超米兰标准肝细胞癌肝移植术后复发预测模型的建立
DOI: 10.3969/j.issn.1001-5256.2022.04.019
Development of a new model for predicting recurrence after liver transplantation for hepatocellular carcinoma beyond Milan criteria
-
摘要:
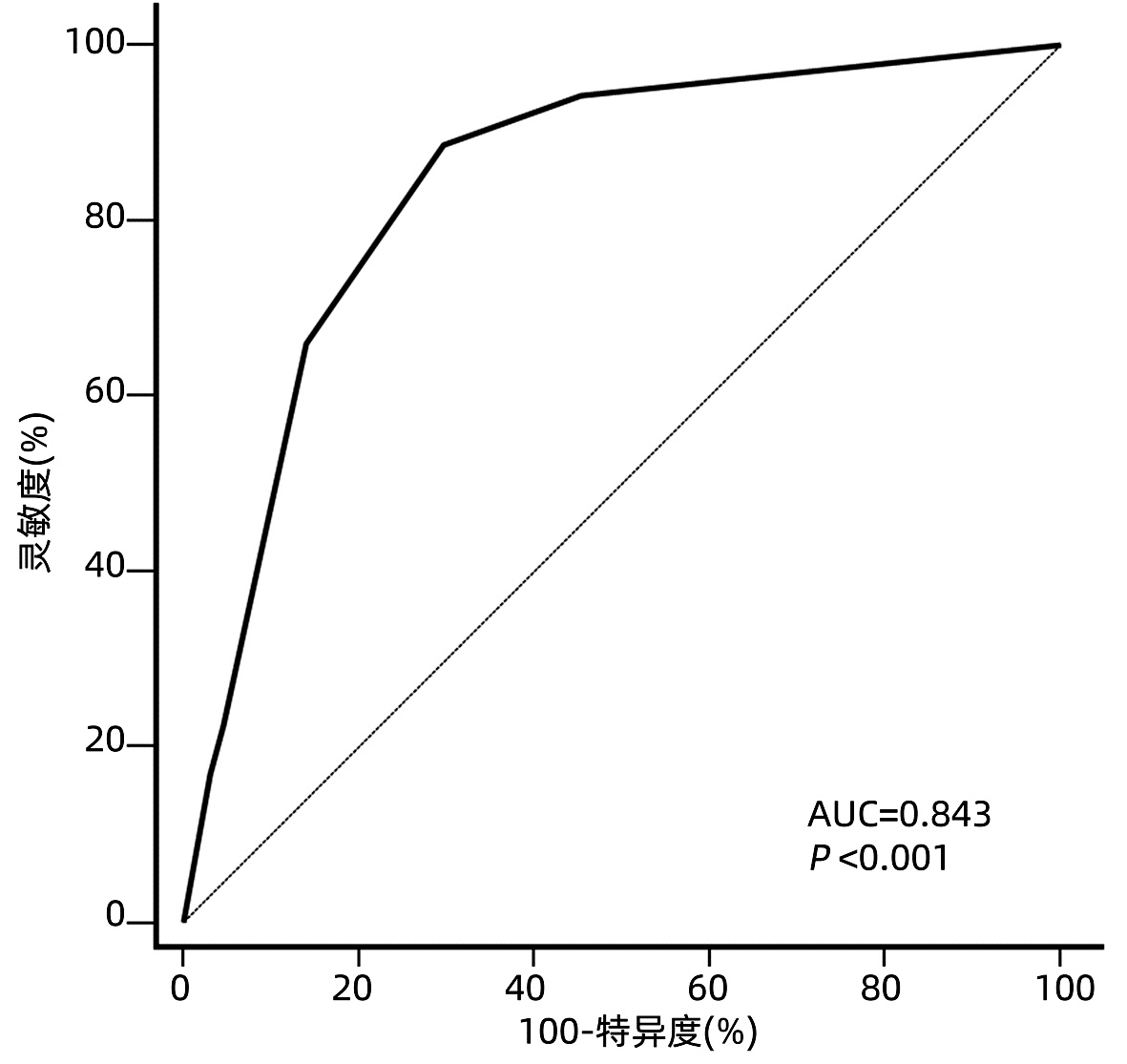
目的 根据患者术前和术后相关指标建立一个预测超米兰标准肝细胞癌(HCC)患者肝移植术后复发的模型。 方法 回顾性分析2014年8月-2018年7月在天津市第一中心医院接受首次原位肝移植的超米兰标准HCC患者的临床资料。根据随访期间肿瘤是否复发, 将患者分为复发组和未复发组。计量资料组间比较采用t检验或Mann-Whitney U检验, 计数资料组间比较使用χ2检验或Fisher精确检验。生存曲线采用Kaplan-Meier法构建, 曲线间差异采用log-rank检验。使用单因素和多因素Cox比例风险回归筛选影响术后无复发生存的危险因素。根据筛选得出的危险因素建立预测超米兰标准HCC患者肝移植术后复发的模型, 使用受试者工作特征曲线(ROC曲线)下面积来判断预测效能, Hosmer-Lemeshow检验用于评估模型的拟合优度。 结果 共纳入117例超米兰标准HCC患者, 中位随访时间24(1~74)个月。共53例(45.3%)患者术后复发, 其中52例(98.1%)于术后3年内复发, 中位复发时间为6(1~52)个月。Cox风险回归分析显示患者术前血清甲胎蛋白(AFP)>769 ng/mL, 中性粒细胞与淋巴细胞比值(NLR)>3.75以及ki67指数>0.25是患者肝移植术后无复发生存的独立危险因素(P值均 < 0.05)。根据这3个危险因素建立的评分模型ROC曲线下面积为0.843, 并且具有良好的灵敏度(88.7%)及特异度(70.3%)。根据约登指数最大化标准选取最佳截断值, 将患者分为低危组(0~1分)和高危组(1.5~4分), log-rank检验显示低危组患者术后3年、5年无复发生存率(84.1%、72.0%)明显高于高危组(10.9%、10.9%)(χ2=29.425, P < 0.001)。 结论 超米兰标准肝癌肝移植要慎重进行, 本研究根据患者术前AFP、NLR以及ki67指数建立的预测模型有助于更精准地把握此类患者的肝移植指征。 Abstract:Objective To develop a new model for predicting recurrence after liver transplantation for hepatocellular carcinoma (HCC) beyond Milan criteria based on related preoperative and postoperative indicators. Methods A retrospective analysis was performed for the clinical data of the patients with HCC beyond Milan criteria who underwent orthotopic liver transplantation for the first time in Tianjin First Central Hospital from August 2014 to July 2018, and according to the presence or absence of recurrence during follow-up, the patients were divided into recurrence group and no-recurrence group. The t-test or the Mann-Whitney U test was used for comparison of continuous data between groups, and the chi-square test or the Fisher's exact test was used for comparison of categorical data between groups. The Kaplan-Meier method was used to plot survival curves, and the log-rank test was used for comparison of survival curves. Univariate and multivariate Cox proportional hazards regression analyses were used to identify the risk factors for recurrence-free survival after surgery. A