419例药物性肝损伤患者临床特点与自身抗体特征分析
DOI: 10.3969/j.issn.1001-5256.2022.01.023
Clinical features and autoantibody characteristics of patients with drug-induced liver injury: An analysis of 419 cases
-
摘要:
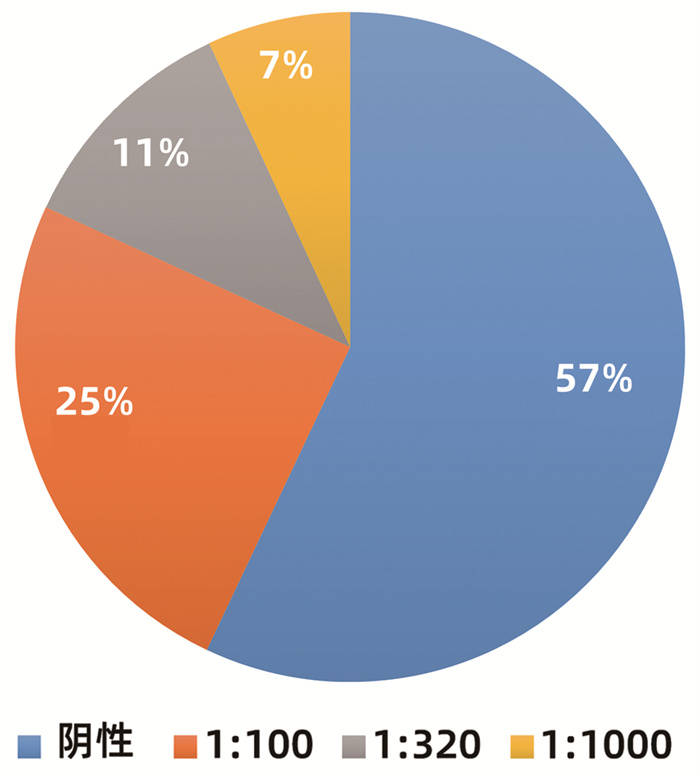
目的 研究药物性肝损伤(DILI)患者的临床特点与自身抗体特征。 方法 回顾性分析2014年9月—2018年9月首都医科大学附属北京地坛医院收治的肝功能异常且通过RUCAM评分临床诊断为DILI的患者,收集入院时的基线肝功能、肾功能、血常规、免疫五项、自身抗体、肝穿刺活检结果。计量资料满足正态性2组间比较使用t检验;计量资料不满足正态性2组间比较使用Mann-Whitney U检验。采用卡方检验研究不同性别、肝损伤类型之间的自身抗体检出率是否存在差异;用logistic回归方法分析自身抗体与性别、年龄、损伤类型之间是否存在回归关系;以各项基线化验结果做自变量,ANA的滴度分级作为因变量做有序logistic回归分析。 结果 共入组419例DILI患者,中位年龄47(35~55)岁,其中男性占32.5%(136/419),女性占67.5%(283/419);肝细胞型有88例(21.5%),混合型87例(21.2%),胆汁淤积型235例(57.3%)。自身抗体检出率为50.6%(212/419),其中抗核抗体检出率为42.9%(180/419),抗体滴度以1∶ 100为主(104/180)。不同性别(χ2=2.658,P=0.103)与不同损伤类型(χ2= 0.859,P=0.651)之间的自身抗体检出率差异均无统计学意义。二元logistics回归提示性别、年龄和损伤类型与自身抗体之间均不存在回归关系(P值均>0.05)。自身抗体阳性组和阴性组患者的PTA和INR存在显著差异(t=2.161,P=0.031;Z=-3.010,P=0.003)。有序logistics回归提示INR和IgG与ANA分级有相关性(OR=3.101,P=0.040; OR=1.043,P=0.014)。 结论 DILI患者自身抗体检出率较高,但自身抗体检出率与性别、损伤类型、年龄无关,自身抗体阳性与阴性患者之间的PTA和INR存在差异,INR和IgG水平与ANA抗体滴度有相关性。 Abstract:Objective To investigate the clinical features and autoantibody characteristics of patients with drug-induced liver injury (DILI). Methods A retrospective analysis was performed for the patients with abnormal liver function who were admitted to Beijing Ditan Hospital, Capital Medical University, from September 2014 to September 2018 and were diagnosed with DILI based on RUCAM score, and related data on admission were collected, including baseline liver function, renal function, routine blood test results, five immune indices, autoantibody, and liver biopsy results. The t-test was used for comparison of normally distributed continuous data between two groups, and the Mann-Whitney U test was used for comparison of non-normally distributed continuous data between two groups; the chi-square test was used to compare the detection rate of autoantibody between the patients with different sexes or types of liver injury. A logistic regression analysis was used to investigate whether autoantibody had a regression relationship with sex, age, and type of injury, and an ordinal logistic regression analysis was performed with baseline laboratory results as independent variables and anti-nuclear antibody (ANA) titer as the dependent variable. Results A total of 419 patients with DILI were enrolled in the study, with a median age of 47 (35-55) years, among whom male patients accounted for 32.5% (136/419) and female patients accounted for 67.5% (283/419). Among these 419 patients, 88 (21.5%) had hepatocellular-type liver injury, 87 (21.2%) had mixed-type liver injury, and 235 (57.3%) had cholestasis-type liver injury. The detection rate of autoantibodies was 