Clinical practice guidelines for the interventional treatment of advanced pancreatic carcinoma (on trial) (6th edition)
-
近年来药物性肝损伤发病率逐渐上升,日益受到人们的关注。引起药物性肝损伤的原因较多,例如中草药、抗菌药、非甾体类抗炎药等,且可能在正常剂量使用下引起药物性肝损伤[1],严重的可导致急性肝衰竭甚至需要肝移植[2],因此药物性肝损伤也逐渐引起全球公共卫生系统关注并成为加重肝病医疗系统经济负担的因素之一[3]。对乙酰氨基酚(Acetaminophen, APAP)是引起药物性肝损伤的主要病因之一,也是导致急性肝衰竭的重要原因[4-5]。肝脏再生对肝损伤修复和预后极为关键,药物性肝损伤后肝再生失败可能会导致严重的后果[6-7]。Toll样受体4(TLR4)是模式识别受体的一种,主要参与机体免疫和炎症反应的调节[8],但Marlini等[9]研究发现,TLR4可能对肝脏修复和再生过程有影响。与野生型小鼠相比,C3H/HeJ小鼠(TLR4基因发生错义突变而导致TLR4表达缺陷型小鼠[10])行部分肝脏切除术后肝脏重量恢复和肝细胞增殖所需时间明显延长。TLR4是否在APAP引起的肝损伤过程肝脏再生中发挥作用,目前研究甚少。TAK-242是目前较常用的小分子TLR4抑制剂,可以特异性阻断TLR4信号传导[11]。因此,本研究利用TAK-242来初步探索TLR4在APAP肝损伤过程肝脏再生中的作用,以期为研究肝再生机制以及药物性肝损伤治疗提供新的见解。
1. 材料与方法
1.1 主要试剂
APAP(HY-66005)、TAK-242(HY-11109)购买于中国MedChemExpress公司;DMSO(D8371)购买于北京索莱宝科技有限公司;ALT检测试剂盒(C009-2-1)购买于南京建成生物工程研究所;RT-PCR逆转录试剂盒(R-202)、SYBR Green染料(Q204)购买于新贝生物科技有限公司;Trizol(15596026)购买于美国Thermo Fisher Scientific公司;GAPDH抗体(TA-08)、免疫组化试剂盒(SP-9001)、DAB显色试剂盒(ZLI-9018)购买于北京中杉金桥生物技术有限公司;4%多聚甲醛组织固定液(BL539A)购买于中国白鲨生物科技有限公司;PCNA抗体(2586)、Ki-67抗体(12202)、STAT3抗体(9139)和p-STAT3抗体(9145)购买于美国Cell Signaling Technology公司;Cyclin D1抗体(26939-1-AP)购买于武汉三鹰生物技术有限公司;WB超敏显影液(K-12043-D10)购买于美国Advansta公司;引物由上海生工生物工程股份有限公司合成。
1.2 动物分组及造模
雄性CD-1(ICR)小鼠78只,6~8周龄,购买于维通利华实验动物技术有限公司[生产许可证编号:SCXK(浙)2019-0001;使用许可证编号:SYXK(皖)2017-006]。采用随机数字表法将小鼠分为9组:对照组(正常对照组、溶剂对照组、抑制剂对照组)每组6只,实验组(APAP 24 h组、TAK-242+APAP 24 h组、APAP 48 h组、TAK-242+APAP 48 h组、APAP 72 h组、TAK-242+APAP 72 h组)每组10只。APAP溶液用生理盐水配置,TAK-242溶液用0.5% DMSO配置。所有小鼠适应性喂养1周后再进行造模。给药前所有小鼠禁食12 h。实验组小鼠给予单剂量腹腔注射APAP(300 mg/kg),TAK-242在APAP注射前3 h以3 mg/kg剂量腹腔注射于相应组小鼠(剂量参照文献[12-13])。正常对照组小鼠腹腔注射与APAP组等体积生理盐水,抑制剂对照组小鼠注射与实验组小鼠等体积TAK-242溶液,溶剂对照组小鼠注射与抑制剂对照组等体积的0.5% DMSO溶液。在注射APAP后24 h、48 h、72 h麻醉后处理小鼠,收集小鼠血液和肝脏并及时冷冻保存,以便进行后续实验。
1.3 实验方法
1.3.1 血清ALT检测
将血液样本在室温下静置2 h,3 000 r/min离心20 min,将血清转移至另一EP管保存。按照ALT检测试剂盒说明测定ALT水平。
1.3.2 肝脏组织病理观察
使用4%多聚甲醛组织固定液对肝左叶进行固定,按照常规步骤进行脱水、浸蜡、包埋和切片。将肝组织切片进行HE染色,然后在显微镜下观察组织损伤情况并进行拍照。
1.3.3 RT-PCR检测mRNA水平
取40 mg肝组织放入玻璃研磨器中,同时加入1 mL Trizol,在冰上充分研磨,经过氯仿、异丙醇、75%乙醇萃取、沉淀和洗涤等步骤,提取总RNA。检测RNA浓度后,按照逆转录试剂盒说明书进行逆转录得到cDNA。以GAPDH为内参,使用RT-PCR方法检测并以2-△△Ct法计算PCNA、Ki-67、Cyclin D1的mRNA水平。引物序列见表 1。
表 1 RT-PCR引物序列Table 1. RT-PCR primer sequences基因 上游(5′-3′) 下游(5′-3′) Cyclin D1 AGGCGGATGAGAACAAGCAG CCTTGTTTAGCCAGAGGCCG Ki-67 CCATCATTGACCGCTCCTT CTGCCAGTGTGCTGTTCTAC PCNA GGGTTGGTAGTTGTCGCTGT CCAAGGAGACGTGAGACGAG GAPDH GACATGCCGCCTGGAGAAAC AGCCCAGGATGCCCTTTAGT 1.3.4 Western blot检测蛋白水平
在玻璃研磨器中加入70 mg肝脏组织及1 mL RIPA裂解液,充分研磨并经过离心后,得到总蛋白。利用BCA法测定蛋白浓度,加入上样缓冲液后将蛋白放在加热器上100 ℃加热10 min使其变性。将蛋白样品加入到SDS-PAGE凝胶样品孔中进行电泳,结束后在冰上进行转膜,将蛋白转移至PVDF膜上。室温下使用脱脂牛奶封闭2~3 h,TBST溶液清洗3次,一抗4 ℃孵育过夜(GAPDH,1∶ 1 000;STAT3,1∶ 1 000;p-STAT3,1∶ 2 000;PCNA,1∶ 1 000;Cyclin D1,1∶ 5 000)。第2天PVDF洗涤干净后,二抗室温孵育1 h。最后显影,使用Image J进行灰度值分析。
1.3.5 免疫组化检测
肝脏组织切片进行常规的烘烤、脱蜡、水化,使用柠檬酸钠进行抗原修复,然后阻断内源性过氧化物酶,经过封闭后,一抗(Ki-67,1∶ 300)4 ℃孵育过夜。第2天室温下复温30 min,洗涤后二抗孵育45 min。DAB显色,最后使用中性树胶封片并拍照。
1.4 统计学方法
采用SPSS 23.0统计软件进行数据分析,Graphpad Prism 8.0软件进行绘图。正态分布的计量资料以x±s表示,两组间比较采用成组t检验;多组间比较采用单因素方差分析,进一步两两比较采用LSD-t检验。非正态分布的计量资料以M(P25~P75)表示,两组间比较采用Mann-Whitney U检验;多组间比较及进一步两两比较均采用Kruskal-Wallis H检验。P<0.05为差异有统计学意义。
2. 结果
2.1 观察抑制剂和溶剂对正常小鼠肝脏的影响
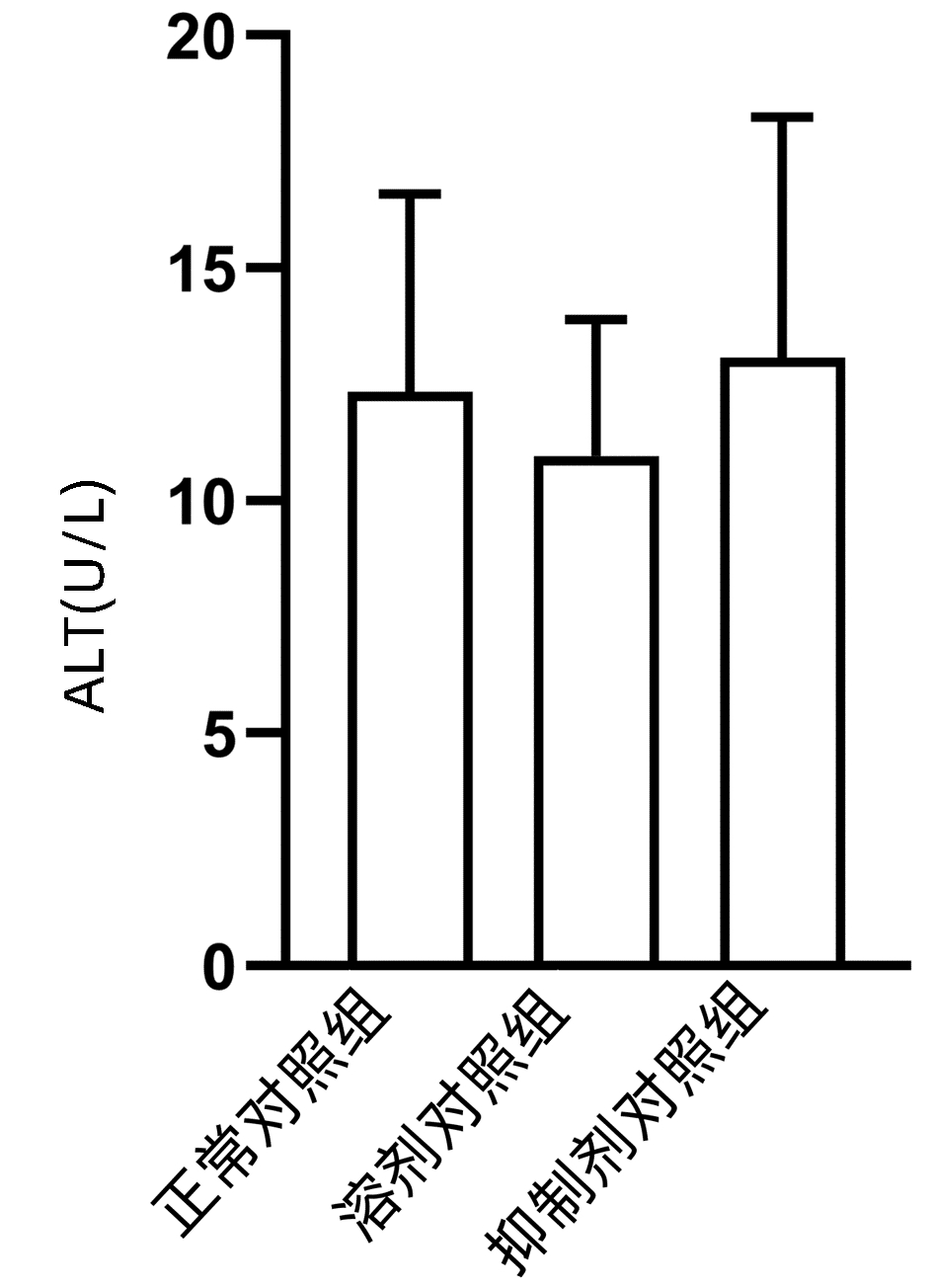
结果显示,正常对照组、溶剂对照组和抑制剂对照组小鼠血清ALT水平差异无统计学意义(P>0.05)(图 1),且与正常对照组相比,溶剂对照组和抑制剂对照组小鼠的肝脏HE染色未发现明显的病理改变(图 2)。
2.2 抑制TLR4可能延迟APAP诱导的肝损伤过程中肝脏的恢复
APAP 24 h组和APAP 48 h组血清ALT水平均明显高于正常对照组(P值均<0.05),而TAK-242+APAP 24 h组及TAK-242+APAP 48 h组的ALT水平明显高于同时间点APAP组(P值均<0.05);小鼠肝组织HE染色结果显示,正常对照组小鼠肝小叶结构清晰,肝细胞排列整齐,APAP处理的小鼠肝脏可见典型的小叶中心性坏死。与相同时间点APAP组相比,TAK-242+APAP 24 h组和TAK-242+APAP 48 h组小鼠肝脏坏死面积明显较大(P值均<0.05)(表 2、3,图 3)。
表 2 APAP对小鼠血清ALT及肝脏组织的影响Table 2. Effects of APAP on serum ALT and liver tissue in mice组别 动物数(只) ALT(U/L) 肝脏坏死面积(%) 正常对照组 6 12.339±4.245 0 APAP 24 h组 10 1 449.848±209.4911) 41.600(38.617~46.502)1) APAP 48 h组 10 281.702±140.5921) 36.050(25.127~45.718)1) APAP 72 h组 10 45.251±4.298 0 (0~1.903) H值 17.857 16.035 P值 <0.001 0.001 注:与正常对照组比较,1)P<0.05。 表 3 抑制TLR4在各时间点对APAP肝损伤小鼠血清ALT及肝脏组织的影响Table 3. Effects of TLR4 inhibition on serum ALT and liver tissue of mice with APAP-induced liver injury at different time point组别 动物数(只) 24 h 48 h 72 h ALT(U/L) APAP组 10 1 449.848±209.491 281.702±140.592 45.251±4.298 TAK-242+APAP组 10 3 484.212±960.336 1 151.505±656.722 56.995±24.763 t值 -4.628 -2.896 -1.045 P值 0.002 0.040 0.352 肝脏坏死面积(%) APAP组 10 41.600(38.617~46.502) 36.050(25.127~45.718) 0(0~1.903) TAK-242+APAP组 10 58.554(55.889~73.409) 59.558(54.856~60.591) 2.713(0~8.289) Z值 -2.610 -2.611 -1.294 P值 0.008 0.008 0.310 2.3 抑制TLR4下调肝脏Cyclin D1、PCNA、Ki-67的mRNA水平和蛋白表达