new model was developed for recurrence after liver transplantation in the patients with HCC beyond Milan criteria based on the risk factors identified. The area under the receiver operating characteristic curve (AUC) was used to evaluate predictive performance, and the Hosmer-Lemeshow test was used to assess the goodness of fit of the model. Results A total of 117 patients with HCC beyond Milan criteria were enrolled in this study, with a median follow-up time of 24 (1-74) months. A total of 53 patients (45.3%) experienced recurrence after surgery, among whom 52 (98.1%) had recurrence within 3 years after surgery, with a median time to recurrence of 6 (1-52) months. The Cox proportional hazards regression analysis showed that preoperative serum alpha-fetoprotein (AFP) >769 ng/mL, neutrophil-lymphocyte ratio (NLR) >3.75, and ki67 index >0.25 were independent risk factors for recurrence-free survival after liver transplantation. The model established based on these three risk factors had an AUC of 0.843, with good sensitivity (88.7%) and specificity (70.3%). The optimal cut-off value was selected according to the maximization of Youden index, and then the patients were divided into low-risk group (0-1 point) and high-risk group (1.5-4 points). The log-rank test showed that the low-risk group had significantly higher 3-and 5-year recurrence-free survival rates than the high-risk group (84.1%/72.0% vs 10.9%/10.9%, χ2=29.425, P < 0.001). Conclusion Liver transplantation for HCC beyond Milan criteria should be performed with caution, and the predictive model established based on preoperative AFP, NLR, and ki67 index can accurately assess the indication for liver transplantation in such patients. -
Key words:
- Carcinoma, Hepatocellular /
- Liver Transplantation /
- Recurrence /
- Models, Theoretical
-
表 1 117例患者的一般资料
Table 1. The general data of 117 patients
