血清miR-486-5p联合CA19-9在可切除和交界性可切除胰腺癌中的预测价值
DOI: 10.3969/j.issn.1001-5256.2021.10.028
Value of serum miR-486-5p combined with carbohydrate antigen 19-9 in predicting resectable or borderline resectable pancreatic cancer
-
摘要:
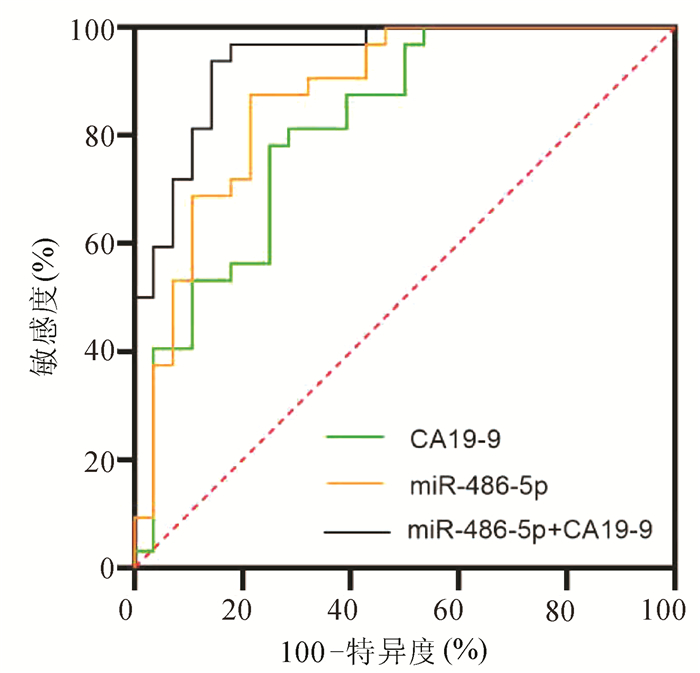
目的 探讨胰腺癌患者血清miR-486-5p的表达水平,以及联合糖链抗原19-9(CA19-9)对胰腺癌可切除性的预测能力。 方法 选取2018年9月—2020年12月青岛市市立医院确诊的胰腺癌60例,其中可切除和交界性可切除胰腺癌共32例(可手术组),不可切除胰腺癌28例(不可手术组),设置胰腺良性疾病组30例和健康对照组44例。采用实时荧光定量聚合酶链反应(qRT-PCR)检测各组血清miR-486-5p的水平,并计算相对表达量,分析miR-486-5p的相对表达量与胰腺癌临床特征(包括年龄、性别、肿瘤位置、大小、TNM分期、淋巴转移和远处转移)的关系。不符合正态分布的计量资料两组间比较采用Mann-Whitney U检验,计数资料组间比较采用χ2检验。构建受试者工作特征曲线(ROC曲线),通过二元logistic回归计算联合预测值,分析血清miR-486-5p联合CA19-9对胰腺癌可切除性的预测价值。 结果 可手术组[2.16(1.38~3.30)]和不可手术组[4.65(2.80~9.90)]血清miR-486-5p相对表达量均显著高于胰腺良性疾病组[1.01(0.52~1.53)]和健康对照组[0.99(0.24~1.01)](P值均<0.001)。在TNM分期、有无淋巴转移和有无远处转移中miR-486-5p的低表达和高表达差异有统计学意义(χ2值分别为13.765、5.157、6.638,P值均<0.05)。miR-486-5p+CA19-9区分可手术组与良性疾病组(AUC=0.87,95%CI:0.760~0.942,敏感度81.3%,特异度83.3%)、区分可手术组与健康对照组(AUC=0.92,95%CI:0.836~0.970,敏感度90.6%,特异度86.4%)以及区分可手术组和不可手术组(AUC=0.94,95%CI:0.884~0.998,敏感度85.7%,特异度93.7%)价值优于CA19-9单独检测(Z值分别为2.841、2.510、2.387,P值均<0.05),最佳临界值分别为3.12、3.21和6.63。 结论 miR-486-5p可作为诊断胰腺癌血清生物标志物,联合CA19-9在胰腺良性疾病和健康人群中预测胰腺癌可切除性的临床价值优于CA19-9。 Abstract:Objective To investigate the expression level of serum miR-486-5p in patients with pancreatic cancer and the value of serum miR-486-5p combined with carbohydrate antigen 19-9 (CA19-9) in predicting the resectability of pancreatic cancer. Methods A total of 60 patients who were diagnosed with pancreatic cancer in Qingdao Municipal Hospital from September 2018 to December 2020 were enrolled, among whom 32 patients had resectable or borderline resectable pancreatic cancer (operable group) and 28 had unresectable pancreatic cancer (non-operable group), and a benign pancreatic disease group with 30 patients and a healthy control group with 44 individuals were also established. Quantitative real-time PCR was used to measure the serum level of miR-486-5p in each group, and the relative expression level of miR-486-5p was calculated to analyze its association with the clinical features of pancreatic cancer, including age, sex, tumor location, tumor size, TNM stage, lymphatic metastasis, and distant metastasis. The Mann-Whitney U test was used for comparison of non-normally distributed continuous variables between two groups, and the chi-square test was used for comparison of categorical variables. The receiver operating characteristic (ROC) curve was plotted, and a binary logistic regression analysis was used to calculate the combined predictive value and then