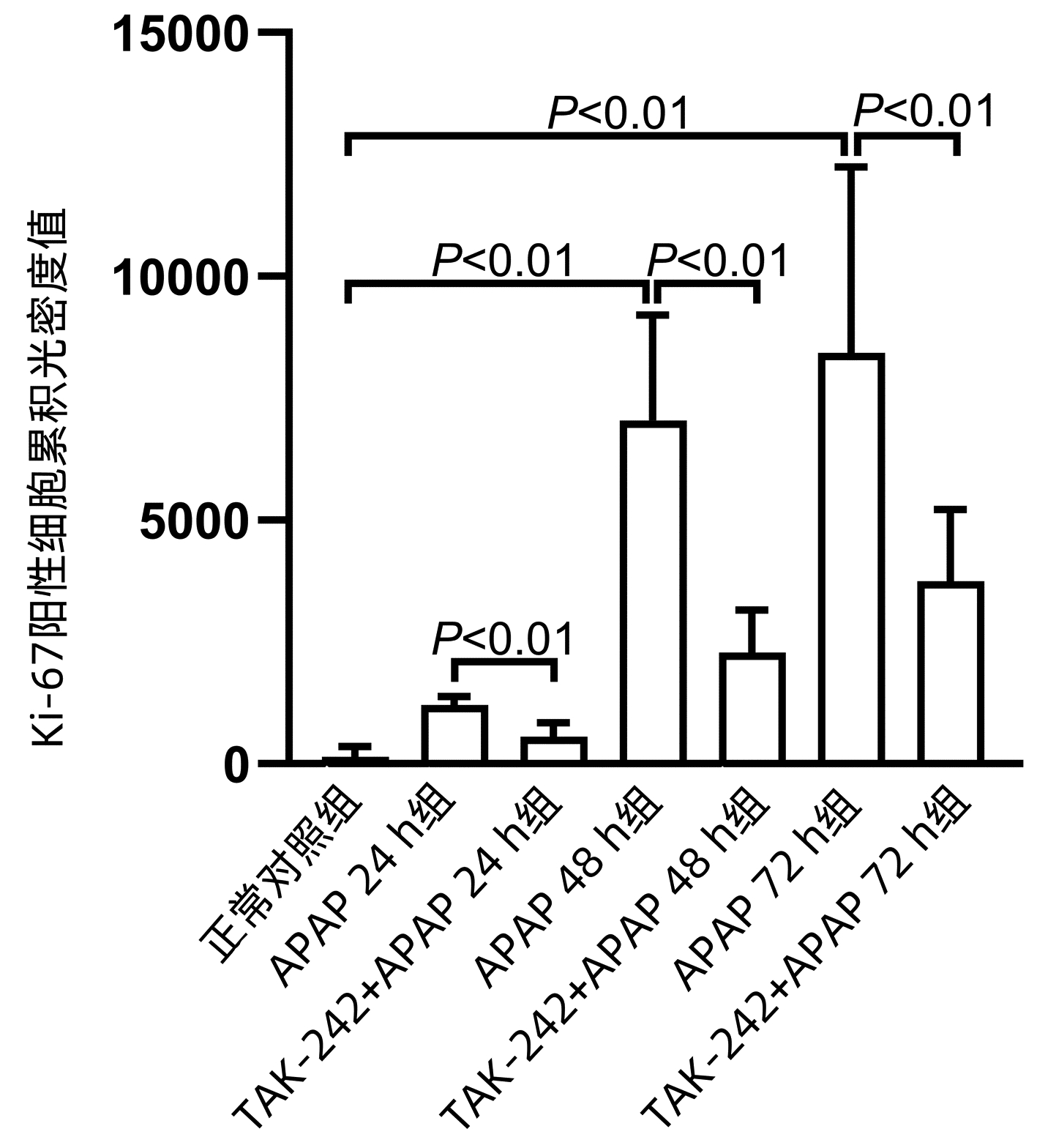
APAP 48 h组和APAP72 h组Cyclin D1、PCNA、Ki-67 mRNA水平均明显高于正常对照组(P值均<0.05),APAP 24 h组PCNA mRNA水平显著高于正常对照组(P<0.05);TAK-242+ APAP 24 h组、TAK-242+APAP 48 h组和TAK-242+APAP 72 h组的Cyclin D1、PCNA mRNA水平均低于同时间点APAP组,TAK-242+APAP 24 h组和TAK-242+APAP 72 h组的Ki-67 mRNA水平均低于同时间点APAP组,差异均有统计学意义(P值均<0.05) (表 4、5)。Western blot检测Cyclin D1、PCNA蛋白表达结果显示,TAK-242+APAP 24 h组和TAK-242+APAP 48 h组的Cyclin D1、PCNA蛋白表达明显低于同时间点APAP组(P值均<0.05)(图 4)。免疫组化染色及Ki-67阳性细胞累积光密度值比较结果显示,APAP 48 h组和APAP 72 h组Ki-67蛋白表达显著高于正常对照组(P值均<0.05),而TAK-242+APAP 24 h组、TAK-242+APAP 48 h组及TAK-242+APAP 72 h组的Ki-67蛋白表达明显低于同时间点APAP组(P值均<0.05)(图 5、6)。
表 4 APAP对小鼠肝组织Ki-67 mRNA、Cyclin D1 mRNA、PCNA mRNA表达的影响Table 4. Effects of APAP on the expression of Ki-67 mRNA, Cyclin D1 mRNA and PCNA mRNA in mouse liver组别 动物数(只) Ki-67 mRNA相对表达量 Cyclin D1 mRNA相对表达量 PCNA mRNA相对表达量 正常对照组 6 1.002±0.072 1.034±0.325 1.009±0.162 APAP 24 h组 10 1.027±0.184 1.061±0.102 1.347±0.1151) APAP 48 h组 10 83.566±23.3621) 3.707±0.2551) 5.327±0.1621) APAP 72 h组 10 39.316±10.9161) 2.417±0.3361) 1.739±0.0861) F值 27.853 88.659 657.231 P值 <0.001 <0.001 <0.001 注:与正常对照组比较,1)P<0.05。 表 5 抑制TLR4对小鼠肝组织Ki-67 mRNA、Cyclin D1 mRNA、PCNA mRNA表达的影响Table 5. Effects of TLR4 inhibition on the expression of Ki-67 mRNA, Cyclin D1 mRNA and PCNA mRNA in mouse liver组别 动物数(只) 24 h 48 h 72 h Ki-67 mRNA相对表达量 APAP组 10 1.027±0.184 83.566±23.362 39.316±10.916 TAK-242+APAP组 10 0.366±0.129 70.145±31.141 5.950±0.678 t值 5.107 0.597 5.284 P值 0.007 0.583 0.006 Cyclin D1 mRNA相对表达量 APAP组 10 1.061±0.102 3.707±0.255 2.417±0.336 TAK-242+APAP组 10 0.678±0.073 2.918±0.487 1.837±0.202 t值 6.116 2.871 2.957 P值 0.001 0.028 0.025 PCNA mRNA相对表达量 APAP组 10 1.347±0.115 5.327±0.162 1.739±0.086 TAK-242+APAP组 10 0.830±0.075 2.937±0.144 0.620±0.035 t值 6.529 19.074 20.909 P值 0.003 <0.001 <0.001 2.4 抑制TLR4下调p-STAT3水平
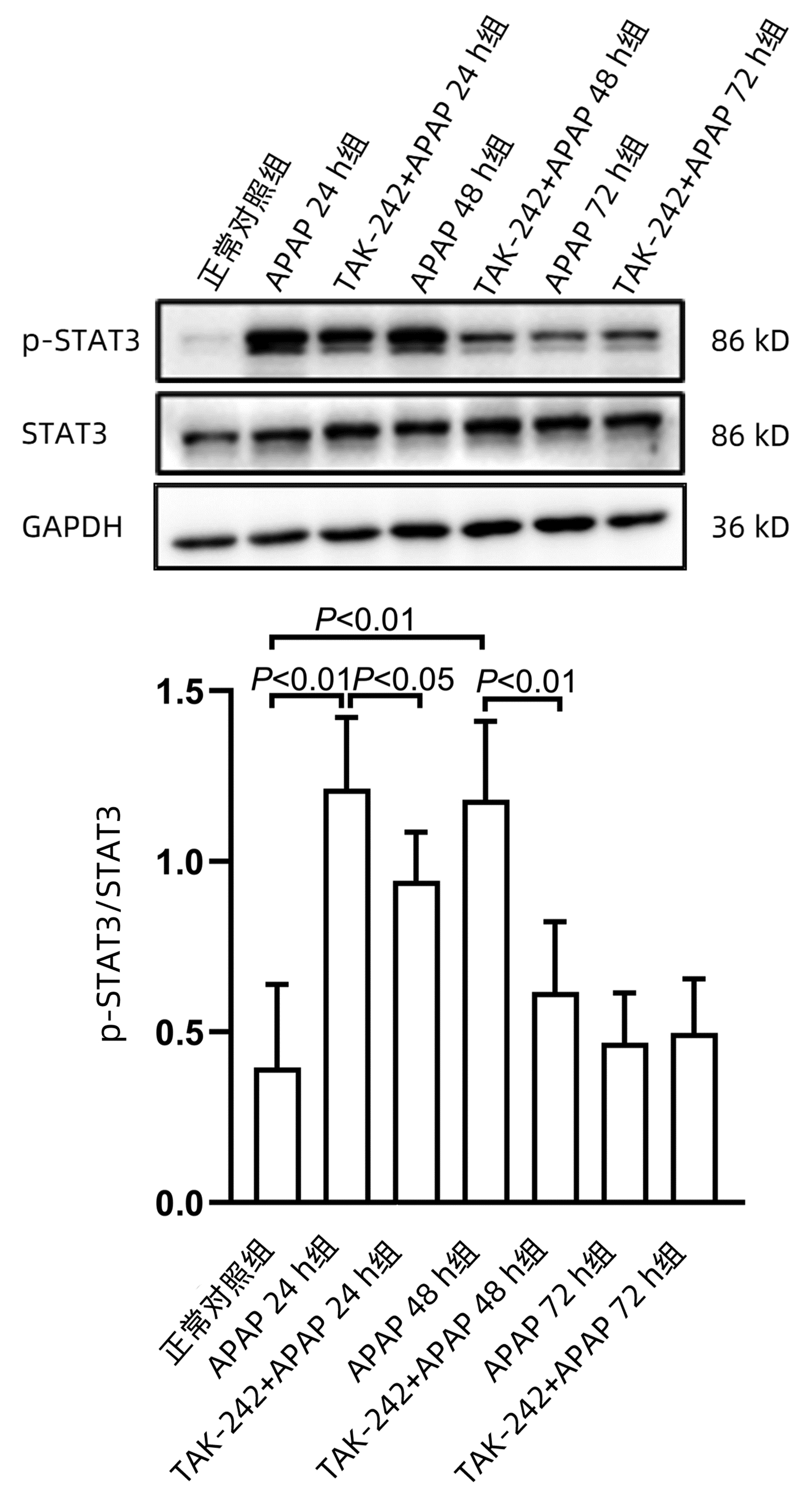
课题组前期体外研究[14]发现,STAT3在APAP肝损伤后肝细胞再生过程中发挥重要作用,为探索TLR4参与肝脏再生过程是否与STAT3有关,应用Western blot方法检测STAT3蛋白磷酸化(p-STAT3)水平。结果显示,APAP 24 h组和APAP 48 h组p-STAT3水平均显著高于正常对照组(P值均<0.01),而TAK-242+APAP 24 h组及TAK-242+APAP 48 h组p-STAT3水平均明显低于同时间点APAP组(P值均<0.05)(图 7)。
3. 讨论
肝脏是具有极强再生能力的器官,也是唯一能够在被部分/大部分切除后恢复原有重量的内脏器官[15]。据报道[16],存在肝脏炎症和肝细胞坏死的肝脏疾病都可能发生肝再生,包括药物性肝损伤。N-乙酰半胱氨酸(NAC)是最常用于治疗药物性肝损伤的药物,特别是对于APAP肝损伤,治疗效果较好。但是NAC的治疗时间窗较短,对晚期发现的肝损伤治疗效果较差,甚至长时间高剂量使用NAC会不利于APAP肝损伤恢复[17]。在毒性化学物引起的肝损伤中,肝损伤和再生是此消彼长的关系。一项急性肝损伤的动态研究[18]发现,在轻度急性肝损伤小鼠体内,肝损伤进展时,肝再生也在增强,当肝再生强于肝损伤,即开始进入恢复阶段;而在重度肝损伤小鼠模型中,肝再生一开始即受到抑制,因肝再生速度不及损伤的速度,损伤加速进展,造成小鼠死亡。据报道[19-20],在APAP肝损伤中,明显的肝再生发现在24 h以后,因此本研究选择分别于24 h、48 h和72 h处死小鼠。
TLR4是一种跨膜多功能分子,可以与来自细胞内外的配体结合,在肝、肾、心、肺、皮肤等多个器官和组织中表达,参与炎症反应、免疫调控和组织修复等过程[21]。有研究[22]报道,早期阻断TLR4可以预防肾小管坏死和急性肾损伤,但是在愈合期阻断TLR4会导致IL-22生成减少,从而损害肾再生。与野生型小鼠相比,TLR4敲除的小鼠对博来霉素引起的肺损伤更敏感,肺泡上皮损伤也更严重,Ⅱ型肺泡上皮细胞增殖数较少[23]。最近一项研究[24]发现,在行部分肝切除术小鼠体内,TLR4与脂多糖结合可以激活肝细胞转化,促进肝再生。TLR4敲除小鼠行部分肝切除术后,细胞增殖指标Ki-67和PCNA的表达明显受到抑制。本文利用TLR4特异性抑制剂TAK-242进行研究发现,与24 h和48 h的APAP组相比,使用抑制剂组处理的小鼠ALT水平和肝脏坏死面积恢复均较慢,这表明抑制TLR4可能会阻碍APAP肝损伤过程肝脏的修复。进一步检测再生相关指标发现,在APAP诱导小鼠肝损伤中,抑制TLR4后小鼠肝脏的Cyclin D1、PCNA和Ki-67 mRNA及蛋白水平均下降,提示TLR4可能参与APAP肝损伤过程中肝再生,并对肝再生起到一定的促进作用。
STAT3是细胞增殖和组织再生过程的重要分子,在肝脏再生过程中发挥重要作用。在行部分肝切除术的小鼠体内,STAT3信号通路激活增加,肝细胞STAT3特异性敲除的小鼠肝脏再生会受到抑制[25-26]。此外,在四氯化碳诱导的肝损伤小鼠模型中发现,STAT3表达显著增加,抑制STAT3后,肝脏增殖指标表达明显降低[27]。在本研究过程中,笔者发现与正常对照组相比,APAP 24 h组和APAP 48 h组STAT3磷酸化水平明显升高,但同时间点TAK-242+APAP组STAT3磷酸化水平降低,这提示p-STAT3可能参与了TLR4在APAP肝损伤中促进肝脏再生的过程。
肝脏再生的机制比较复杂,涉及的分子及通路众多。本研究仅对TLR4在APAP肝损伤过程肝再生中的作用进行了初步探讨,更深层的机制有待课题组后期进一步研究。
综上所述,TLR4可能通过STAT3信号通路对APAP诱导的小鼠肝损伤过程中的肝脏再生起到一定的促进作用。
-
TNM分期 内容 TNM分期 T分期 N分期 M分期 原发肿瘤(T) Tx:原发肿瘤无法评估
T0:无原发肿瘤证据
Tis:原位癌
T1: 肿瘤最大径≤2.0 cm
T1a:肿瘤最大径≤0.5 cm
T1b: 肿瘤最大径>0.5 cm且<1.0 cm
T1c:肿瘤最大径≥1.0 cm且≤2.0 cm
T2: 肿瘤最大径>2.0 cm且≤4.0 cm
T3: 肿瘤最大径>4.0 cm
T4: 肿瘤不论大小,累及腹腔干、
肠系膜上动脉,和/或肝总动脉0 Tis N0 M0 ⅠA T1 N0 M0 ⅠB T2 N0 M0 ⅡA T3 N0 M0 ⅡB T1~3 N1 M0 Ⅲ T4 Any N M0 Any T N2 M0 Ⅳ Any T Any N M1 区域淋巴结(N) Nx: 区域淋巴结无法评估
N0: 无区域淋巴结转移
N1: 1~3枚区域淋巴结转移
N2: 4枚以上区域淋巴结转移远处转移(M) M0: 无远处转移M1: 有远处转移 表 2 射频参量及相关参数
Table 2. RF parameters and related parameters