项目 复发组(n=53) 未复发组(n=64) 统计值 P值 年龄(岁) 52.02±8.28 56.16±10.11 t=2.388 0.019 男/女(例) 45/8 59/5 χ2=1.556 0.212 肝硬化(有/无,例) 47/6 61/3 0.296 病因(乙型肝炎/非乙型肝炎,例) 48/5 54/10 χ2=0.994 0.319 术前局部区域治疗(有/无,例) 34/19 41/23 χ2=0.001 1.000 卫星灶(有/无,例) 15/38 9/55 χ2=3.605 0.058 门静脉癌栓(有/无,例) 23/30 17/47 χ2=3.651 0.056 肿瘤个数(单个/多个,例) 12/41 16/48 χ2=0.089 0.766 肿瘤最大直径(≤5 cm/>5 cm,例) 21/32 48/16 χ2=14.997 <0.001 微血管侵犯(有/无,例) 12/41 32/32 χ2=9.248 0.002 肿瘤分化程度(高/中/低,例) 1/39/13 0/54/10 χ2=2.801 0.246 术前AFP(>769 ng/mL/≤769 ng/mL,例) 31/22 14/50 χ2=16.422 <0.001 MELD评分 7.80(6.05~18.39) 8.36(6.09~14.16) Z=-0.134 0.893 NLR(>3.75/≤3.75,例) 28/25 8/56 χ2=22.137 <0.001 免疫组化 AFP(-/+,例) 31/22 47/17 χ2=2.915 0.088 CK19(-/+,例) 45/8 59/5 χ2=1.556 0.212 CK(-/+,例) 5/48 9/55 χ2=0.590 0.443 P53(-/+,例) 25/28 32/32 χ2=0.093 0.760 GS(-/+,例) 6/47 12/52 χ2=1.229 0.268 VEGF(-/+,例) 12/41 14/50 χ2=0.010 0.921 EGFR(-/+,例) 9/44 9/55 χ2=0.190 0.663 GPC3(-/+,例) 5/48 13/51 χ2=2.636 0.104 KLI(>0.25/≤0.25,例) 35/18 18/46 χ2=16.817 <0.001 注:MELD,终末期肝病模型;CK, 角蛋白;GS,谷氨酰胺酶;VEGF,血管内皮生长因子;EGFR,表皮生长因子受体;GPC3,磷脂酰肌醇蛋白聚糖3;KLI,ki67指数。 表 2 单因素及多因素Cox回归分析结果
Table 2. The results of univariate and multivariate Cox regression analysis
变量 单因素分析 多因素分析 HR(95%CI) P值 HR(95%CI) P值 年龄(>54岁) 0.738(0.410~1.328) 0.311 性别(男性) 0.750(0.353~1.593) 0.454 肝硬化 0.900(0.384~2.109) 0.809 病因(乙型肝炎) 1.095(0.428~2.804) 0.850 术前局部区域治疗 0.948(0.540~1.667) 0.854 卫星灶 1.879(1.030~3.428) 0.040 1.625(0.833~3.171) 0.285 门静脉癌栓 2.107(1.214~3.659) 0.008 0.707(0.360~1.389) 0.931 肿瘤个数(多个) 1.176(0.617~2.242) 0.623 肿瘤最大直径(>5 cm) 3.202(1.800~5.700) <0.001 1.508(0.744~3.053) 0.306 微血管侵犯 2.845(1.485~5.448) 0.002 1.468(0.713~3.022) 0.272 肿瘤分化程度 低分化 0.710(0.092~5.471) 0.743 中分化 0.384(0.052~2.834) 0.348 AFP>769 ng/mL 3.687(2.097~6.481) <0.001 4.429(2.438~8.045) <0.001 NLR>3.75 3.901(2.246~6.774) <0.001 3.877(2.119~7.097) <0.001 免疫组化 KLI>0.25 4.351(2.375~7.974) <0.001 2.655(1.356~5.200) 0.004 AFP(+) 1.774(1.024~3.072) 0.041 0.720(0.348~1.490) 0.247 CK19(+) 2.641(1.227~5.681) 0.013 2.157(0.911~5.106) 0.075 CK(+) 1.599(0.635~4.027) 0.319 P53(+) 1.288(0.750~2.212) 0.358 GS(+) 0.940(0.624~1.416) 0.768 VEGF(+) 0.658(0.345~1.257) 0.205 EGFR(+) 0.783(0.382~1.605) 0.504 GPC3(+) 1.974(0.785~4.965) 0.149 注:HR,风险比。 表 3 基于多因素Cox回归分析β系数的模型得分
Table 3. The score of the model based on β coefficient of multivariate Cox regression analysis
变量 β系数 得分 AFP>769 ng/mL 1.488 1.5 NLR>3.75 1.355 1.5 KLI>0.25 0.977 1.0 -