50.6% (212/419), and the detection rate of ANA was 42.9% (180/419), with a titer of mainly 1∶ 100 (104/180). There was no significant difference in the detection rate of autoantibodies between the patients with different sexes (χ2=2.658, P=0.103) or different types of injury (χ2=0.859, P=0.651). The binary logistic regression analysis showed that autoantibody did not have a regression relationship with sex, age, and type of injury (all P > 0.05) There were significant differences in prothrombin time activity (PTA) and international normalized ratio (INR) between the positive autoantibody group and the negative autoantibody group (t=2.161, P=0.031; Z=-3.010, P=0.003). The ordinal logistic regression analysis showed that INR (odds ratio [OR]=3.101, P=0.040) and IgG (OR=1.043, P=0.014) were associated with ANA grade. Conclusion There is a relatively high detection rate of autoantibodies in patients with DILI, and the detection rate of autoantibodies is not associated with sex, age, or type of injury. There are differences in PTA and INR between autoantibody-positive patients and autoantibody-negative patients, and the levels of INR and IgG are correlated with antibody titer. -
Key words:
- Drug-Induced Liver Injury /
- Autoantibodies /
- Immunoglobulin G
-
表 1 自身抗体分布情况
抗体种类 例数 抗体种类 例数 ANA 180 CENP 8 AMA 2 nRNP/Sm 2 ANCA 15 RO-52 36 dsDNA 10 SSA 17 LKM 4 SSB 10 PCNA 5 抗Sm 8 SMA 2 表 2 logistics回归分析结果
指标 B值 Wald OR 95%CI P值 性别 -0.318 0.219 2.098 0.728 0.474~1.119 0.148 年龄 0.002 0.008 0.104 1.002 0.988~1.017 0.747 损伤类型 肝细胞型 Ref 混合型 0.064 0.251 0.064 1.066 0.651~1.744 0.800 胆汁淤积型 0.185 0.255 0.529 1.203 0.731~1.982 0.467 表 3 自身抗体阳性组与阴性组基线情况
指标 所有患者(n=419) 阴性组(n=207) 阳性组(n=212) 统计值 P值 女性(例) 283 132 151 χ2=2.658 0.103 年龄(岁) 47(35~55) 46(35~54) 47(35~56) Z=-0.871 0.384 ALT (U/L) 365.0(121.0~751.0) 401.0(108.1~802.0) 342.5(133.5~716.3) Z=-0.478 0.633 AST (U/L) 206.0(93.0~446.4) 200.2(87.4~446.4) 216.9(101.3~445.3) Z=-0.826 0.409 TBil (μmol/L) 33.2(6.9~109.3) 36.5(18.0~117.0) 28.5(16.0~101.7) Z=-1.641 0.101 DBil (μmol/L) 21.0(16.0~83.2) 26.0(7.5~88.0) 16.0(6.3~73.2) Z=-1.241 0.214 ALP (U/L) 129.4(96.0~172.9) 134.4(96.5~177.9) 127.0(95.5~170.0) Z=-0.749 0.454 BUN (mmol/L) 3.9(3.1~5.0) 4.05(3.05~5.13) 3.72(3.06~4.74) Z=-1.636 0.102 CREA (μmol/L) 58.7(51.0~67.1) 59.0(51.1~68.8) 58.0(51.0~65.6) Z=-1.526 0.127 PTA(%) 89.00±21.82 91.35±21.76 86.74±21.69 t=2.161 0.031 INR 1.03(0.97~1.17) 1.02(0.96~1.12) 1.05(0.99~1.19) Z=-3.010 0.003 WBC (×109/L) 4.92(4.08~6.03) 4.97(4.11~6.15) 4.88(4.07~5.93) Z=-0.923 0.356 Hb (g/L) 131.0(120.0~237.0) 131.0(121.9~141.0) 131.0(119.0~141.0) Z=-0.472 0.637 IgG(g/L) 13.8(11.4~18.0) 13.4(11.4~16.7) 14.8(11.2~20.1) Z=-1.215 0.224 IgA(g/L) 2.49(1.77~3.47) 2.49(1.77~3.65) 2.49(1.78~3.33) Z=-0.138 0.890 IgM(g/L) 1.03(0.72~1.65) 1.01(0.71~1.62) 1.11(0.72~1.69) Z=-0.874 0.382 C3(g/L) 0.89(0.69~1.06) 0.93(0.71~1.06) 0.89(0.68~1.06) Z=-1.452 0.146 C4(g/L) 0.20(0.14~0.23) 0.20(0.15~0.23) 0.19(0.14~0.23) Z=-0.516 0.606 表 4 DILI患者随访1个月与基线检查结果差值对比
