protease/Phosphatase Inhibitor Cocktail的2×SDS buffer,于冰上裂解细胞15 min,再于95 ℃孵育10 min后,于12 000 r/min离心2 min,收集上清。BCA法进行蛋白定量。配置SDS-PAGE(5%浓缩胶、10%分离胶),以30 μg/泳道上样、电泳,电转移至PVDF膜,5%脱脂奶粉/TBST封闭,洗涤后加入兔抗STAT3、兔抗STAT3(phospho Y705)、兔抗Src、兔抗Src(phospho Y418)、兔抗GAPDH,4 ℃孵育过夜,洗涤后加入山羊抗兔IgG室温孵育2 h,洗涤后加入ECL,曝光。
1.3 伦理学审查
本研究方案经陕西省人民医院医学伦理委员会(批号:省医伦2017-082号)和空军军医大学第二附属医院(第四军医大学唐都医院)医学伦理委员会批准(批号:TDLL-201505-013),所有入组志愿者均签署知情同意书。
1.4 统计学方法
统计数据采用SPSS 21.0软件分析。计量资料采用x±s表示,两组间比较采用t检验或配对t检验。计数资料组间比较采用χ2检验。P<0.05为差异具有统计学意义。
2. 结果
2.1 一般资料
HCC组和HC组一般资料见表 2。两组在性别比例、年龄之间的差异均无统计学意义(P值均>0.05)。
表 2 入组志愿者一般资料资料 HC组
(n=19)HCC组
(n=44)统计值 P值 男/女(例) 11/8 28/16 χ2=0.728 0.537 年龄(岁) 54.2±7.9 58.7±12.1 t=1.486 0.142 AFP(ng/mL) 6.1±1.8 871.2±243.0 t=15.444 <0.000 1 HBV/HCV感染史(例) 0 38 - - 2.2 两组患者血浆IL-6表达水平的比较
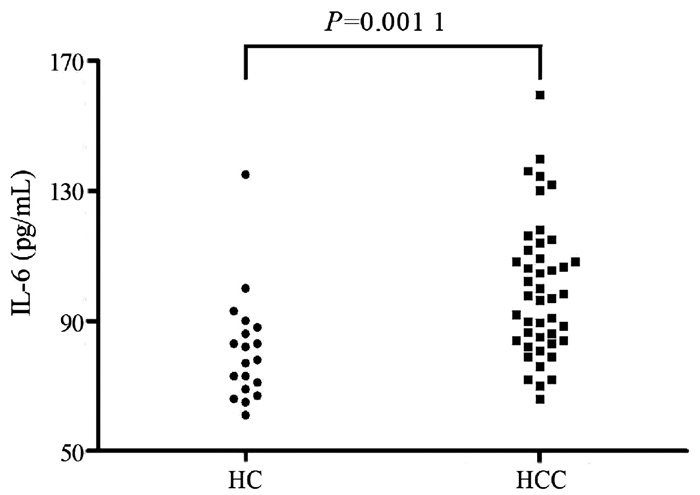
HCC组血浆IL-6的水平较HC组[(99.67±20.92)pg/mL vs (81.05 ±16.76)pg/mL]显著升高(t=3.427,P=0.001 1)(图 1)。
2.3 两组患者外周血CD8+T淋巴细胞比例与PD-1阳性比例的比较
HC组和HCC组CD3+CD8+T淋巴细胞以及PD-1在CD8+T淋巴细胞中表达的典型流式散点图见图 2a。外周血CD3+CD8+T淋巴细胞的比例在HC组和HCC组之间的差异无统计学意义(26.37%±6.59% vs 29.43%±6.99%,t=1.618,P=0.111)(图 2b),但HCC组患者中PD-1+CD8+细胞的比例(3.79% ±1.36%)则显著高于HC组(2.20%±0.47%),差异具有统计学意义(t=5.335,P<0.000 1)(图 2c)。
2.4 HCC患者外周血CD8+T淋巴细胞在抗人IL-6中和抗体处理后细胞增殖、PD-1表达以及分泌细胞因子的变化
CCK-8结果提示,抗人IL-6中和抗体对CD8+T淋巴细胞增殖无明显影响(t=1.285,P=0.206)(图 3a),但PD-1+细胞占CD8+T淋巴细胞的比例在抗人IL-6中和抗体处理后显著下降(3.33%±1.12% vs 2.67%±0.91%,t=2.177,P=0.035)(图 3b)。抗人IL-6中和抗体处理后CD8+T淋巴细胞分泌IFNγ的水平显著升高[(10.82±1.37)pg/mL vs (13.50±3.82)pg/mL,t=3.170,P=0.002 8](图 3c),但TNFα在抗人IL-6中和抗体处理与未处理组中分泌水平的差异无统计学意义[(96.66±15.78)pg/mL vs(103.20±16.06)pg/mL,t=1.385,P=0.173](图 3d)。
2.5 HCC患者外周血CD8+T淋巴细胞在抗人IL-6中和抗体处理后穿孔素、颗粒酶B和颗粒溶素mRNA以及STAT3和Src蛋白磷酸化的变化
取上述刺激后的CD8+T淋巴细胞,分别提取mRNA和蛋白,实时定量PCR结果提示,抗人IL-6中和抗体处理后CD8+T淋巴细胞中穿孔素和颗粒酶B mRNA的相对表达量均显著升高(t值分别为6.161、14.140,P值均<0.000 1)(图 4a、b),但颗粒溶素mRNA的相对表达量在抗人IL-6中和抗体处理和未处理的CD8+T淋巴细胞之间的差异无统计学意义(t=1.493,P=0.143)(图 4c)。对Western blot结果进行半定量分析发现,抗人IL-6中和抗体处理后总STAT3和总Src的水平均无显著变化,对Src的磷酸化亦无明显影响,但STAT3磷酸化水平在抗人IL-6中和抗体处理后显著降低(P<0.000 1)(图 4d、e)。
3. 讨论
在免疫功能紊乱和肿瘤发生发展之间,细胞因子/趋化因子、免疫细胞和免疫分子所构成的免疫网络发挥重要作用。HCC的发生与不同病因(包括慢性乙型肝炎、慢性丙型肝炎、酒精性肝病、非酒精性脂肪性肝病等)造成的慢性炎症和纤维化密切相关,以TNFα和IL-6为代表的炎症细胞因子及其下游信号转导通路不但可诱导HCC发生,还能促进炎症,加重肝损伤[9]。同时,促炎细胞因子亦可调控T淋巴细胞表面免疫检查点分子PD-1的表达,参与机体免疫和炎症应答调控[10]。因此,本研究探讨了促炎因子IL-6对HCC患者CD8+T淋巴细胞功能的调节作用和初步机制。结果显示,HCC患者外周血IL-6水平以及CD8+T淋巴细胞表面PD-1水平均显著升高,阻断IL-6介导的信号通路可降低HCC患者CD8+T淋巴细胞PD-1表达,增加IFNγ分泌和穿孔素、颗粒酶B mRNA相对表达量,提示抑制IL-6可能增强了HCC患者CD8+T淋巴细胞的功能。
