Expression level of serum HBV RNA in HBeAg-positive chronic hepatitis B patients at different periods and its value of measurement
-
摘要:
目的 探讨血清HBV RNA在HBeAg阳性慢性乙型肝炎(CHB)患者不同时期的表达水平及潜在临床价值。 方法 选取2019年8月—2020年12月于杭州市西溪医院肝病科门诊及住院部就诊的CHB患者61例,根据HBeAg阳性CHB患者的抗病毒治疗情况分为3组:HBeAg阳性CHB[HBeAg(+)、HBV DNA(+)]未治患者(A组),HBeAg血清学转换前[HBeAg(+)、HBV DNA(-)]经治患者(B组),HBeAg血清学转换后[HBeAg(-)、HBV DNA(-)]经治患者(C组),检测不同时期患者外周血HBV RNA载量,并分析其与HBsAg、HBV D NA的相关性。符合正态分布的计量资料2组间比较采用t检验;非正态分布的计量资料2组间比较采用Mann-Whitney U检验;计数资料2组间比较采用χ2检验;采用Pearson或Spearman相关分析描述两变量间的相关性。 结果 3组患者HBV RNA阳性率分别为100%(22/22)、88.2%(15/17)、22.7%(6/22)。HBeAg阳性CHB未治患者HBV RNA与HBsAg、HBV DNA均呈正相关(r值分别为0.612、0.922,P值均<0.01),在HBeAg血清学转换前和HBeAg血清学转换后的经治患者中,HBV RNA与HBsAg无相关性。经治HBeAg阳性组的HBV RNA、HBsAg均高于HBeAg阴性组,差异均有统计学意义(Z值分别为-4.44、-2.41,P值均<0.05)。HBV DNA阳性组HBV RNA显著高于HBV DNA阴性组,差异有统计学意义(Z=-6.16, P<0.01)。 结论 CHB患者经核苷(酸)类药物抗病毒治疗HBV DNA阴转后仍有部分患者能检测出血清HBV RNA;且HBV RNA只能来自肝内cccDNA, 因此HBV RNA比HBV DNA更能反映肝内病毒复制活性,对CHB人群管理有着一定的临床价值。 Abstract:Objective To investigate the expression level and potential clinical value of serum HBV RNA in HBeAg-positive chronic hepatitis B (CHB) patients at different periods. Methods A total of 61 CHB patients who attended the outpatient and inpatient services of Department of Hepatology, Hangzhou Xixi Hospital, from August 2019 to December 2020 were enrolled, and according to the antiviral therapy for HBeAg-positive CHB patients, they can be divided into group A with untreated HBeAg-positive CHB (HBeAg+ and HBV DNA+) patients, group B with treatment-experienced patients before HBeAg seroconversion (HBeAg+ and HBV DNA-), and group C with treatment-experienced patients after HBeAg seroconversion (HBeAg- and HBV DNA-). Peripheral blood HBV RNA load was measured at different periods, and its correlation with HBsAg and HBV DNA was analyzed. The t-test was used for comparison of normally distributed continuous data between groups, and the Mann-Whitney U test was used for comparison of non-normally distributed continuous data between two groups; the chi-square test was used for