指标 阳性组(n=207) 阴性组(n=212) Z值 P值 基线 1个月 差值 基线 1个月 差值 ALT(U/L) 342.5(133.5~716.3) 74.7(34.4~139.6) -270.0(-665.3~-26.8) 401.0(108.1~802.0) 74.7(34.4~132.8) -299.5(-746.05~-1.5) -0.357 0.721 AST(U/L) 216.9(101.3~445.3) 80.7(39.0~242.0) -137.0(-383.1~17.8) 200.2(87.4~446.4) 77.6(44.5~235.5) -87.7(-348.4~46.2) -0.898 0.369 TBil(μmol/L) 28.5(16.0~101.7) 18.0(13.3~33.6) -10.3(-84.0~4.9) 36.5(18.0~117.0) 17.0(12.2~29.2) -13.9(-84.7~5.1) -0.158 0.874 表 5 ANA滴度多元有序logistic回归分析
变量 B值 SE Wald OR 95%CI P值 ALT 0.000 0.000 2.464 1.000 0.999~1.001 0.117 TBil -0.004 0.006 0.353 0.996 0.984~1.009 0.552 DBil 0.003 0.008 0.096 1.003 0.986~1.019 0.756 INR 1.132 0.552 4.206 3.101 1.051~9.145 0.040 IgG 0.042 0.017 6.038 1.043 1.008~1.078 0.014 -
[1] SHEN T, LIU Y, SHANG J, et al. Incidence and etiology of drug-induced liver injury in mainland China[J]. Gastroenterology, 2019, 156(8): 2230-2241. e11. DOI: 10.1053/j.gastro.2019.02.002. [2] ANDRADE RJ, CHALASANI N, BJÖRNSSON ES, et al. Drug-induced liver injury[J]. Nat Rev Dis Primers, 2019, 5(1): 58. DOI: 10.1038/s41572-019-0105-0. [3] KE L, LU C, SHEN R, et al. Knowledge mapping of drug-induced liver injury: A scientometric investigation (2010—2019)[J]. Front Pharmacol, 2020, 11: 842. DOI: 10.3389/fphar.2020.00842. [4] YU YC, MAO YM, CHEN CW, et al. CSH guidelines for the diagnosis and treatment of drug-induced liver injury[J]. Hepatol Int, 2017, 11(3): 221-241. DOI: 10.1007/s12072-017-9793-2. [5] CHALASANI N, FONTANA RJ, BONKOVSKY HL, et al. Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States[J]. Gastroenterology, 2008, 135(6): 1924-1934, 1934. e1-4. DOI: 10.1053/j.gastro.2008.09.011. [6] FONTANA RJ. Pathogenesis of idiosyncratic drug-induced liver injury and clinical perspectives[J]. Gastroenterology, 2014, 146(4): 914-928. DOI: 10.1053/j.gastro.2013.12.032. [7] YOKOI T, ODA S. Models of idiosyncratic drug-induced liver injury[J]. Annu Rev Pharmacol Toxicol, 2021, 61: 247-268. DOI: 10.1146/annurev-pharmtox-030220-015007. [8] STRAVITZ RT, LEE WM. Acute liver failure[J]. Lancet, 2019, 394(10201): 869-881. DOI: 10.1016/S0140-6736(19)31894-X. [9] CHEN QQ, LU HH, SUN FF, et al. Analysis on chronic factors of patients with drug-induced liver injury[J/CD]. Chin J Liver Dis(Electronic Version), 2020, 12(1): 68-75. DOI: 10.3969/j.issn.1674-7380.2020.01.012.陈琦琪, 陆慧慧, 孙芳芳, 等. 药物性肝损伤慢性化相关因素分析[J/CD]. 