IL-6是固有免疫应答中重要的细胞因子之一,主要由单核巨噬细胞分泌,发挥促炎作用。IL-6还能调控巨噬细胞极化,在HCC发生过程中,IL-6/STAT3信号通路可抑制M1型巨噬细胞、活化M2型巨噬细胞,从而促进HCC细胞活性、增殖、迁移和侵袭[11]。因此,无论是经典的IL-6信号通路还是IL-6跨信号通路(trans-signaling)均可通过不同的机制(包括:抑制p53抑癌基因、增强β-连环蛋白活性、保护肿瘤细胞免受DNA损伤所致的凋亡、直接促进内皮细胞增殖等)诱导HCC发生发展[12]。IL-6/STAT3信号通路还可通过调控肝脏肿瘤干细胞活性诱导针对索拉非尼耐药的肝癌细胞[13],降低IL-6的表达则可增强肝癌细胞对索拉非尼的敏感性[14]。本研究发现,HCC患者外周血IL-6的水平较健康志愿者显著升高,进一步提示IL-6参与了HCC的发生发展,但其中的机制仍有待深入研究。
通过流式细胞术检测发现,HCC患者外周血CD8+T淋巴细胞所占比例与健康对照者的差异无统计学意义。但是,HCC患者CD8+T淋巴细胞中PD-1水平显著升高,PD-1作为重要的免疫检查点分子,已证实在肿瘤、感染性疾病、自身免疫性疾病中发挥重要的免疫调控作用[15],PD-1的表达升高可能诱导CD8+T淋巴细胞功能衰竭,使HCC疾病恶化。在炎症性关节病中,以IL-6为代表的促炎细胞因子可增加膜型和可溶型PD-1的表达[10]。在肿瘤微环境中,抗IL-6中和抗体和抗PD-1/PD-L1抗体亦可发挥协同作用,打破肿瘤微环境中的免疫耐受,抑制肿瘤生长[16-17]。这提示IL-6和PD-1之间可能存在相互作用。新近的研究[18]发现,在HCC发病过程中,IL-6可通过抑制受体型蛋白酪氨酸磷酸酶O促进单核巨噬细胞中PD-L1表达。由于本研究发现HCC患者IL-6和PD-1的表达均升高,因此,本研究利用抗人IL-6中和抗体处理HCC患者分选的CD8+T淋巴细胞,观察抑制IL-6对CD8+T淋巴细胞的影响,并分析其初步机制。结果发现,抑制IL-6可增强HCC患者CD8+T淋巴细胞的功能,主要表现为PD-1的表达降低、IFNγ分泌增加、穿孔素和颗粒酶B mRNA的相对表达量增加。这一结果与在病毒感染中所发现的IL-6促进病毒特异性细胞毒性CD8+T淋巴细胞增殖和分化的结果[19-20]不尽相同,这可能与不同的疾病类型有关。因此,进一步对IL-6的下游信号通路进行了分析,结果发现,抑制IL-6主要影响HCC患者CD8+T淋巴细胞中的JNK-STAT3信号通路,而对Src信号通路无显著影响。但值得注意的是,本研究纳入的样本量相对较少,且肿瘤免疫网络的调控非常复杂,本研究结果仍需扩大样本量并进行体内动物实验加以证实。
综上,本文研究表明HCC患者中IL-6和CD8+T淋巴细胞中PD-1的表达升高,抑制IL-6可能通过降低PD-1表达、增加IFNγ分泌和升高穿孔素及颗粒酶B水平发挥增强HCC患者CD8+T淋巴细胞的作用。
-
表 1 实时定量PCR引物序列
基因名称 上游引物(5′-3′) 下游引物(5′-3′) 穿孔素 CGCCTACCTCAGGCTTATCTC CCTCGACAGTCAGGCAGTC 颗粒酶B TGGGGGACCCAGAGATTAAAA TTTCGTCCATAGGAGACAATGC 颗粒溶素 ACTGAAGATGGTGGATAAGCC GCCCTGGGTAACTCTAGACTG GAPDH GCACCGTCAAGGCTGAGAAC TGGTGAAGACGCCAGTGG 表 2 入组志愿者一般资料
资料 HC组
(n=19)HCC组
(n=44)统计值 P值 男/女(例) 11/8 28/16 χ2=0.728 0.537 年龄(岁) 54.2±7.9 58.7±12.1 t=1.486 0.142 AFP(ng/mL) 6.1±1.8 871.2±243.0 t=15.444 <0.000 1 HBV/HCV感染史(例) 0 38 - - -