[1] BRAY F, FERLAY J, SOERJOMATARAM I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2018, 68(6): 394-424. DOI: 10.3322/caac.21492. [2] MAZZAFERRO V, REGALIA E, DOCI R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis[J]. N Engl J Med, 1996, 334(11): 693-699. DOI: 10.1056/NEJM199603143341104. [3] XU X, LU D, LING Q, et al. Liver transplantation for hepatocellular carcinoma beyond the Milan criteria[J]. Gut, 2016, 65(6): 1035-1041. DOI: 10.1136/gutjnl-2014-308513. [4] SAPISOCHIN G, BRUIX J. Liver transplantation for hepatocellular carcinoma: Outcomes and novel surgical approaches[J]. Nat Rev Gastroenterol Hepatol, 2017, 14(4): 203-217. DOI: 10.1038/nrgastro.2016.193. [5] ZAVAGLIA C, de CARLIS L, ALBERTI AB, et al. Predictors of long-term survival after liver transplantation for hepatocellular carcinoma[J]. Am J Gastroenterol, 2005, 100(12): 2708-2716. DOI: 10.1111/j.1572-0241.2005.00289.x. [6] REN A, LI Z, ZHOU X, et al. Evaluation of the alpha-fetoprotein model for predicting recurrence and survival in patients with hepatitis B virus (HBV)-related cirrhosis who received liver transplantation for hepatocellular carcinoma[J]. Front Surg, 2020, 7: 52. DOI: 10.3389/fsurg.2020.00052. [7] ZHANG X, WU Z, PENG Y, et al. Correlationship between Ki67, VEGF, and p53 and hepatocellular carcinoma recurrence in liver transplant patients[J]. Biomed Res Int, 2021, 2021: 6651397. DOI: 10.1155/2021/6651397. [8] FENG J, ZHU R, FENG D, et al. Prediction of early recurrence of solitary hepatocellular carcinoma after orthotopic liver transplantation[J]. Sci Rep, 2019, 9(1): 15855. DOI: 10.1038/s41598-019-52427-8. [9] COUSSENS LM, WERB Z. Inflammation and cancer[J]. Nature, 2002, 420(6917): 860-867. DOI: 10.1038/nature01322. [10] COFFELT SB, de VISSER KE. Cancer: Inflammation lights the way to metastasis[J]. Nature, 2014, 507(7490): 48-49. DOI: 10.1038/nature13062. [11] AINO H, SUMIE S, NⅡZEKI T, et al. The systemic inflammatory response as a prognostic factor for advanced hepatocellular carcinoma with extrahepatic metastasis[J]. Mol Clin Oncol, 2016, 5(1): 83-88. DOI: 10.3892/mco.2016.879. [12] MOTOMURA T, SHIRABE K, MANO Y, et al. Neutrophil-lymphocyte ratio reflects hepatocellular carcinoma recurrence after liver