investigate the value of serum miR-486-5p combined with CA19-9 in predicting the resectability of pancreatic cancer. Results The relative expression level of serum miR-486-5p in the operable group [2.16 (1.38~3.30)] and the non-operable group [4.65 (2.80~9.90)] was significantly higher than that in the benign pancreatic disease group [1.01 (0.52~1.53)] and the healthy control group [0.99 (0.24~1.01)] (all P < 0.001). There were significant differences in the number of patients with low or high expression of miR-486-5p between the patients with different TNM stages, presence or absence of lymphatic metastasis, and presence or absence of distant metastasis (χ2=13.765, 5.157, and 6.638, all P < 0.05). Compared with CA19-9 alone, miR-486-5p+CA19-9 had a significantly better value in distinguishing the operable group from the benign pancreatic disease group (area under the ROC curve [AUC]=0.87, 95% confidence interval [CI]: 0.760-0.942; with a sensitivity of 81.3% and a specificity of 83.3%), distinguishing the operable group from the healthy control group (AUC=0.92, 95% CI: 0.836-0.970; with a sensitivity of 90.6% and a specificity of 86.4%), and distinguishing the operable group from the non-operable group (AUC=0.94, 95%CI: 0.884-0.998; with a sensitivity of 85.7% and a specificity of 93.7%) (Z=2.841, 2.510, and 2.387, all P < 0.05), and the optimal cut-off values were 3.12, 3.21, and 6.63, respectively. Conclusion MiR-486-5p can be used as a serum biomarker for the diagnosis of pancreatic cancer, and miR-486-5p combined with CA19-9 has a better clinical value than CA19-9 alone in predicting the resectability of pancreatic cancer in the patients with benign pancreatic diseases and the healthy population. -
Key words:
- Pancreatic Neoplasms /
- MicroRNAs /
- CA-19-9 Antigen
-
表 1 各组血清miR-486-5p水平比较
组别 例数 miR-486-5p相对表达量 良性疾病组 30 1.01(0.52~1.53) 健康对照组 44 0.99(0.24~1.01) 可手术组 32 2.16(1.38~3.30)1)2) 不可手术组 28 4.65 (2.80~9.90)1)2)3) 注:与良性疾病组比较,1)P<0.001;与健康对照组比较,2)P<0.001;与可手术组比较,3)P<0.001。 表 2 PC血清miR-486-5p与临床特征的关系
临床参数 例数 miR-486-5p χ2值 P值 低表达组 高表达组 性别(例) 0.558 0.455 男 34 15 19 女 26 14 12 年龄(例) 1.714 0.190 ≥60岁 35 15 20 <60岁 25 15 10 肿瘤位置(例) 0.617 0.432 胰头 35 16 19 胰体/尾 25 14 11 肿瘤大小(例) 3.128 0.077 ≥4 cm 33 12 21 <4 cm 27 16 11 TNM分期(例) 13.765 <0.001 Ⅰ~Ⅱ期 21 17 4 Ⅲ~Ⅳ期 39 12 27 淋巴转移(例) 5.157 0.023 有 37 13 24 无 23 15 8 远处转移(例) 6.638 0.010 有 25 8 17 无 35 23 12 表 3 miR-486-5p、CA19-9和联合后对可切除和交界性可切除PC的预测价值
组别 敏感度
(%)特异度