[1] MCLANE LM, ABDEL-HAKEEM MS, WHERRY EJ. CD8 T cell exhaustion during chronic viral infection and cancer[J]. Annu Rev Immunol, 2019, 37: 457-495. DOI: 10.1146/annurev-immunol-041015-055318. [2] MILLER BC, SEN DR, AL ABOSY R, et al. Subsets of exhausted CD8(+) T cells differentially mediate tumor control and respond to checkpoint blockade[J]. Nat Immunol, 2019, 20(3): 326-336. DOI: 10.1038/s41590-019-0312-6. [3] GALLUZZI L, HUMEAU J, BUQUÉ A, et al. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors[J]. Nat Rev Clin Oncol, 2020, 17(12): 725-741. DOI: 10.1038/s41571-020-0413-z. [4] DOLLADILLE C, EDERHY S, SASSIER M, et al. Immune checkpoint inhibitor rechallenge after immune-related adverse events in patients with cancer[J]. JAMA Oncol, 2020, 6(6): 865-871. DOI: 10.1001/jamaoncol.2020.0726. [5] ERIKSSON E, MILENOVA I, WENTHE J, et al. IL-6 signaling blockade during CD40-mediated immune activation favors antitumor factors by reducing TGF-β, collagen type I, and PD-L1/PD-1[J]. J Immunol, 2019, 202(3): 787-798. DOI: 10.4049/jimmunol.1800717. [6] LOKAU J, SCHOEDER V, HAYBAECK J, et al. Jak-stat signaling induced by interleukin-6 family cytokines in hepatocellular carcinoma[J]. Cancers (Basel), 2019, 11(11): 1704. DOI: 10.3390/cancers11111704. [7] Bureau of Medical Administrationnational Health Commission of the People's Republic of China. Guidelines for diagnosis and treatment of primary liver cancer in China (2019 edition)[J]. J Clin Hepatol, 2020, 36(2): 277-292. DOI: 10.3969/j.issn.1001-5256.2020.02.007.中华人民共和国国家卫生健康委员会医政医管局. 原发性肝癌诊疗规范(2019年版)[J]. 临床肝胆病杂志, 2020, 36(2): 277-292. DOI: 10.3969/j.issn.1001-5256.2020.02.007. [8] HOU H, KANG Y, ZENG Y, et al. Interleukin-7 augments CD8(+) T cells function and promotes viral clearance in chronic hepatitis C virus infection[J]. Cytokine, 2018, 102: 26-33. DOI: 10.1016/j.cyto.2017.12.014. [9] YANG YM, KIM SY, SEKI E. Inflammation and liver cancer: Molecular mechanisms and therapeutic targets[J]. Semin Liver Dis, 2019, 39(1): 26-42. DOI: 10.1055/s-0038-1676806. [10] BOMMARITO D, HALL C, TAAMS LS, et al. Inflammatory cytokines compromise programmed cell death-1 (PD-1)-mediated T cell suppression in inflammatory arthritis through up-regulation of soluble PD-1[J]. Clin Exp Immunol, 2017, 188(3): 455-466. DOI: 10.1111/cei.12949. [11] YIN Z, MA T, LIN Y, et al. IL-6/STAT3 pathway intermediates M1/M2 macrophage polarization during the development of hepatocellular carcinoma[J]. J Cell Biochem, 2018, 119(11): 9419-9432. DOI: 10.1002/jcb.27259. [12] BERGMANN