中国肝脏病杂志(电子版), 2020, 12(1): 68-75. DOI: 10.3969/j.issn.1674-7380.2020.01.012. [10] HASSAN A, FONTANA RJ. The diagnosis and management of idiosyncratic drug-induced liver injury[J]. Liver Int, 2019, 39(1): 31-41. DOI: 10.1111/liv.13931. [11] MADDUKURI VC, BONKOVSKY HL. Herbal and dietary supplement hepatotoxicity[J]. Clin Liver Dis (Hoboken), 2014, 4(1): 1-3. DOI: 10.1002/cld.364. [12] CHOW HC, SO TH, CHOI H, et al. Literature review of traditional Chinese medicine herbs-induced liver injury from an oncological perspective with RUCAM[J]. Integr Cancer Ther, 2019, 18: 1534735419869479. DOI: 10.1177/1534735419869479. [13] MANNS MP, LOHSE AW, VERGANI D. Autoimmune hepatitis—Update 2015[J]. J Hepatol, 2015, 62(1 Suppl): S100-S111. DOI: 10.1016/j.jhep.2015.03.005. [14] CHRISTEN U, HINTERMANN E. Pathogens and autoimmune hepatitis[J]. Clin Exp Immunol, 2019, 195(1): 35-51. DOI: 10.1111/cei.13203. [15] LIU X, DENG GY, YANG SF, et al. Microorganism infection and autoimmune diseases[J]. Chin J Immunol, 2020, 36(17): 2169-2173, the cover 4. DOI: 10.3969/j.issn.1000-484X.2020.17.025.刘欣, 邓国英, 杨淑凤, 等. 病原微生物感染与自身免疫病[J]. 中国免疫学杂志, 2020, 36(17): 2169-2173, 封4. DOI: 10.3969/j.issn.1000-484X.2020.17.025. [16] Chinese Society of Hepatology, Chinese Medical Association; Chinese Society of Gastroenterology, Chinese Medical Association; Chinese Society of Infectious Diseases, Chinese Medical Association. Consensus on the diagnosis and management of autoimmune hepatitis(2015)[J]. J Clin Hepatol, 2016, 32(1): 9-22. DOI: 10.3969/j.issn.1001-5256.2016.01.002.中华医学会肝病学分会, 中华医学会消化病学分会, 中华医学会感染病学分会. 自身免疫性肝炎诊断和治疗共识(2015)[J]. 临床肝胆病杂志, 2016, 32(1): 9-22. DOI: 10.3969/j.issn.1001-5256.2016.01.002. [17] SEBODE M, SCHULZ L, LOHSE AW. "Autoimmune(-Like)" drug and herb induced liver Injury: New insights into molecular pathogenesis[J]. Int J Mol Sci, 2017, 18(9): 1954. DOI: 10.3390/ijms18091954. [18] WEBER S, BENESIC A, BUCHHOLTZ ML, et al. Antimitochondrial rather than antinuclear antibodies correlate with severe drug-induced liver injury[J]. Dig Dis, 2021, 39(3): 275-282. DOI: 10.1159/000511635. [19] ALVAREZ F, BERG PA, BIANCHI FB, et al. International Autoimmune Hepatitis Group Report: Review of criteria for diagnosis of autoimmune hepatitis[J]. J Hepatol, 1999, 31(5): 929-938. DOI: 10.1016/s0168-8278(99)80297-9. [20] WEBER S, BENESIC A, ROTTER I, et al. Early ALT response to corticosteroid treatment distinguishes idiosyncratic drug-induced liver injury from autoimmune hepatitis[J]. Liver Int, 2019, 39(10): 1906-1917. DOI: 10.1111/liv.14195. [21] HISAMOCHI A, KAGE M, IDE T, et al. An analysis of drug-induced liver injury, which showed histological findings similar to autoimmune hepatitis[J]. J Gastroenterol, 2016, 51(6): 597-607. DOI: 10.1007/s00535-015-1131-7. [22] DEVARBHAVI H, SINGH R, PATIL M, et al. Outcome and determinants of mortality in 269 patients with combination anti-tuberculosis drug-induced liver injury[J]. J