(%)AUC 95%CI 可手术组vs不可手术组 miR-486-5p 75.0 78.1 0.82 0.707~0.925 CA19-9 78.6 87.5 0.87 0.779~0.964 miR-486-5p +CA19-9 85.7 93.7 0.94 0.884~0.998 可手术组vs良性疾病组 miR-486-5p 71.9 71.7 0.80 0.675~0.888 CA19-9 62.5 66.7 0.73 0.606~0.838 miR-486-5p +CA19-9 81.3 83.3 0.87 0.760~0.942 可手术组vs健康对照组 miR-486-5p 87.5 81.8 0.86 0.763~0.930 CA19-9 62.5 75.0 0.78 0.670~0.867 miR-486-5p+CA19-9 90.6 86.4 0.92 0.836~0.970 -
[1] BRAY F, FERLAY J, SOERJOMATARAM I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2018, 68(6): 394-424. DOI: 10.3322 /caac.21492. [2] VENKATESAN P. Biomarkers for subtypes of pancreatic ductal adenocarcinoma[J]. Lancet Oncol, 2017, 18(12): e718. DOI: 10.1016 /S1470-2045(17)30842-2. [3] ZHOU J, QIE S, FANG H, et al. MiR-487a-3p suppresses the malignant development of pancreatic cancer by targeting SMAD7[J]. Exp Mol Pathol, 2020, 116: 104489. DOI: 10.1016 /j.yexmp.2020.104489. [4] SPRINGETT GM, HOFFE SE. Borderline resectable pancreatic cancer: On the edge of survival[J]. Cancer Control, 2008, 15(4): 295-307. DOI: 10.1177 /107327480801500404. [5] BLOOMSTON M, FRANKEL WL, PETROCCA F, et al. MicroRNA expression patterns to differentiate pancreatic adenocarcinoma from normal pancreas and chronic pancreatitis[J]. JAMA, 2007, 297(17): 1901-1908. DOI: 10.1001/jama.297.17.1901. [6] NCCN Clinical Practice Guidelines in Oncology: Pancreatic Adenocarcinoma(Version 1.2020)[EB/OL]. NCCN 2020[2019-11-26]. http://www.nccn.org. [7] GABLO NA, PROCHAZKA V, KALA Z, et al. Cell-free microRNAs as non-invasive diagnostic and prognostic bio- markers in pancreatic cancer[J]. Curr Genomics, 2019, 20(8): 569-580. DOI: 10.2174/1389202921666191217095017. [8] SIEGEL RL, MILLER KD, JEMAL A. Cancer statistics, 2019[J]. CA Cancer J Clin, 2019, 69(1): 7-34. DOI: 10.3322/caac.21551. [9] USHIDA Y, INOUE Y, ITO H, et al. High CA19-9 level in resectable pancreatic cancer is a potential indication of neoadjuvant treatment[J]. Pancreatology, 2021, 21(1): 130-137. DOI: 10.1016/j.pan.2020.11.026. [10] CHANG JC, KUNDRANDA M. Novel diagnostic and predictive biomarkers in pancreatic adenocarcinoma[J]. Int J Mol Sci, 2017, 18(3): 667. DOI: 10.3390/ijms18030667. [11] MIZRAHI JD, SURANA R, VALLE JW, et al. Pancreatic cancer[J]. Lancet, 2020, 395(10242): 2008-2020. DOI: 10.1016/s0140-6736(20)30974-0. [12] PORUK KE, GAY DZ, BROWN K, et al. The clinical utility of CA 19-9 in pancreatic adenocarcinoma: Diagnostic and prognostic updates[J]. Curr Mol Med, 2013, 13(3): 340-351. DOI: 10.2174/1566524011313030003. [13] MAHER TM, OBALLA E, SIMPSON JK, et al. An epithelial biomarker signature for idiopathic pulmonary fibrosis: An analysis from the multicentre PROFILE cohort study[J]. Lancet Respir Med, 2017, 5(12): 946-955. DOI: 10.1016/S2213-2600(17)30430-7. [14] BARTEL DP. MicroRNAs: Genomics, biogenesis, mechanism, and function[J]. Cell, 2004, 116(2): 281-297. DOI: 10.1016/s0092-8674(04)00045-5. [15] GARZON R, CALIN GA, CROCE CM. MicroRNAs in cancer[J]. Annu Rev Med, 2009, 60: 167-179. DOI: 10.1146/annurev.med.59.053006.104707. [16] HUMMEL R, HUSSEY DJ, HAIER J. MicroRNAs: Predictors and modifiers of chemo- and radiotherapy in different tumour types[J]. Eur J Cancer, 2010, 46(2): 298-311. DOI: 10.1016/j.ejca.2009.10.027. [17] LANG B, ZHAO S. miR-486 functions as a tumor suppressor in esophageal cancer by targeting CDK4/BCAS2[J]. Oncol Rep, 2018, 39(1): 71-80. DOI: 10.3892/or.2017.6064. [18] XU J, CAO Z, LIU W, et al. Plasma miRNAs effectively distinguish patients with pancreatic cancer from controls: A multicenter study[J]. Ann Surg, 2016, 263(6): 1173-1179. DOI: 10.1097/SLA.0000000000001345. [19] WANG W, LIU B, SUN S, et al. Downregulation of miR-486-5p enhances the anti-tumor effect of 5-fluorouracil on pancreatic cancer cells[J]. Onco Targets Ther, 2020, 13: 1649-1659. DOI: 10.2147/OTT.S231153. [20] YANG Q, WANG S, HUANG J, et al. Serum miR-20a and miR-486 are potential biomarkers for discriminating colorectal neoplasia: A pilot study[J]. J Cancer Res Ther, 2018, 14(7): 1572-1577. DOI: 10.4103/jcrt.JCRT_1198_16. [21] SUN H, CUI C, XIAO F, et al. miR-486 regulates metastasis and chemosensitivity in hepatocellular carcinoma by targeting CLDN10 and CITRON[J]. Hepatol Res, 2015, 45(13): 1312-1322. DOI: 10.1111/hepr.12500. [22] MEES ST, MARDIN WA, SIELKER S, et al. Involvement of CD40 targeting miR-224 and miR-486 on the progression of pancreatic ductal adenocarcinomas[J]. Ann Surg Oncol, 2009, 16(8): 2339-2350. DOI: 10.1245/s10434-009-0531-4. [23] WANG Y, ZHENG D, TAN Q, et al. Nanopore-based detection of circulating microRNAs in lung cancer patients[J]. Nat Nanotechnol, 2011, 6(10): 668-674. DOI: 10.1038/nnano.2011.147. 期刊类型引用(8)
1. 蒋丽莎,马洪升. 日归手术——中国日间手术的升华. 华西医学. 2022(02): 161-164 .  百度学术
百度学术2. 雷甜甜,梁鹏,马洪升,蒋丽莎. 四川大学华西医院日归手术管理实践. 广东医学. 2022(10): 1222-1228 .  百度学术
百度学术3. 方宁波,陈伟力. 腹腔镜胆囊切除术日间手术围术期指标观察及患者延迟出院原因分析. 江西医药. 2022(10): 1524-1527 .  百度学术
百度学术4. 刘力玮,郑亚民,刘东斌,王悦华,刘家峰,梁阔,江华,高崇崇,于志浩,徐大华. 日间腹腔镜胆囊切除术的可行性分析. 腹腔镜外科杂志. 2021(05): 359-362 .  百度学术
百度学术5. 杨奕夫,黎柏峰,肖艳. 日间腹腔镜胆囊切除术质量控制的研究进展. 腹腔镜外科杂志. 2020(10): 796-798+800 .  百度学术
百度学术6. 李航,赵礼金. 老年患者腹腔镜胆囊切除日间手术的安全性分析. 中国普通外科杂志. 2019(08): 1012-1017 .  百度学术
百度学术7. 石鑫,秦琦瑜,刘维丽,胥静,王威. “三镜”联合治疗胆囊结石合并肝外胆管结石手术效果及安全性研究. 临床军医杂志. 2018(06): 710-712 .  百度学术
百度学术8. 张少成,张亚辉,徐小梦. 年龄因素对上胆道疾病患者行腹腔镜胆囊切除术安全性的影响. 深圳中西医结合杂志. 2018(06): 184-186 .  百度学术
百度学术其他类型引用(1)
-




 PDF下载 ( 2657 KB)
PDF下载 ( 2657 KB)


 下载:
下载:

 百度学术
百度学术