J, MVLLER M, BAUMANN N, et al. IL-6 trans-signaling is essential for the development of hepatocellular carcinoma in mice[J]. Hepatology, 2017, 65(1): 89-103. DOI: 10.1002/hep.28874. [13] LI Y, CHEN G, HAN Z, et al. IL-6/STAT3 signaling contributes to sorafenib resistance in hepatocellular carcinoma through targeting cancer stem cells[J]. Onco Targets Ther, 2020, 13: 9721-9730. DOI: 10.2147/OTT.S262089. [14] YANG J, WANG J, LUO J. Decreased IL-6 induces sensitivity of hepatocellular carcinoma cells to sorafenib[J]. Pathol Res Pract, 2019, 215(10): 152565. DOI: 10.1016/j.prp.2019.152565. [15] ALSAAB HO, SAU S, ALZHRANI R, et al. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: Mechanism, combinations, and clinical outcome[J]. Front Pharmacol, 2017, 8: 561. DOI: 10.3389/fphar.2017.00561. [16] TSUKAMOTO H, FUJIEDA K, MIYASHITA A, et al. Combined blockade of IL6 and PD-1/PD-L1 signaling abrogates mutual regulation of their immunosuppressive effects in the tumor microenvironment[J]. Cancer Res, 2018, 78(17): 5011-5022. DOI: 10.1158/0008-5472.CAN-18-0118. [17] LIU H, SHEN J, LU K. IL-6 and PD-L1 blockade combination inhibits hepatocellular carcinoma cancer development in mouse model[J]. Biochem Biophys Res Commun, 2017, 486(2): 239-244. DOI: 10.1016/j.bbrc.2017.02.128. [18] ZHANG W, LIU Y, YAN Z, et al. IL-6 promotes PD-L1 expression in monocytes and macrophages by decreasing protein tyrosine phosphatase receptor type O expression in human hepatocellular carcinoma[J]. J Immunother Cancer, 2020, 8(1): e000285. DOI: 10.1136/jitc-2019-000285. [19] WU W, DIETZE KK, GIBBERT K, et al. TLR ligand induced IL-6 counter-regulates the anti-viral CD8(+) T cell response during an acute retrovirus infection[J]. Sci Rep, 2015, 5: 10501. DOI: 10.1038/srep10501. [20] ZHOU X, HOPKINS JW, WANG C, et al. IL-2 and IL-6 cooperate to enhance the generation of influenza-specific CD8 T cells responding to live influenza virus in aged mice and humans[J]. Oncotarget, 2016, 7(26): 39171-39183. DOI: 10.18632/oncotarget.10047. 期刊类型引用(1)
1. 张殿亮,孙童,闫森,宁尚昆,马翔宇,刘吉兵. 白介素-6与肝细胞癌治疗的研究进展. 中华肿瘤防治杂志. 2024(21): 1340-1346 .  百度学术
百度学术其他类型引用(1)
-




 PDF下载 ( 3487 KB)
PDF下载 ( 3487 KB)

 下载:
下载:




 下载:
下载:



 百度学术
百度学术