Gastroenterol Hepatol, 2013, 28(1): 161-167. DOI: 10.1111/j.1440-1746.2012.07279.x. [23] STEPHENS C, ROBLES-DIAZ M, MEDINA-CALIZ I, et al. Comprehensive analysis and insights gained from long-term experience of the Spanish DILI Registry[J]. J Hepatol, 2021, 75(1): 86-97. DOI: 10.1016/j.jhep.2021.01.029. [24] NORMAN BH. Drug Induced Liver Injury (DILI). Mechanisms and medicinal chemistry avoidance/mitigation strategies[J]. J Med Chem, 2020, 63(20): 11397-11419. DOI: 10.1021/acs.jmedchem.0c00524. [25] LUCENA MI, SANABRIA J, GARCÍA-CORTES M, et al. Drug-induced liver injury in older people[J]. Lancet Gastroenterol Hepatol, 2020, 5(9): 862-874. DOI: 10.1016/S2468-1253(20)30006-6. [26] SUTTI S, TACKE F. Liver inflammation and regeneration in drug-induced liver injury: Sex matters![J]. Clin Sci (Lond), 2018, 132(5): 609-613. DOI: 10.1042/CS20171313. [27] CIRULLI ET, NICOLETTI P, ABRAMSON K, et al. A missense variant in PTPN22 is a risk factor for drug-Induced liver injury[J]. Gastroenterology, 2019, 156(6): 1707-1716. e2. DOI: 10.1053/j.gastro.2019.01.034. [28] WILLIAMS DP, LAZIC SE, FOSTER AJ, et al. Predicting drug-induced liver injury with bayesian machine learning[J]. Chem Res Toxicol, 2020, 33(1): 239-248. DOI: 10.1021/acs.chemrestox.9b00264. [29] LIU A, WALTER M, WRIGHT P, et al. Prediction and mechanistic analysis of drug-induced liver injury (DILI) based on chemical structure[J]. Biol Direct, 2021, 16(1): 6. DOI: 10.1186/s13062-020-00285-0. [30] CHEN Z, MENG X, ZOU L, et al. A dual-emissive phosphorescent polymeric probe for exploring drug-induced liver injury via imaging of peroxynitrite elevation in vivo[J]. ACS Appl Mater Interfaces, 2020, 12(11): 12383-12394. DOI: 10.1021/acsami.9b18135. 期刊类型引用(10)
1. 邓江丽,蒋锐,杜飞舟,盛金平,伍发. 肝硬化合并原发性肝癌患者CT动态增强扫描变化及诊断价值. 西部医学. 2024(04): 589-593 .  百度学术
百度学术2. 罗宇君,张雅敏. 肝部分切除术后连续监测吲哚菁绿15分钟滞留率联合标准残肝体积对肝细胞癌患者肝功能不全的预测价值. 临床肝胆病杂志. 2024(06): 1162-1168 .  本站查看
本站查看3. 葛书怡,李斯曼,刘聪聪,邓雪飞,阎博华. 基于医院信息系统真实世界扶正消瘤贴对肝癌患者的临床疗效获益的回顾性队列研究. 中医临床研究. 2024(16): 31-36 .  百度学术
百度学术4. 周子惠,何娜,张思思,许皓彤,卢慧平,庄萍萍,李莉莉. 乙肝肝硬化住院患者衰弱现状及其影响因素分析. 当代护士(中旬刊). 2024(07): 153-157 .  百度学术
百度学术5. 杨旭宏,杨勇,王明磊,刘文潇,马万龙,王敏行,丁向春,王晓东. 血氨及胆碱酯酶对肝硬化伴轻微肝性脑病的早期诊断价值. 临床肝胆病杂志. 2023(02): 339-344 .  本站查看
本站查看6. 李坤,李璐璐,李楠楠,胡卫红,周建超. 成人肾移植术后血糖控制和CYP3A5基因多态性对他克莫司谷浓度的影响. 中国临床药理学与治疗学. 2023(07): 767-774 .  百度学术
百度学术7. 李莉莉,何娜,邓淑敏,淦伟强,谢日华,高志良. 乙肝肝硬化住院患者衰弱风险预测模型的构建及验证. 现代临床护理. 2023(06): 1-8 .  百度学术
百度学术8. 宋春荣,席奇,刘永刚,刘亚珠,宋粉莉,闫瑞娟,南然. 当归芍药散加味联合甘遂臌胀贴对血瘀水停证乙肝肝硬化腹水患者的临床疗效. 中成药. 2023(09): 3155-3158 .  百度学术
百度学术9. 邢恩涛,赵孟杰,金鹏飞,武振东,刘志春. 肝硬化门静脉高压TIPS术后HVPG下降相关因素. 青岛大学学报(医学版). 2023(05): 759-762 .  百度学术
百度学术10. 刘小伟,秦文羚,陈敏,陈雪梅,顾文,杨丽华. 二维超声剪切波弹性成像对慢性乙型肝炎患者肝纤维化程度的评估价值. 中国当代医药. 2022(28): 143-147 .  百度学术
百度学术其他类型引用(3)
-




 PDF下载 ( 2079 KB)
PDF下载 ( 2079 KB)

 下载:
下载:
 百度学术
百度学术