电极种类 数量 间距(mm) 目标能量(kJ) 功率设定(W) 治疗长度(mm) 治疗直径(mm) 治疗体积(cm3) T20 1 9 20 22 20 5 T20 2 7 14 40 25 20~27 7 T20 3 15 24 60 25 20~30 12 T30 1 15 30 32 22 5.5 T30 2 13 25 60 35 25 8 T30 3 15 15~35 90 35 20~30 14 T40 1 29 40 44 25 14 T40 2 13.3 39 80 45 25~33 19 T40 3 20 35~70 120 50 30~40 22 T40 3 25 70~130 120 55 40~50 47 T40 3 30 130~250 120 60 50~60 87 表 3 微波能量及相关参数
Table 3. Microwave energy and related parameters
消融时间 主机功率 50 W 55 W 60 W 70 W 5 min 40×25×5 40×26×5 42×27×6 44×30×7 8 min 42×26×5 43×27×6 44×30×6 46×32×7 10 min 45×28×5 48×30×6 49×32×7 50×34×8 12 min 48×30×6 49×32×7 50×34×7 52×36×10 15 min 50×34×6 51×36×7 52×38×7 54×40×10 表 4 晚期胰腺癌介入方法选择方案
Table 4. Selection of interventional methods for advanced pancreatic cancer
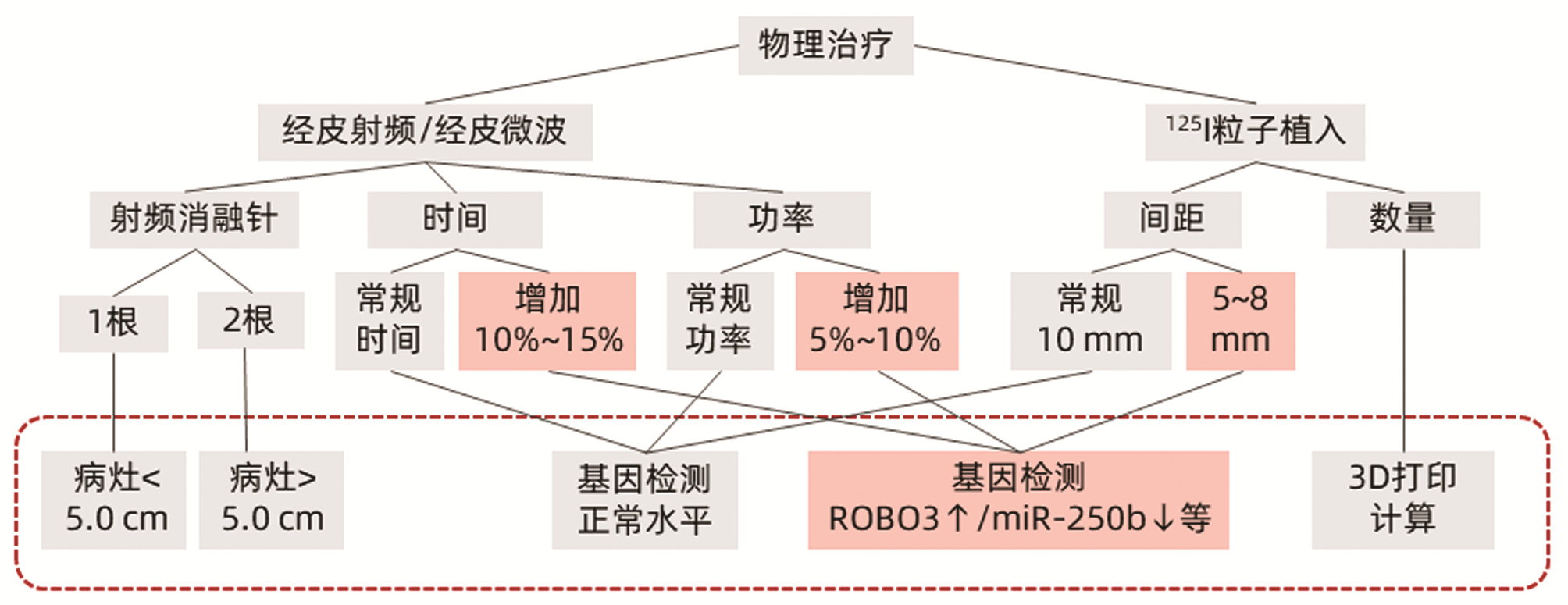
cTAI治疗 低/未分化腺癌、血供丰富、基因敏感ROBO3↑/miR-250b↓等 胰头部:胃十二指肠动脉、肠系膜上动脉 胰体尾部:脾短动脉、胃网膜右动脉 优势动脉:化疗栓塞;非优势动脉:栓塞 物理治疗 中/高分化腺癌、血供不丰富、基因不敏感ROBO3/miR-250b等 胰头部:125I粒子植入 体尾部:射频/微波消融 表 5 三组晚期胰腺癌患者基本资料
Table 5. Basic data of three groups of patients with advanced pancreatic cancer
参数 对症治疗(n=406) 对症+介入(n=422) 对症+化疗(n=190) 统计值 P值 年龄(岁) 71.84±11.58 66.39±11.37 62.25±10.05 F=1.290 0.075 性别[例(%)] χ2=0.955 0.620 男 252(62.07) 255(60.43) 110(57.89) 女 154(37.93) 167(39.57) 80(42.11) TNM分期[例(%)] χ2=2.012 0.366 T3N1MO 55(13.55) 45(10.66) 20(10.53) T3N1M1 351(86.45) 377(89.34) 170(89.47) 肿瘤大小[例(%)] χ2=8.753 0.068 <3.0 cm 10(2.46) 9(2.13) 8(4.21) 3.0~5.0 cm 38(9.36) 50(11.85) 31(16.31) >5.0 cm 358(88.18) 363(86.02) 151(79.48) 有无转移[例(%)] χ2=0.075 0.963 有 345(84.98) 360(85.31) 152(80.00) 无 61(15.02) 62(14.69) 38(20.00) 腹腔积液[例(%)] χ2=25.823 0.000 有 80(19.70) 32(7.58) 28(14.74) 无 326(80.30) 390(92.42) 162(85.26) 黄疸[例(%)] χ2=83.009 0.000 有 122(30.05) 250(59.24) 110(57.89) 无 284(69.95) 172(40.76) 80(42.11) Child-Pugh分级[例(%)] χ2=72.295 0.000 A级 16(3.94) 8(1.90) 35(18.42) B级 94(23.15) 105(24.88) 48(25.26) C级 296(72.90) 309(73.22) 107(56.32) CA19-9[例(%)] χ2=3.355 0.187 正常 88(21.67) 105(24.88) 54(28.42) 异常 318(78.33) 317(75.12) 136(71.58) 表 6 三组临床缓解率比较
Table 6. Comparison of clinical remission rates among the three groups
mRECIST 对症治疗(n=406) 对症+介入(n=422) 对症+化疗(n=190) 总计(n=1018) P值 完全缓解+部分缓解[例(%)] 80(19.7) 198(46.9) 79(41.6) 341(33.5) 0.000 疾病稳定[例(%)] 105(25.9) 147(34.8) 59(31.1) 418(41.1) 疾病进展[例(%)] 221(54.4) 77(18.2) 55(28.9) 259(25.4) 表 7 三组间6、12、18个月生存率比较
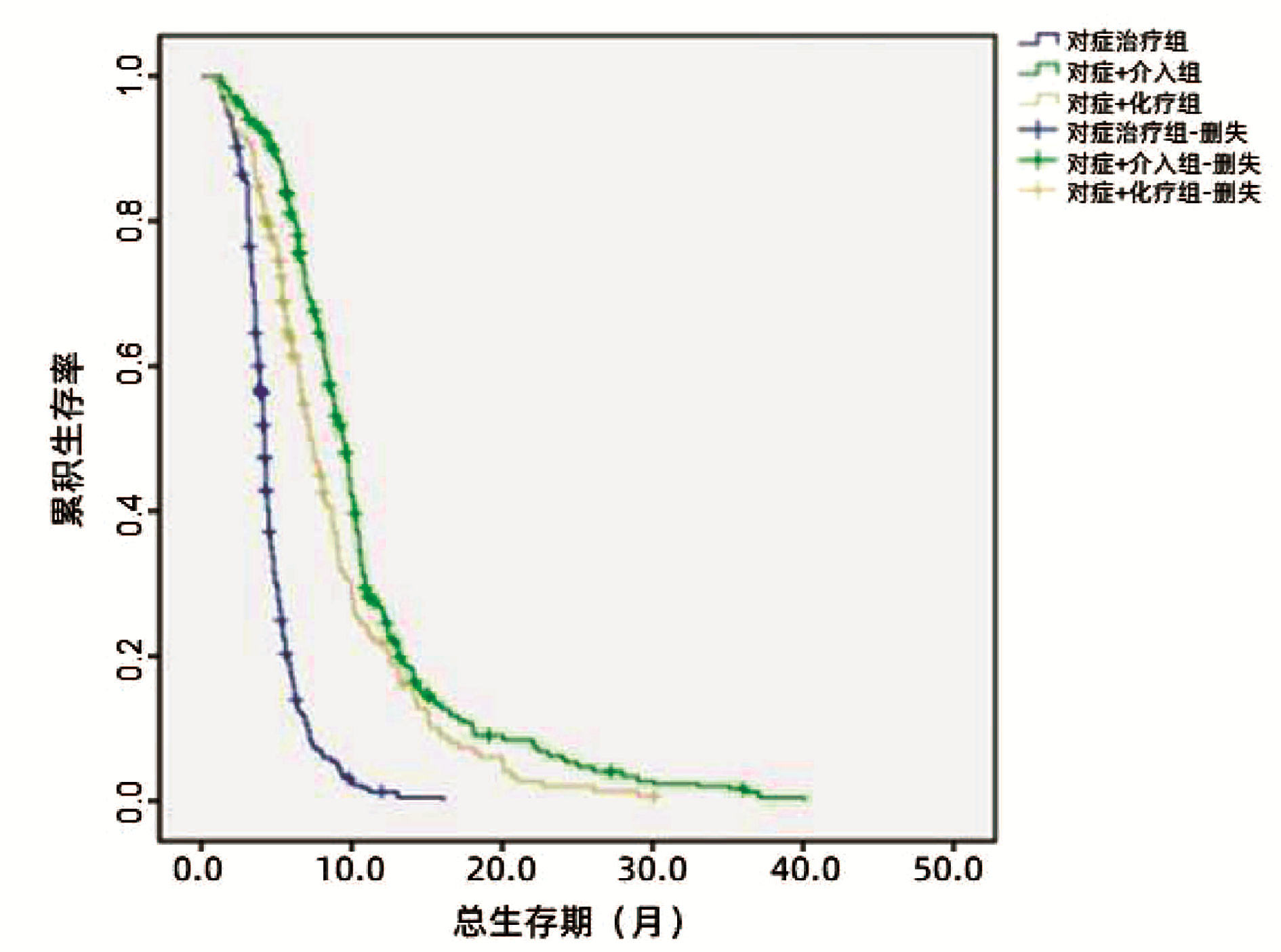
Table 7. Comparison of 6, 12 and 18 month survival rates among the three groups
时间 结局 对症治疗(n=406) 对症+介入(n=422) 对症+化疗(n=190) 总计(n=1018) P值 6个月 0.000 存活[例(%)] 77(19.0) 341(80.8) 120(63.2) 538(52.8) 死亡[例(%)] 329(81.0) 81(19.2) 70(36.8) 480(47.2) 12个月 0.000 存活[例(%)] 8(2.0) 114(27.0) 42(22.1) 164(16.1) 死亡[例(%)] 398(98.0) 308(73.0) 148(77.9) 854(83.9) 18个月 0.000 存活[例(%)] 0 46(10.9) 15(7.9) 61(6.0) 死亡[例(%)] 406(100.0) 376(89.1) 175(92.1) 957(94.0) -
[1] International Agency for Research on Cancer, World Health Organization. Global cancer observatory 2018[DB/OL]. http://gco.iarc.fr/. [2] CABASAG CJ, FERLAY J, LAVERSANNE M, et al. Pancreatic cancer: an increasing global public health concern[J]. Gut, 2021. DOI: 10.1136/gutjnl-2021-326311. [3] ALLEN PJ, KUK D, CASTILLO CF, et al. Multi-institutional validation study of the american joint commission on cancer (8th edition) changes for T and N staging in patients with pancreatic adenocarcinoma[J]. Ann Surg, 2017, 265(1): 185-191. DOI: 10.1097/SLA.0000000000001763. [4] SOBIN LH, GOSPODAROWICZ MK, WITTEKIND C, et al. TNM classification of malignant tumours[M]. 7th ed. Chichester, West Sussex, UK; Hoboken, NJ: Wiley-Blackwell, 2010. [5] KRUGER S, SCHIRLE K, HAAS M, et al. Prolonged time to treatment initiation in advanced pancreatic cancer patients has no major effect on treatment outcome: a retrospective cohort study controlled for lead time bias and waiting time paradox[J]. J Cancer Res Clin Oncol, 2020, 146(2): 391-399. DOI: 10.1007/s00432-019-03061-4. [6] HIDAGOM M. Pancreatic cancer[J]. N Engl J Med, 2010, 362(17): 1605-1617. DOI: 10.1056/NEJMra0901557 [7] HEINRICH S, KRAFT D, STAIB-SEBLER E, et al. Phase Ⅱ study on combined intravenous and intra-arterial chemotherapy with gemcitabine and mitomycin C in patients with advanced pancreatic cancer[J]. Hepatogastroenterology, 2013, 60(126): 1492-1496. DOI: 10.5754/hge11805. [8] Pancreatic Cancer Committee of Chinese Anti-Cancer Association. Comprehensive guidelines for the diagnosis and treatment of pancreatic cancer (2018 version)[J]. J Clin Hepatol, 2018, 34(10): 2109-2120. DOI: 10.3969/j.issn.1001-5256.2018.10.011.