comparison of categorical data between groups; a Pearson or Spearman correlation analysis was used to describe the correlation between two variables. Results The positive rates of HBV RNA in these three groups were 100% (22/22), 88.2% (15/17), and 22.7% (6/22), respectively. In group A, HBV RNA was positively correlated with HBsAg and HBV DNA (r=0.612 and 0.922, both P < 0.01), while in groups B and C, there was no correlation between HBV RNA and HBsAg. Group B had significantly higher levels of HBV RNA and HBsAg than group C (Z=-4.44 and -2.41, both P < 0.05). The HBV DNA-positive group had a significantly higher level of HBV RNA than the HBV DNA-negative group (Z=-6.16, P < 0.01). Conclusion After HBV DNA clearance achieved by antiviral therapy with nucleos(t)ide analogues in CHB patients, serum HBV RNA can still be detected in some of these patients. Since HBV RNA only comes from cccDNA in the liver, it can better reflect viral replication activity in the liver than HBV DNA and thus has a certain clinical value in the management of CHB patients. -
Key words:
- Hepatitis B, Chronic /
- Hepatitis B virus /
- RNA, Viral /
- DNA, Viral /
- Hepatitis B e Antigens
-
全球有超过2.4亿慢性HBV感染者,若不及时进行有效、规范的治疗,15%~40%的患者会进展为肝硬化,最终导致肝衰竭和肝细胞癌(HCC)[1]。虽然乙型肝炎疫苗的接种使HBV感染的患病率逐年下降,但多数亚洲地区仍归为中至高流行区。我国HBsAg流行率为5%~6%,但因为人口基数大,所以仍存在许多的慢性HBV感染者,其中需要治疗的慢性乙型肝炎(CHB)患者约有2000万~3000万[2-3];因此慢性HBV感染依然是我国的重大公共卫生问题。当前用于抗病毒治疗的核苷(酸)类似物(nucleos(t)ide analogues, NAs)虽可有效抑制病毒复制,延缓疾病进展,但对肝细胞核内的共价闭合环状DNA(covalently closed circular DNA,cccDNA)均无明确作用,使得病毒无法完全清除。cccDNA作为HBV复制的原始模板,检测肝内cccDNA水平是评价抗病毒疗效及停止治疗的重要指标[4-5],由于肝活检为侵入性操作,cccDNA在肝组织内分布不均,松弛环状双链DNA(relaxed circularDNA,rcDNA)的存在影响cccDNA含量等因素导致cccDNA检测难以在临床广泛开展[6-7]。因此需要寻找能反映肝内cccDNA活性且方便操作的临床替代指标。近年来HBV RNA作为新的血清学标志物被广泛提出,因为其只能来自肝内cccDNA, 所以能更好的反映HBV转录活性,本研究主要探讨血清HBV RNA在HBeAg阳性CHB患者不同时期的表达水平及检测价值。
1. 资料与方法
1.1 研究对象
收集2019年8月—2020年12月在杭州市西溪医院肝病科门诊及住院部诊治的CHB患者,纳入标准:(1)诊断符合《慢性乙型肝炎防治指南(2019年版)》[8];(2)准备接受或已接受NAs抗病毒治疗。排除标准:(1)合并HAV、HCV、巨细胞病毒等其他嗜肝病毒感染;(2)合并HIV感染;(3)HCC患者;(4)代谢性肝病、自身免疫性肝病及近期使用肝损伤性药物者;(5)合并其他系统恶性肿瘤或严重疾病者;(6)研究者认为不适合入组的其他情况。