transplantation via inflammatory microenvironment[J]. J Hepatol, 2013, 58(1): 58-64. DOI: 10.1016/j.jhep.2012.08.017. [13] XU ZG, YE CJ, LIU LX, et al. The pretransplant neutrophil-lymphocyte ratio as a new prognostic predictor after liver transplantation for hepatocellular cancer: A systematic review and meta-analysis[J]. Biomark Med, 2018, 12(2): 189-199. DOI: 10.2217/bmm-2017-0307. [14] SULLIVAN LM, MASSARO JM, SR DRB. Presentation of multivariate data for clinical use: The Framingham Study risk score functions[J]. Stat Med, 2004, 23(10): 1631-1660. DOI: 10.1002/sim.1742. [15] JOU Y, HUANG C, CHO H. A VIF-based optimization model to alleviate collinearity problems in multiple linear regression[J]. Computational Statistics, 2014, 29(6): 1515-1541. DOI: 10.1007/s00180-014-0504-3. [16] MA E, LI J, XING H, et al. Development of a predictive nomogram for early recurrence of hepatocellular carcinoma in patients undergoing liver transplantation[J]. Ann Transl Med, 2021, 9(6): 468. DOI: 10.21037/atm-21-334. [17] MA KW, SHE WH, CHAN A, et al. Validated model for prediction of recurrent hepatocellular carcinoma after liver transplantation in Asian population[J]. World J Gastrointest Oncol, 2019, 11(4): 322-334. DOI: 10.4251/wjgo.v11.i4.322. [18] SHIMAMURA T, AKAMATSU N, FUJIYOSHI M, et al. Expanded living-donor liver transplantation criteria for patients with hepatocellular carcinoma based on the Japanese nationwide survey: The 5-5-500 rule-a retrospective study[J]. Transpl Int, 2019, 32(4): 356-368. DOI: 10.1111/tri.13391. [19] SHI K, LI P, XUE D, et al. Neutrophil-lymphocyte ratio and the risk of hepatocellular carcinoma in patients with hepatitis B-caused cirrhosis[J]. Eur J Gastroenterol Hepatol, 2021, 33(1S Suppl 1): e686-e692. DOI: 10.1097/MEG.0000000000002217. [20] SILVA TH, SCHILITHZ A, PERES W, et al. Neutrophil-lymphocyte ratio and nutritional status are clinically useful in predicting prognosis in colorectal cancer patients[J]. Nutr Cancer, 2020, 72(8): 1345-1354. DOI: 10.1080/01635581.2019.1679198. [21] CHEN L, QI L, ZHANG J, et al. Neutrophil-lymphocyte ratio as a prognostic factor for minute clear cell renal cell carcinoma diagnosed using multi-slice spiral CT[J]. Medicine (Baltimore), 2021, 100(23): e26292. DOI: 10.1097/MD.0000000000026292. [22] KOTEISH A, THULUVATH PJ. Screening for hepatocellular carcinoma[J]. J Vasc Interv Radiol, 2002, 13(9 Pt 2): s185-s190. DOI: 10.1016/s1051-0443(07)61785-0. [23] JIANG N, ZENG KN, DOU KF, et al. Preoperative alfa-fetoprotein and fibrinogen predict hepatocellular carcinoma recurrence after liver transplantation regardless of the Milan criteria: model development with external validation[J]. Cell Physiol Biochem, 2018, 48(1): 317-327. DOI: 10.1159/000491731. [24] HALAZUN KJ, NAJJAR M, ABDELMESSIH RM, et al. Recurrence after liver transplantation for hepatocellular carcinoma: A new MORAL to the story[J]. Ann Surg, 2017, 265(3): 557-564. DOI: 10.1097/SLA.0000000000001966. [25] FENG J, WU J, ZHU R, et al. Simple risk score for prediction of early recurrence of hepatocellular carcinoma within the Milan criteria after orthotopic liver transplantation[J]. Sci Rep, 2017, 7: 44036. DOI: 10.1038/srep44036. [26] MEHTA N, HEIMBACH J, HARNOIS DM, et al. Validation of a risk estimation of tumor recurrence after transplant (RETREAT) score for hepatocellular carcinoma recurrence after liver transplant[J]. JAMA Oncol, 2017, 3(4): 493-500. DOI: 10.1001/jamaoncol.2016.5116. [27] JIANG P, JIA M, HU J, et al. Prognostic value of Ki67 in patients with stage 1-2 endometrial cancer: Validation of the cut-off value of Ki67 as a predictive factor[J]. Onco Targets Ther, 2020, 13: 10841-10850. DOI: 10.2147/OTT.S274420. [28] CSERNI G, VÖRÖS A, LIEPNIECE-KARELE I, et al. Distribution pattern of the Ki67 labelling index in breast cancer and its implications for choosing cut-off values[J]. Breast, 2014, 23(3): 259-263. DOI: 10.1016/j.breast.2014.02.003. [29] WILKINS AC, GUSTERSON B, SZIJGYARTO Z, et al. Ki67 Is an independent predictor of recurrence in the largest randomized trial of 3 radiation fractionation schedules in localized prostate cancer[J]. Int J Radiat Oncol Biol Phys, 2018, 101(2): 309-315. DOI: 10.1016/j.ijrobp.2018.01.072. [30] GRANT L, BANERJI S, MURPHY L, et al. Androgen receptor and Ki67 expression and survival outcomes in non-small cell lung cancer[J]. Horm