中国抗癌协会胰腺癌专业委员会. 胰腺癌综合诊治指南(2018版)[J]. 临床肝胆病杂志, 2018, 34(10): 2109-2120. DOI: 10.3969/j.issn.1001-5256.2018.10.011. [9] Pancreatic Surgery Group, Branch of Surgery, Chinese Medical Association, Editorial Department of Chinese Journal of Surgery. Expert consensus on prevention and treatment of common surgical complications after pancreatic surgery (2010)[J]. Chin J Surg, 2010, 48(18): 1365-1368. DOI: 10.3760/cma.j.issn.0529-5815.2010.18.002.中华医学会外科学分会胰腺外科学组, 中华外科杂志编辑部. 胰腺术后外科常见并发症预防及治疗的专家共识(2010)[J]. 中华外科杂志, 2010, 48(18): 1365-1368. DOI: 10.3760/cma.j.issn.0529-5815.2010.18.002. [10] NI XQ, YU XR, LIU L. Clinical diagnostic criteria for pancreatic cancer in China[J]. China Oncol, 2012, 22(2): 81-87. DOI: 10.3969/j.issn.1007-3969.2012.02.001.倪泉兴, 虞先濬, 刘亮. 中国胰腺癌临床诊断标准的探讨[J]. 中国癌症杂志, 2012, 22(2): 81-87. DOI: 10.3969/j.issn.1007-3969.2012.02.001. [11] ARCELLI A, GUIDO A, BUWENGE M, et al. Higher biologically effective dose predicts survival in SBRT of pancreatic cancer: a multicentric analysis (PAULA-1)[J]. Anticancer Res, 2020, 40(1): 465-472. DOI: 10.21873/anticanres.13975. [12] SUKER M, NUYTTENS JJ, ESKENS F, et al. Efficacy and feasibility of stereotactic radiotherapy after folfirinox in patients with locally advanced pancreatic cancer (LAPC-1 trial)[J]. E Clinical Medicine, 2019, 17: 100200. DOI: 10.1016/j.eclinm.2019.10.013. [13] KIRTLAND HB Jr. A safe method of pancreatic biopsy; a preliminary report[J]. Am J Surg, 1951, 82(4): 451-457. DOI: 10.1016/0002-9610(51)90372-8. [14] SMITH EH, BARTRUM RJ Jr, CHANG YC. Ultrasonically guided percutaneous aspiration biopsy of the pancreas[J]. Radiology, 1974, 112(3): 737-738. DOI: 10.1148/112.3.737. [15] LVNING M, KURSAWE R, SCHÖPKE W, et al. CT guided percutaneous fine-needle biopsy of the pancreas[J]. Eur J Radiol, 1985, 5(2): 104-108. [16] DELMASCHIO A, VANZULLI A, SIRONI S, et al. Pancreatic cancer versus chronic pancreatitis: diagnosis with CA 19-9 assessment, US, CT, and CT-guided fine-needle biopsy[J]. Radiology, 1991, 178(1): 95-99. DOI: 10.1148/radiology.178.1.1984331. [17] ZECH CJ, HELMBERGER T, WICHMANN MW, et al. Large core biopsy of the pancreas under CT fluoroscopy control: results and complications[J]. J Comput Assist Tomogr, 2002, 26(5): 743-749. DOI: 10.1097/00004728-200209000-00014. [18] KLASSEN DK, WEIR MR, CANGRO CB, et al. Pancreas allograft biopsy: safety of percutaneous biopsy-results of a large experience[J]. Transplantation, 2002, 73(4): 553-555. DOI: 10.1097/00007890-200202270-00011. [19] SYED A, BABICH O, RAO B, et al. Endoscopic ultrasound guided fine-needle aspiration vs core needle biopsy for solid pancreatic lesions: Comparison of diagnostic accuracy and procedural efficiency[J]. Diagn Cytopathol, 2019, 47(11): 1138-1144. DOI: 10.1002/dc.24277. [20] D'ONOFRIO M, MALAGÒ R, ZAMBONI G, et al. Ultrasonography of the pancreas. 5. Interventional procedures[J]. Abdom Imaging, 2007, 32(2): 182-190. DOI: 10.1007/s00261-006-9011-5. [21] RAYMOND S, YUGAWA D, CHANG K, et al. Metastatic neoplasms to the pancreas diagnosed by fine-needle aspiration/biopsy cytology: A 15-year retrospective analysis[J]. Diagn Cytopathol, 2017, 45(9): 771-783. DOI: 10.1002/dc.23752. [22] National Comprehensive Cancer Network. NCCN guidelines for pancreatic adenocarcinoma[DB/OL]. https://www.nccn.org/professionals/physician_gls/default.aspx#pancreatic. [23] MOULLA Y, PETERSEN TO, MAIWALD B, et al. Ablative treatment options for locally advanced unresectable and borderline resectable pancreatic carcinoma[J]. Chirurg, 2020, 91(4): 319-328. DOI: 10.1007/s00104-019-01072-y. [24] UYANIK SA, ÖǦÜŞLÜ U, YILMAZ B, et al. Percutaneous intraductal microwave ablation of malignant biliary strictures: initial experience[J]. AJR Am J Roentgenol, 2020, 215(3): 753-759. DOI: 10.2214/AJR.19.21897. [25] YANG SY, LIU F, LIU Y, et al. Stent insertion with high-intensity focused ultrasound ablation for distal biliary obstruction secondary to pancreatic carcinoma[J]. Medicine (Baltimore), 2020, 99(6): e19099. DOI: 