1.2 实验室检测
本研究使用的HBV RNA定量检测试剂盒由湖南圣湘科技有限公司提供,检测原理通过逆转录HBV核酸中的pgRNA(经DNA酶消化),利用针对HBV pgRNA序列设计的一组特异性引物与荧光探针,配以PCR反应液,在荧光定量PCR仪上,应用一步法RT实时荧光定量PCR检测技术,通过荧光信号的变化实现HBV pgRNA的定量检测,HBV RNA检测下限为50拷贝/mL。应用化学发光免疫分析法在美国雅培Alinity i全自动化学发光免疫分析仪上行HBV血清学标志物检测;用HBV DNA定量检测试剂盒,在ABI7500荧光定量PCR仪上行HBV DNA检测;HBV DNA检测下限为30 IU/mL;用Beckman Coulter AU5831全自动生化分析仪检测ALT(正常范围9~50 U/L)、AST(正常范围15~40 U/L)。
1.3 伦理学审查
本研究经杭州市西溪医院伦理委员会批准, 批号:2019年(科)伦审第21号,所有患者均签署知情同意书。
1.4 统计学方法
采用SPSS 25.0进行统计学处理,正态分布的计量资料用x ±s表示,2组间比较采用独立样本t检验;非正态分布的计量资料用M(P25~P75)表示,2组间比较采用Mann-Whitney U检验;计数资料组间比较采用χ2检验;采用Pearson或Spearman相关分析描述两变量间的相关性。P<0.05为差异有统计学意义。
2. 结果
2.1 一般资料
本研究共纳入61例CHB患者,平均年龄(39.95±9.88)岁。按HBeAg及HBV DNA状态分为3组:HBeAg阳性CHB[HBeAg(+)、HBV DNA(+)]未治患者,HBeAg血清学转换前[HBeAg(+)、HBV DNA(-)]经治患者,HBeAg血清学转换后[HBeAg(-)、HBV DNA(-)]经治患者(表 1)。
表 1 纳入患者分组情况及基线资料组别 例数 男 女 年龄(岁) HBeAg(+)、HBV DNA(+) 22 13 9 35.36±7.55 HBeAg(+)、HBV DNA(-) 17 11 6 39.35±7.98 HBeAg(-)、HBV DNA(-) 22 13 9 45.00±11.16 2.2 不同时期血清HBV RNA的表达水平
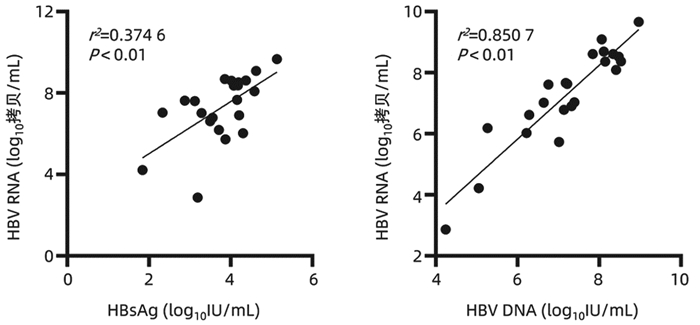
HBeAg阳性CHB未治患者血清HBV RNA阳性率100%(22/22),HBV RNA载量最大值为9 log10拷贝/mL,平均为7 log10拷贝/mL;HBeAg血清学转换前经治患者血清HBV RNA阳性率88.2%(15/17),HBV RNA载量最大值为5 log10拷贝/mL,平均4 log10拷贝/mL;HBeAg血清学转换后经治患者血清HBV RNA阳性率22.7%(6/22),HBV RNA载量最大值为4 log10拷贝/mL。
2.3 经治HBeAg阳性组与HBeAg阴性组血清学指标比较
经治HBeAg阳性组的HBV RNA阳性率显著高于HBeAg阴性组,差异有统计学意义(P<0.001), 2组间HBV RNA、HBsAg水平比较,差异均有统计学意义(P值均<0.05)(表 2)。
表 2 经治患者HBeAg阳性组与HBeAg阴性组临床资料比较项目 HBeAg阳性组(n=17) HBeAg阴性组(n=22) 统计值 P值 男/女(例) 11/6 13/9 0.753 年龄(岁) 39.35±7.98 45.00±11.16 t=-1.77 0.086 HBV RNA阳性[例(%)] 15(88.2) 6(27.3) <0.001 HBV RNA(log10拷贝/mL) 4.05(3.01~5.18) 1.40(1.40~1.83) Z=-4.44 <0.001 HBsAg(log10IU/mL) 3.24(2.86~3.50) 2.76(2.07~3.35) Z=-2.41 0.016 ALT(U/L) 22.24±10.83 25.09±11.76 t=-0.78 0.442 AST(U/L) 23.00±4.48 23.9±14.23 t=-0.65 0.521 2.4 HBV DNA阳性组与HBV DNA阴性组血清学指标比较
CHB患者NAs治疗实现HBV DNA阴转时,肝功能复常,ALT、AST水平降低,HBV RNA、HBsAg水平也均有所降低(P值均<0.001)(表 3)。对于治疗后HBV DNA阴转的CHB患者,Spearman相关分析示,血清HBV RNA与血清HBsAg无相关性(P=0.091)。