Cancer, 2018, 9(4): 288-294. DOI: 10.1007/s12672-018-0336-7. [31] JACOBSEN F, KOHSAR J, GEBAUER F, et al. Loss of p16 and high Ki67 labeling index is associated with poor outcome in esophageal carcinoma[J]. Oncotarget, 2020, 11(12): 1007-1016. DOI: 10.18632/oncotarget.27507. [32] TEMRAZ S, SHAMSEDDINE A, MUKHERJI D, et al. Ki67 and P53 in relation to disease progression in metastatic pancreatic cancer: A single institution analysis[J]. Pathol Oncol Res, 2019, 25(3): 1059-1066. DOI: 10.1007/s12253-018-0464-y. 期刊类型引用(19)
1. 夏艳军. 腹腔镜再次胆道手术治疗胆总管结石的有效性及安全性分析. 辽宁医学杂志. 2021(04): 64-66 .  百度学术
百度学术2. 叶春凤,缪珊珊,许斌. 利胆排石汤在预防腹腔镜术后胆管再发结石中的应用价值. 肝胆外科杂志. 2021(03): 208-211 .  百度学术
百度学术3. 熊良昆,马鹏,汪茂鸣,刘浩,余开焕. 胆囊切除术后残留胆总管结石的临床特点及腹腔镜治疗. 医学研究杂志. 2019(03): 120-123 .  百度学术
百度学术4. 校晗,张鑫. 胆囊结石合并肝外胆管结石患者采取腹腔镜与选择性辅助小切口手术治疗的差异性分析. 人人健康. 2019(12): 63 .  百度学术
百度学术5. 范波,李志坚,陈咏梅,严俊杰,贺学红,吕超波,田义,张丽. 腹腔镜Ⅰ期联合ERCP治疗高龄胆囊胆管结石的临床研究. 河北医药. 2019(15): 2311-2313+2317 .  百度学术
百度学术6. 刘世洲,田彦璋. LCBDE和EST治疗胆总管结石疗效比较. 中华肝脏外科手术学电子杂志. 2019(06): 542-546 .  百度学术
百度学术7. 刘志刚,孙礼侠,陈圣林,付明凤,刘丹峰,刘昌阔. 上腹部手术史患者行LCBDE的技术难点与对策. 肝胆外科杂志. 2018(02): 140-142+153 .  百度学术
百度学术8. 郭德胜. 老年肝外胆管结石患者应用腹腔镜联合胆道镜疗效分析. 肝胆外科杂志. 2018(03): 216-218 .  百度学术
百度学术9. 吕发凯. 用规则性肝部分切除术对老年肝内胆管结石患者进行治疗的效果研析. 当代医药论丛. 2018(11): 1-2 .  百度学术
百度学术10. 辛莘,郐大余,徐宝宏. ERCP、十二指肠乳头小切开联合乳头括约肌气囊扩张术在肝外胆管结石治疗中的临床应用. 中国医刊. 2018(04): 388-389 .  百度学术
百度学术11. 俞勇鸿,王征. 内镜逆行胰胆管造影术(ERCP)在胆漏诊治中的应用价值. 浙江创伤外科. 2018(01): 116-117 .  百度学术
百度学术12. 刘鹏. 胆道内镜微创手术治疗胆管结石的临床效果分析. 中国实用医药. 2018(30): 28-29 .  百度学术
百度学术13. 严伟,陈辉. 十二指肠镜、腹腔镜联合应用于高龄胆囊结石并肝外胆管结石的效果分析. 中国老年保健医学. 2018(06): 33-35 .  百度学术
百度学术14. 潘书贵,张亚运. 腹腔镜二次胆道手术治疗胆总管结石的临床研究. 肝胆外科杂志. 2018(06): 460-463 .  百度学术
百度学术15. 唐炳林. 完全腹腔镜下胆总管探查术治疗老年复杂性肝胆管结石的效果及对炎性应激反应指标与免疫功能的影响. 标记免疫分析与临床. 2017(11): 1247-1251+1262 .  百度学术
百度学术16. 李继东,冯帆,耿天翔. 腹腔镜手术与传统开放手术治疗老年残余胆囊胆管结石的疗效对比. 肝胆外科杂志. 2017(04): 278-281 .  百度学术
百度学术17. 王勇,刘会春,金浩,周磊,满忠然. 腹腔镜胆道再次手术与开腹手术治疗胆总管结石临床疗效对比研究. 现代医药卫生. 2017(19): 2987-2990 .  百度学术
百度学术18. 兰军,黄涛. 腹腔镜下胆管一期缝合与T管引流治疗肝外胆管结石的临床观察. 医学理论与实践. 2017(24): 3655-3656 .  百度学术
百度学术19. 陈勇军,蒋清平. 腹腔镜胆道再次手术对肝外胆管结石老年患者的效果分析. 浙江创伤外科. 2017(05): 971-972 .  百度学术
百度学术其他类型引用(1)
-




 PDF下载 ( 2530 KB)
PDF下载 ( 2530 KB)


 下载:
下载:

 百度学术
百度学术