10.1097/MD.0000000000019099. [26] HE XF, XIAO YY, ZHANG X, et al. Preliminary clinical application of the robot-assisted CT-guided irreversible electroporation ablation for the treatment of pancreatic head carcinoma[J]. Int J Med Robot, 2020, 16(4): e2099. DOI: 10.1002/rcs.2099. [27] FEI Q, PAN Y, LIN W, et al. High-dimensional single-cell analysis delineates radiofrequency ablation induced immune microenvironmental remodeling in pancreatic cancer[J]. Cell Death Dis, 2020, 11(7): 589. DOI: 10.1038/s41419-020-02787-1. [28] SONG HB. Possible involvement of HSP70 in pancreatic cancer cell proliferation after heat exposure and impact on RFA postoperative patient prognosis[J]. Biochem Biophys Rep, 2019, 20: 100700. DOI: 10.1016/j.bbrep.2019.100700. [29] TONGUC T, STRUNK H, GONZALEZ-CARMONA MA, et al. US-guided high-intensity focused ultrasound (HIFU) of abdominal tumors: outcome, early ablation-related laboratory changes and inflammatory reaction. A single-center experience from Germany[J]. Int J Hyperthermia, 2021, 38(2): 65-74. DOI: 10.1080/02656736.2021.1900926. [30] TONGUC T, STRUNK H, GONZALEZ-CARMONA MA, et al. US-guided high-intensity focused ultrasound (HIFU) of abdominal tumors: outcome, early ablation-related laboratory changes and inflammatory reaction. A single-center experience from Germany[J]. Int J Hyperthermia, 2021, 38(2): 65-74. DOI: 10.1080/02656736.2021.1900926. [31] FILIPPIADIS D, PTOHIS N, EFTHYMIOU E, et al. A Technical report on the performance of percutaneous cryoneurolysis of splanchnic nerves for the treatment of refractory abdominal pain in patients with pancreatic cancer: initial experience[J]. Cardiovasc Intervent Radiol, 2021, 44(5): 789-794. DOI: 10.1007/s00270-020-02756-3. [32] XIN Y, YANG Y, CHEN Y, et al. Safety and efficacy of ultrasound-guided percutaneous coaxial core biopsy of pancreatic lesions: a retrospective study[J]. J Ultrasound, 2021, 24(3): 269-277. DOI: 10.1007/s40477-020-00487-2. [33] LACOMB JF, PLENKER D, TIRIAC H, et al. Single-pass vs 2-pass endoscopic ultrasound-guided fine-needle biopsy sample collection for creation of pancreatic adenocarcinoma organoids[J]. Clin Gastroenterol Hepatol, 2021, 19(4): 845-847. DOI: 10.1016/j.cgh.2020.02.045. [34] DING D, JAVED AA, CUNNINGHAM D, et al. Challenges of the current precision medicine approach for pancreatic cancer: A single institution experience between 2013 and 2017[J]. Cancer Lett, 2021, 497: 221-228. DOI: 10.1016/j.canlet.2020.10.039. [35] SHI HB, YANG MJ, WANG XL, et al. Feeding arteries of advanced pancreatic cancer: evaluation of 218 patients by digital subtract angiography[J]. Chin J Clinl Med, 2014, 21(1): 61-62. https://www.cnki.com.cn/Article/CJFDTOTAL-LCYX201401023.htm施惠斌, 杨敏捷, 王小林, 等. 晚期胰腺癌患者的肿瘤血供来源: 218例患者的DSA评价[J]. 中国临床医学, 2014, 21(1): 61-62. https://www.cnki.com.cn/Article/CJFDTOTAL-LCYX201401023.htm [36] DAOUD AZ, MULHOLLAND EJ, COLE G, et al. MicroRNAs in Pancreatic Cancer: biomarkers, prognostic, and therapeutic modulators[J]. BMC Cancer, 2019, 19(1): 1130. DOI: 10.1186/s12885-019-6284-y. [37] RUARUS A, VROOMEN L, PUIJK R, et al. Locally advanced pancreatic cancer: a review of local ablative therapies[J]. Cancers (Basel), 2018, 10(1): 16. DOI: 10.3390/cancers10010016. [38] HESS V, GLIMELIUS B, GRAWE P, et al. CA 19-9 tumour-marker response to chemotherapy in patients with advanced pancreatic cancer enrolled in a randomised controlled trial[J]. Lancet Oncol, 2008, 9(2): 132-138. DOI: 10.1016/S1470-2045(08)70001-9. [39] MAITHEL SK, MALONEY S, WINSTON C, et al. Preoperative CA 19-9 and the yield of staging laparoscopy in patients with radiographically resectable pancreatic adenocarcinoma[J]. Ann Surg Oncol, 2008, 15(12): 3512-3520. DOI: 10.1245/s10434-008-0134-5. [40] TAKAHASHI H, OHIGASHI H, ISHIKAWA O, et al. Serum CA19-9 alterations during preoperative gemcitabine-based chemoradiation therapy for resectable invasive ductal carcinoma of the pancreas as an indicator for therapeutic selection and survival[J]. Ann Surg, 2010, 251(3): 461-469. DOI: 10.1097/SLA.0b013e3181cc90a3. [41] GOONETILLEKE KS, SIRIWARDENA AK. Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer[J]. Eur J Surg Oncol, 2007, 33(3): 266-270. DOI: 10.1016/j.ejso.2006.10.004. [42] TEMPERO MA, MALAFA MP, AL-HAWARY M, et al. Pancreatic adenocarcinoma, version 2. 