表 3 HBV DNA阳性组与HBV DNA阴性组临床资料比较项目 HBV DNA阳性组(n=22) HBV DNA阴性组(n=39) 统计值 P值 男/女(例) 13/9 24/15 χ2=0.035 0.851 年龄(岁) 35.36±7.55 42.54±10.18 t=-2.88 0.005 HBV RNA(log10拷贝/mL) 7.62(6.51~8.55) 1.94(1.40~4.04) Z=-6.16 <0.001 HBsAg(log10IU/mL) 3.94(3.26~4.23) 2.90(2.61~3.44) Z=-4.07 <0.001 ALT(U/L) 249.50(114.25~664.50) 20.00(16.00~31.00) Z=-6.45 <0.001 AST(U/L) 106.00(63.75~418.75) 23.00(21.00~27.00) Z=-6.45 <0.001 HBV DNA(log10IU/ml) 7.21±1.23 - 2.5 不同时期HBV RNA与HBsAg、HBV DNA的相关性
在HBeAg阳性CHB未治患者中,HBV RNA与HBsAg(r=0.612,P<0.01)、HBV DNA(r=0.922, P<0.01)均具有较强的相关性(图 1);在HBeAg血清学转换前和HBeAg血清学转换后的经治患者中,HBV RNA与HBsAg无相关性(P>0.05);3组患者HBV RNA与年龄、ALT、AST均无相关性(P值均>0.05)。
3. 讨论
CHB是导致肝硬化、肝衰竭、HCC的主要危险因素,每年约有80万人死于HBV感染相关性疾病[9],因此如何有效的管理CHB患者仍是当前临床工作的热点和难点。随着对HBV RNA的研究深入,已知HBV感染患者血清中的HBV RNA就是前基因组RNA(pgRNA),即3.5 kb mRNA;pgRNA是HBV复制的中间产物,利用cccDNA作为模板在病毒核衣壳内转录,最后释放完整的子代病毒,因此血清HBV RNA与HBsAg不同,其只能来自于肝内cccDNA;而NAs是通过取代HBV复制过程中聚合酶区的核苷来抑制病毒复制,并不能影响cccDNA转录生成mRNA,由此可以推断HBV RNA与肝内cccDNA相关,检测外周血HBV RNA水平可以反映肝内cccDNA的转录活性。因此相比于HBsAg、HBV DNA等传统的血清学指标,血清HBV RNA水平可以更好的反映肝内病毒复制水平,其能作为CHB患者管理的新型标志物[10-11]。
HBeAg阳性CHB患者在接受抗病毒药物治疗后会逐步实现HBV DNA阴转、HBeAg血清学转换等目标,本研究通过观察HBeAg阳性的CHB患者不同时期的HBV RNA表达水平,发现未治疗的22例CHB患者血清中均能检测出较高的血清HBV RNA水平;39例治疗后HBV DNA阴转的CHB患者中仍有21例可以检测出HBV RNA,这表明在评估病毒复制方面HBV RNA比HBV DNA更灵敏,即使是在HBV DNA持续低于检测下限且发生HBeAg血清学转换也有部分患者血清HBV RNA阳性,意味HBeAg的消失只能表示病毒复制减弱,肝内cccDNA仍可能存在低水平的转录。
对HBeAg阳性CHB患者而言,发生HBeAg血清学转换是比较满意的终点,是发生HBsAg阴转的基本条件。本文通过比较治疗后HBV DNA阴转的HBeAg阳性组与HBeAg阴性组,发现HBeAg阳性组HBV RNA阳性率显著高于HBeAg阴性组(88.2% vs 27.3%),两组间HBV RNA、HBsAg水平差异也具有统计学意义,因HBV RNA只能来自于肝内cccDNA, 提示HBeAg阳性CHB患者肝内cccDNA转录活性更高。因此临床上对HBeAg阳性的CHB患者要努力实现HBeAg血清学转换。
通过分析HBV DNA阳性组与HBV DNA阴性组患者,发现接受NAs治疗后HBV DNA、HBV RNA均会发生下降,这是因为NAs类药物抑制pgRNA的逆转录,致使DNA合成受阻,而长时间接受NAs治疗使rcDNA的形成受到抑制,影响cccDNA池的回补,被感染的肝细胞数量减少,进一步导致HBV RNA的生成也减少;另外接受NAs治疗的CHB人群在病毒清除的过程中可能会促进机体免疫应答的能力从而减少cccDNA池。但是在临床上发现即使CHB患者经过长期NAs抗病毒治疗,能实现完全治愈的患者极为罕见,可能是只需要极少部分逆转录酶存在活性就能对cccDNA池进行补充,因此对大部分CHB患者而言需要长期口服NAs抗病毒治疗。
本研究发现,未接受治疗的CHB患者HBV RNA与HBsAg(r=0.612,P<0.01)、HBV DNA(r=0.922, P<0.01)均具有较强的正相关,这种血清学间良好的相关性有助于基层医院或者医疗资源匮乏地区,使用HBsAg定量水平反映HBeAg阳性CHB患者体内病毒复制水平,而另外两组HBV DNA阴性的CHB患者HBV RNA与HBsAg无相关性,这与Mak等[12]的研究结果一致,随着治疗时间的延长,HBV RNA与HBsAg相关性逐渐下降,从一定程度反映了治疗后的HBsAg水平不能准确反映肝内cccDNA转录活性。
综上所述,对未接受抗病毒治疗的CHB患者,血清HBV RNA与其他血清学标志物有一定的相关性,对NAs治疗后HBV DNA阴转的患者有望使用HBV RNA继续监测肝内病毒复制活性,为CHB患者长期抗病毒治疗提供依据。但本研究样本量较少,相关结论有待进一步证实,后续可以在未治疗组继续纳入合适患者并进行前瞻性队列研究,观察HBeAg阳性CHB患者在NAs治疗过程中HBV RNA的动态变化。
-
表 1 纳入患者分组情况及基线资料
组别 例数 男 女 年龄(岁) HBeAg(+)、HBV DNA(+) 22 13 9 35.36±7.55 HBeAg(+)、HBV DNA(-) 17 11 6 39.35±7.98 HBeAg(-)、HBV DNA(-) 22 13 9 45.00±11.16 表 2 经治患者HBeAg阳性组与HBeAg阴性组临床资料比较
项目 HBeAg阳性组(n=17) HBeAg阴性组(n=22) 统计值 P值 男/女(例) 11/6 13/9 0.753 年龄(岁) 39.35±7.98 45.00±11.16 t=-1.77 0.086 HBV RNA阳性[例(%)] 15(88.2) 6(27.3) <0.001 HBV RNA(log10拷贝/mL) 4.05(3.01~5.18) 1.40(1.40~1.83) Z=-4.44 <0.001 HBsAg(log10IU/mL) 3.24(2.86~3.50) 2.76(2.07~3.35) Z=-2.41 0.016 ALT(U/L) 22.24±10.83 25.09±11.76 t=-0.78 0.442 AST(U/L) 23.00±4.48 23.9±14.23 t=-0.65 0.521 表 3 HBV DNA阳性组与HBV DNA阴性组临床资料比较
项目 HBV DNA阳性组(n=22) HBV DNA阴性组(n=39) 统计值 P值 男/女(例) 13/9 24/15 χ2=0.035 0.851 年龄(岁) 35.36±7.55 42.54±10.18 t=-2.88 0.005 HBV RNA(log10拷贝/mL) 7.62(6.51~8.55) 1.94(1.40~4.04) Z=-6.16 <0.001 HBsAg(log10IU/mL) 3.94(3.26~4.23) 2.90(2.61~3.44) Z=-4.07 <0.001 ALT(U/L) 249.50(114.25~664.50) 20.00(16.00~31.00) Z=-6.45 <0.001 AST(U/L) 106.00(63.75~418.75) 23.00(21.00~27.00) Z=-6.45 <0.001 HBV DNA(log10IU/ml) 7.21±1.23 - -
[1] TANG L, COVERT E, WILSON E, et al. Chronic hepatitis B infection: A review[J]. JAMA, 2018, 319(17): 1802-1813. DOI: 10.1001/jama.2018.3795. [2] LIU J, LIANG W, JING W, et al. Countdown to 2030: Eliminating hepatitis B disease, China[J]. Bull World Health Organ, 2019, 97(3): 230-238. DOI: 10.2471/BLT.18.219469. [3] CUI FQ. Achievements in prevention and control of viral hepatitis since the founding of the people's Republic of China[J]. Int J Virol, 2019, 26(5): 289-292. DOI: 10.3760/cma.j.issn.1673-4092.2019.05.001.崔富强. 中国建国以来病毒性肝炎的防控成就[J]. 