2017, NCCN clinical practice guidelines in oncology[J]. J Natl Compr Canc Netw, 2017, 15: 1028-1061. DOI: 10.6004/jnccn.2017.0131. [43] NIMURA Y, NAGINO M, TAKAO S, et al. Standard versus extended lymphadenectomy in radical pancreatoduodenectomy for ductal adenocarcinoma of the head of the pancreas: long-term results of a Japanese multicenter randomized controlled trial[J]. J Hepatobiliary Pancreat Sci, 2012, 19(3): 230-241. DOI: 10.1007/s00534-011-0466-6. [44] JANG JY, KANG MJ, HEO JS, et al. A prospective randomized controlled study comparing outcomes of standard resection and extended resection, including dissection of the nerve plexus and various lymph nodes, in patients with pancreatic head cancer[J]. Ann Surg, 2014, 259(4): 656-664. DOI: 10.1097/SLA.0000000000000384. [45] SIEGEL R, MA J, ZOU Z, et al. Cancer statistics, 2014[J]. CA Cancer J Clin, 2014, 64(1): 9-29. DOI: 10.3322/caac.21208. [46] National Cancer Network. Clinical practice guidelines in oncology. pancreatic adenocarcinoma. version 2.2014[DB/OL]. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. [47] TOL JA, GOUMA DJ, BASSI C, et al. Definition of a standard lymphadenectomy in surgery for pancreatic ductal adenocarcinoma: a consensus statement by the International Study Group on Pancreatic Surgery (ISGPS)[J]. Surgery, 2014, 156(3): 591-600. DOI: 10.1016/j.surg.2014.06.016. [48] BOCKHORN M, UZUNOGLU FG, ADHAM M, et al. Borderline resectable pancreatic cancer: a consensus statement by the International Study Group of Pancreatic Surgery (ISGPS)[J]. Surgery, 2014, 155(6): 977-988. DOI: 10.1016/j.surg.2014.02.001. [49] HARTWIG W, VOLLMER CM, FINGERHUT A, et al. Extended pancreatectomy in pancreatic ductal adenocarcinoma: definition and consensus of the International Study Group for Pancreatic Surgery (ISGPS)[J]. Surgery, 2014, 156(1): 1-14. DOI: 10.1016/j.surg.2014.02.009. [50] ASBUN HJ, CONLON K, FERNANDEZ-CRUZ L, et al. When to performance pancreatoduodenectomy in the absence of positive histology? A consensus statement by the International Study Group of Pancreatic Surgery[J]. Surgery, 2014, 155(5): 887-892. DOI: 10.1016/j.surg.2013.12.032. [51] GRECO SH, AUGUST DA, SHAH MM, et al. Neoadjuvant therapy is associated with lower margin positivity rates after Pancreaticoduodenectomy in T1 and T2 pancreatic head cancers: An analysis of the National Cancer Database[J]. Surg Open Sci, 2021, 3: 22-28. DOI: 10.1016/j.sopen.2020.12.001. [52] BLOMSTRAND H, GREEN H, FREDRIKSON M, et al. Clinical characteristics and blood/serum bound prognostic biomarkers in advanced pancreatic cancer treated with gemcitabine and nab-paclitaxel[J]. BMC Cancer, 2020, 20(1): 950. DOI: 10.1186/s12885-020-07426-8. [53] Interventional Group, Radiology Branch, Chinese Medical Association. Guide to intra-arterial infusion chemotherapy for pancreatic cancers (draft text)[J]. J Intervent Radiol, 2012, 21(5): 353-355. DOI: 10.3969/j.issn.1008-794X.2012.05.001.中华医学会放射学分会介入学组. 胰腺癌经动脉灌注化疗指南(草案)[J]. 介入放射学杂志, 2012, 21(5): 353-355. DOI: 10.3969/j.issn.1008-794X.2012.05.001. [54] Japan Pancreas Society. Classification of pancreatic carcinoma[M]. Second English ed. Tokyo: Kanehara & Co., Ltd., 2003. [55] PHILIP PA, BENEDETTI J, CORLESS CL, et al. Phase Ⅲ study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group-directed intergroup trial S0205[J]. J Clin Oncol, 2010, 28(22): 3605-3610. DOI: 10.1200/JCO.2009.25.7550. [56] KINDLER HL, NIEDZWIECKI D, HOLLIS D, et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase Ⅲ trial of the Cancer and Leukemia Group B (CALGB 80303)[J]. J Clin Oncol, 2010, 28(22): 3617-3622. DOI: 10.1200/JCO.2010.28.1386. [57] MOORE MJ, GOLDSTEIN D, HAMM J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase Ⅲ trial of the National Cancer Institute of Canada Clinical Trials Group[J]. J Clin Oncol, 2007, 25(15): 1960-1966. DOI: 10.1200/JCO.2006.07.9525. [58] 58. TANAKA T, SAKAGUCHI H, SHO M, et al. A novel interventional radiology technique for arterial infusion chemotherapy against advanced pancreatic cancer[J]. AJR Am J Roentgenol, 2009, 192(4): W168-W177. DOI: 10.2214/AJR.08.1392. [59] CONROY T, DESSEIGNE F, YCHOU M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer[J]. N Engl J Med, 2011, 364(19): 1817-1825. DOI: 10.1056/NEJMoa1011923. [60] KULKE MH, TEMPERO MA, NIEDZWIECKI D, et al. Randomized phase Ⅱ study of gemcitabine administered at a fixed dose rate or in combination with cisplatin, docetaxel, or irinotecan in patients with metastatic pancreatic cancer: CALGB 89904[J]. J Clin Oncol, 2009, 27(33): 5506-5512. DOI: 10.1200/JCO.2009.22.1309. [61] CUNNINGHAM D, CHAU I, STOCKEN DD, et al. Phase Ⅲ randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer[J]. J Clin Oncol, 2009, 27(33): 5513-5518. DOI: 10.1200/JCO.2009.24.2446. [62] LOUVET C, LABIANCA R, HAMMEL P, et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase Ⅲ trial[J]. J Clin Oncol, 2005, 23(15): 3509-3516. DOI: 10.1200/JCO.2005.06.023. [63] OETTLE H, RICHARDS D, RAMANATHAN RK, et al. A phase Ⅲ trial of pemetrexed plus gemcitabine versus gemcitabine in patients with unresectable or metastatic pancreatic cancer[J]. Ann Oncol, 2005, 16(10): 1639-1645. DOI: 10.1093/annonc/mdi309. [64] ABOU-ALFA GK, LETOURNEAU R, HARKER G, et al. Randomized phase Ⅲ study of exatecan and gemcitabine compared with gemcitabine alone in untreated advanced pancreatic cancer[J]. J Clin Oncol, 2006, 24(27): 4441-4447. DOI: 10.1200/JCO.2006.07.0201. [65] TEMPERO M, PLUNKETT W, RUIZ VAN HAPEREN V, et al. Randomized phase Ⅱ comparison of dose-intense gemcitabine: thirty-minute infusion and fixed dose rate infusion in patients with pancreatic adenocarcinoma[J]. J Clin Oncol, 2003, 21(18): 3402-3408. DOI: 10.1200/JCO.2003.09.140. [66] MENON KV, GOMEZ D, SMITH AM, et al. Impact of margin status on survival following pancreatoduo-denectomy for cancer: the Leeds Pathology Protocol (LEEPP)[J]. HPB(Oxford), 2009, 11(1): 18-24. DOI: 10.1111/j.1477-2574.2008.00013.x. [67] WANG JJ, ZHANG FJ. Tumor radioactive particle therapy specification[M]. Beijing: People's Health Publishing House, 2016.王俊杰, 张福君. 肿瘤放射性粒子治疗规范[M]. 北京: 人民卫生出版社, 2016. [68] WANG CF, ZHAO P, LI YX, et al. Role of 125I seed implantation in treatment of unresectable pancreatic cancer[J]. Natl Med J China, 2012, 90(2): 92-95. DOI: 10.3760/cma.j.issn.0376-2491.2010.02.007.王成锋, 赵平, 李晔雄, 等. 125I粒子植入治疗局部进展期胰腺癌[J]. 中华医学杂志, 2012, 90(2): 92-95. DOI: 10.3760/cma.j.issn.0376-2491.2010.02.007. [69] FUJⅡ T, SATOI S, YAMADA S, et al. Clinical benefits of neoadjuvant chemoradiotherapy for adenocarcinoma of the pancreatic head: an observational study using inverse probability of treatment weighting[J]. J Gastroenterol, 2017, 52(1): 81-93. DOI: 10.1007/s00535-016-1217-x. [70] LUNDIN J, ROBERTS PJ, KUUSELA P, et al. Prognostic significance of serum CA242 in pancreatic cancer. A comparison with CA19-9[J]. Anticancer Res, 1995, 15(5): 2181-2186. [71] MA J, LUO J, GU J, et al. Malignant obstructive jaundice treated with intraluminal placement of Iodine-125 seed strands and metal stents: An analysis of long-term outcomes and prognostic features[J]. Brachytherapy, 2018, 17(4): 689-695. DOI: 10.1016/j.brachy.2018.04.001. [72] YANG M, YAN Z, LUO J, et al. A pilot study of intraluminal brachytherapy using 125I seed strand for locally advanced pancreatic ductal adenocarcinoma with obstructive jaundice[J]. Brachytherapy, 2016, 15(6): 859-864. DOI: 10.1016/j.brachy.2016.05.004. [73] YU BF. Intratumoral injection of alcohol saturated solution of anticancer drugs and its pharmacokinetics — a new concept of tumor self therapeutic coagulation block as sustained-release Library of anticancer drugs[J]. Chin J Cancer Prev Treat, 1994, 2: 97-100. https://www.cnki.com.cn/Article/CJFDTOTAL-QLZL402.006.htm于保法. 抗癌药物酒精饱和液肿瘤内注射疗法及其药物动力学研究——一种肿瘤自身治疗性凝固块作为抗癌药物缓释库的新概念[J]. 中华肿瘤防治杂志, 1994, 2: 97-100. https://www.cnki.com.cn/Article/CJFDTOTAL-QLZL402.006.htm [74] Committee of Experts on Radioactive Particle Implantation Technology, Chinese Medical Doctor Association; Particle Treatment Branch, Committee of Minimally Invasive Therapy in Oncology, Chinese Anti-cancer Association. Chinese expert consensus on treatment of pancreatic cancer by radioactive 125I particle implantation (2017)[J]. J Clin Hepatol, 2018, 34(4): 716-723. DOI: 10.3969/j.issn.1001-5256.2018.04.007.中国医师协会放射性粒子植入技术专家委员会, 中国抗癌协会肿瘤微创治疗专业委员会粒子治疗分会. 放射性125I粒子植入治疗胰腺癌中国专家共识(2017年版)[J]. 临床肝胆病杂志, 2018, 34(4): 716-723. DOI: 10.3969/j.issn.1001-5256.2018.04.007. [75] ZHU HD, GUO JH, HUANG MH, et al. Irradiation stents vs. conventional metal stents for unresectable malignant biliary obstruction: A multicenter trial[J]. J Hepatol, 2018, 68(5): 970-977. DOI: 10.1016/j.jhep.2017.12.028. [76] BU JQ, YU BF. Slow intra-tumor release of drugs on B16 melonoma in mice[J]. J Shandong Univ (Health Sciences), 2007, 45(10): 988-991. DOI: 10.3969/j.issn.1671-7554.2007.10.005.卜洁琼, 于保法. 瘤内缓释治疗对B16黑色素瘤小鼠影响的实验研究[J]. 山东大学学报(医学版), 2007, 45(10): 988-991. DOI: 10.3969/j.issn.1671-7554.2007.10.005. [77] YU BF. Tumor interventional chemoimmunotherapy[M]. Beijing: Military Medical Press, 2014.于保法. 肿瘤介入化学免疫治疗学[M]. 北京: 军事医学出版社, 2014. [78] CAI P, CHEN GM, YANG XG, et al. Clinical efficacy of local hyperthermia in the treatment of advanced pancreatic cancer[J]. Mod Oncol, 2015, 23(5): 655-657. DOI: 10.3969/j.issn.1672-4992.2015.05.23.蔡鹏, 陈桂明, 杨先国, 等. 局部热疗联合化疗治疗晚期胰腺癌的疗效观察[J]. 现代肿瘤医学, 2015, 23(5): 655-657. DOI: 10.3969/j.issn.1672-4992.2015.05.23. [79] ZONG Y, NI SX, ZHANG XQ. Clinical analysis of oxaliplatin treatment for the advanced pancreatic cancer through regional arterial infusion chemotherapy[J]. Pract Pharm Clin Remedies, 2013, 16(4): 284-285. DOI: 10.3969/j.issn.1673-0070.2013.04.006.宗煜, 倪绍祥, 张宪庆. 奥沙利铂经区域性动脉灌注化疗治疗晚期胰腺癌临床分析[J]. 实用药物与临床, 2013, 16(4): 284-285. DOI: 10.3969/j.issn.1673-0070.2013.04.006. [80] REN XH, LIU L, LIU PP, et al. Changes of T lymphocyte subgroups in cancer patients and its relationship with tumor staging[J]. J Third Milit Med Univ, 2006, 28(18): 1906-1908. DOI: 10.3321/j.issn:1000-5404.2006.18.024.任秀红, 刘莉, 刘平平, 等. 恶性肿瘤患者T细胞亚群变化及其与肿瘤分期的关系[J]. 第三军医大学学报, 2006, 28(18): 1906-1908. DOI: 10.3321/j.issn:1000-5404.2006.18.024. [81] BALZANO G, DI CARLO V. Is CA 19-9 useful in the management of pancreatic cancer?[J]. Lancet Oncol, 2008, 9(2): 89-91. DOI: 10.1016/S1470-2045(08)70011-1. [82] HAN S, CAO C, TANG T, et al. ROBO3 promotes growth and metastasis of pancreatic carcinoma[J]. Cancer Lett, 2015, 366(1): 61-70. DOI: 10.1016/j.canlet.2015.06.004. [83] LIU T, WU G, CHENG J, et al. Multifunctional lymph-targeted platform based on Mn@ mSiO2 nanocomposites: Combining PFOB for dual-mode imaging and DOX for cancer diagnose and treatment[J]. Nano Research, 2016, 9: 473-489. DOI: 10.1007/s12274-015-0929-1 [84] LIU LX, JI W, WANG JH, et al. A retrospective analysis of patients with unresectable pancreatic cancer treated with combined transarterial intervention and three dimensional conformal radiotherapy[J]. China Oncol, 2011, 21(1): 46-51. DOI: 10.3969/j.issn.1007-3969.2011.01.009.刘凌晓, 姬巍, 王建华, 等. 介入治疗联合三维适形放疗治疗不能手术切除的胰腺癌患者疗效分析[J]. 中国癌症杂志, 2011, 21(1): 46-51. DOI: 10.3969/j.issn.1007-3969.2011.01.009. [85] VINCENT A, HERMAN J, SCHULICK R, et al. Pancreatic cancer[J]. Lancet, 2011, 378(9791): 607-620. DOI: 10.1016/S0140-6736(10)62307-0. [86] KHORANA AA, MANGU PB, BERLIN J, et al. Potentially curable pancreatic cancer: american society of clinical oncology clinical practice guideline update[J]. J Clin Oncol, 2017, 35(20): 2324-2328. DOI: 10.1200/JCO.2017.72.4948. [87] BERGER AC, GARCIA M Jr, HOFFMAN JP, et al. Postresection CA 19-9 predicts overall survival in patients with pancreatic cancer treated with adjuvant chemoradiation: a prospective validation by RTOG 9704[J]. J Clin Oncol, 2008, 26(36): 5918-5922. DOI: 10.1200/JCO.2008.18.6288. [88] CHEN Y, WANG XL, WANG JH, et al. Transarterial infusion with gemcitabine and oxaliplatin for the treatment of unresectable pancreatic cancer[J]. Anticancer Drugs, 2014, 25(8): 958-963. DOI: 10.1097/CAD.0000000000000120. [89] HUANG M, FEI Y, YANG YS, et al. Transarterial infusion chemotherapy of gemcitabine for the management of advanced pancreatic carcinoma[J]. West China Med J, 2004, 19(3): 373-375. DOI: 10.3969/j.issn.1002-0179.2004.03.012.黄明, 飞勇, 杨银山, 等. 经动脉灌注健择治疗中晚期胰腺癌临床疗效观察[J]. 华西医学, 2004, 19(3): 373-375. DOI: 10.3969/j.issn.1002-0179.2004.03.012. [90] RYAN DP, HONG TS, BARDEESY N. Pancreatic adenocarcinoma[J]. N Engl J Med, 2014, 371(11): 1039-1049. DOI: 10.1056/NEJMra1404198 期刊类型引用(1)
1. 努尔孜叶·阿布里克木,热娜古丽·努尔,李静,陆晨. Toll样受体9水平与免疫球蛋白A肾病患者临床病理特征及预后关系. 临床军医杂志. 2025(01): 64-68 .  百度学术
百度学术其他类型引用(0)
-




 PDF下载 ( 2941 KB)
PDF下载 ( 2941 KB)

 下载:
下载:







 下载:
下载:
 百度学术
百度学术