国际病毒学杂志, 2019, 26(5): 289-292. DOI: 10.3760/cma.j.issn.1673-4092.2019.05.001. [4] NASSAL M. HBV cccDNA: Viral persistence reservoir and key obstacle for a cure of chronic hepatitis B[J]. Gut, 2015, 64(12): 1972-1984. DOI: 10.1136/gutjnl-2015-309809. [5] KUMAR R, PÉREZ-DEL-PULGAR S, TESTONI B, et al. Clinical relevance of the study of hepatitis B virus covalently closed circular DNA[J]. Liver Int, 2016, 36(Suppl 1): 72-77. DOI: 10.1111/liv.13001. [6] ROUSSELET MC, MICHALAK S, DUPRÉ F, et al. Sources of variability in histological scoring of chronic viral hepatitis[J]. Hepatology, 2005, 41(2): 257-264. DOI: 10.1002/hep.20535. [7] LU FM, DOU XG, ZHANG WH, et al. Clinical significance of serum HBV RNA measurement in chronic hepatitis B patients[J]. J Clin Hepatol, 2018, 34(5): 934-938. DOI: 10.3969/j.issn.1001-5256.2018.05.005.鲁凤民, 窦晓光, 张文宏, 等. 慢性乙型肝炎患者血清HBV RNA检测的临床意义[J]. 临床肝胆病杂志, 2018, 34(5): 934-938. DOI: 10.3969/j.issn.1001-5256.2018.05.005. [8] Chinese Society of Infectious Diseases, Chinese Medical Association; Chinese Society of Hepatology, Chinese Medical Association. Guidelines for the prevention and treatment of chronic hepatitis B (version 2019)[J]. J Clin Hepatol, 2019, 35(12): 2648-2669. DOI: 10.3969/j.issn.1001-5256.2019.12.007.中华医学会感染病学分会, 中华医学会肝病学分会. 慢性乙型肝炎防治指南(2019年版)[J]. 临床肝胆病杂志, 2019, 35(12): 2648-2669. DOI: 10.3969/j.issn.1001-5256.2019.12.007. [9] ROTH GA, ABATE D, ABATE KH, et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: A systematic analysis for the Global Burden of Disease Study 2017[J]. Lancet, 2018, 392(10159): 1736-1788. DOI: 10.1016/S0140-6736(18)32203-7. [10] GIERSCH K, ALLWEISS L, VOLZ T, et al. Serum HBV pgRNA as a clinical marker for cccDNA activity[J]. J Hepatol, 2017, 66(2): 460-462. DOI: 10.1016/j.jhep.2016.09.028. [11] TSUGE M, MURAKAMI E, IMAMURA M, et al. Serum HBV RNA and HBeAg are useful markers for the safe discontinuation of nucleotide analogue treatments in chronic hepatitis B patients[J]. J Gastroenterol, 2013, 48(10): 1188-1204. DOI: 10.1007/s00535-012-0737-2. [12] MAK LY, CLOHERTY G, WONG DK, et al. HBV RNA profiles in patients with chronic hepatitis B under different disease phases and antiviral therapy[J]. Hepatology, 2021, 73(6): 2167-2179. DOI: 10.1002/hep.31616. -




 PDF下载 ( 1906 KB)
PDF下载 ( 1906 KB)

 下载:
下载:

 下载:
下载: