非酒精性脂肪性肝病患者粪便短链脂肪酸含量测定的临床意义
DOI: 10.3969/j.issn.1001-5256.2022.06.016
Clinical significance of the determination of fecal short-chain fatty acids in patients with nonalcoholic fatty liver disease
-
摘要:
目的 通过分析非酒精性脂肪性肝病(NAFLD)各疾病谱患者粪便短链脂肪酸(SCFA)含量及非酒精性脂肪性肝炎(NASH)高危患者治疗前后粪便SCFA含量的差异,初步探讨SCFA肠道代谢与NAFLD各疾病谱发生发展的关系。 方法 选取2020年7月—2021年7月青海大学附属医院临床诊断为NAFLD的患者共90例,分为单纯性脂肪肝(NAFL)组(n=30)、NASH组(n=30)、非酒精性脂肪性肝纤维化组(n=30),选取同期健康体检者40例作为对照组,收集4组研究对象病例资料、粪便SCFA含量, 以及NASH高危患者10例治疗干预3个月后临床指标和粪便SCFA含量。满足正态分布的计量资料多组间比较采用方差分析,组内比较采用配对样本t检验;不满足正态分布的计量资料采用Kruskal-Wallis H检验,组内比较采用配对样本的Wilcoxon符号秩和检验,相关性分析采用Spearman相关分析,诊断性评价采用ROC曲线分析。 结果 非酒精性脂肪性肝纤维化组戊酸、己酸含量显著高于健康对照组,NAFL组戊酸、己酸含量显著低于健康对照组(P值均<0.05);非酒精性脂肪性肝纤维化组戊酸、己酸含量显著高于NAFL组(P值均<0.05);非酒精性脂肪性肝纤维化组戊酸含量显著高于NASH组(P<0.05);NASH组己酸含量显著高于NAFL组(P<0.05)。NASH组高危患者治疗后糖化血红蛋白、空腹血糖(FPG)、TG、TC、ALT、AST、GGT、总胆汁酸(TBA)、PT、尿酸(UA)、受控衰减参数(CAP)、肝脏硬度值(LSM)均明显低于治疗前(Z值分别为-2.805、-2.703、-2.193、-2.599、-2.805、-2.701、-2.803、-1.988、-2.807、-2.803、-2.803、-2.668,P值均<0.05);NASH组高危患者治疗后乙酸、丙酸含量均显著高于治疗前(Z值分别为-2.803、-2.803,P值均<0.05);异丁酸含量低于治疗前(Z=-2.803,P<0.05);戊酸诊断非酒精性脂肪性肝纤维化的AUC为0.842,最佳界值为141.42 μg/g,灵敏度86.7%,特异度70%;己酸诊断非酒精性脂肪性肝纤维化的AUC为0.819,最佳界值为6.93 μg/g,灵敏度70%,特异度85%。 结论 戊酸、己酸可能促进NAFLD疾病谱发展;乙酸、丙酸对NAFLD患者肝脏可能存在一定程度的保护作用,异丁酸可能促进NASH的发生发展;乙酸、丙酸对肝脏的保护作用可能进一步导致糖化血红蛋白、FPG、TG、TC、ALT、AST、GGT、TBA、PT、UA、CAP、LSM等指标的降低;戊酸、己酸诊断价值劣于三型前胶原肽,但优于四型胶原、透明质酸,推测以戊酸为141.42 μg/g、己酸为6.93 μg/g为临界值可作为早期筛查非酒精性脂肪性肝纤维化的辅助诊断指标。 Abstract:Objective To investigate the association of the metabolism of intestinal short-chain fatty acids (SCFAs) with the development and progression of the disease spectrum of nonalcoholic fatty liver disease (NAFLD) by determining the content of fecal SCFAs in patients with different NAFLD diseases and the change in the content of fecal SCFAs after treatment in patients at a high risk of nonalcoholic steatohepatitis (NASH). Methods A total of 90 patients who were diagnosed with NAFLD in The Affiliated Hospital of Qinghai University from July 2020 to July 2021 were enrolled and divided into simple nonalcoholic fatty liver (NAFL) group with 30 patients, NASH group with 30 patients, and nonalcoholic fatty liver fibrosis group with 30 patients, and 40 individuals who underwent physical examination during the same period of time were enrolled as control group. Related case data and fecal SCFAs content were collected for the four groups, and related clinical indices and fecal SCFAs content were collected for 10 patients at a high risk of NASH after 3 months of intervention. The analysis of variance was used for comparison of normally distributed continuous data between multiple groups, and the paired samples t-test was used for comparison within each group; the Kruskal-Wallis H test was used for comparison of non-normally distributed continuous data between multiple groups, and the paired samples Wilcoxon signed rank sum test was used for comparison within each group; a Spearman correlation analysis was used to investigate the correlation between variables; the receiver operating characteristic (ROC) curve analysis was used for diagnostic evaluation. Results Compared with the control group, the nonalcoholic fatty liver fibrosis group had significantly higher contents of valeric acid and caproic acid, and the NAFL group had significantly lower contents of valeric acid and caproic acid (all P < 0.05); the nonalcoholic fatty liver fibrosis group had significantly higher contents of valeric acid and caproic acid than the NAFL group (P < 0.05); the nonalcoholic fatty liver fibrosis group had a significantly higher content of valeric acid than the NASH group (P < 0.05); the NASH group had a significantly higher content of caproic acid than the NAFL group (P < 0.05). After treatment, the high-risk patients in the NASH group had significant reductions in HbA1c, fasting plasma glucose (FPG), triglyceride (TG), total cholesterol (TC), alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transpeptidase (GGT), total bile acid (TBA), prothrombin time (PT), uric acid (UA), controlled attenuation parameter (CAP), and liver stiffness measurement (LSM) (Z=-2.805, -2.703, -2.193, -2.599, -2.805, -2.701, -2.803, -1.988, -2.807, -2.803, -2.803, and -2.668, all P < 0.05); for these patients, the contents of acetic acid and propionic acid after treatment were significantly higher than those before treatment (Z=-2.803 and -2.803, both P < 0.05), and the content of isobutyric acid after treatment was significantly lower than that before treatment (Z=-2.803, P < 0.05). In the diagnosis of nonalcoholic fatty liver fibrosis, valeric acid had an area under the ROC curve (AUC) of 0.842, with a sensitivity of 86.7% and a specificity of 70% at the optimal cut-off value of 141.42 μg/g; caproic acid had an AUC of 0.819, with a sensitivity of 70% and a specificity of 85% at the optimal cut-off value of 6.93 μg/g. Conclusion Valeric acid and caproic acid may promote the development of NAFLD disease spectrum. Acetic acid and propionic acid may have a certain protective effect on the liver of NAFLD patients, and isobutyric acid may promote the development and progression of NASH. The protective effect of acetic acid and propionic acid on the liver may further lead to the reductions in HbA1c, FPG, TG, TC, ALT, AST, GGT, TBA, PT, UA, CAP, and LSM. Valeric acid and caproic acid have an inferior diagnostic value to PIIIP N-P and a superior diagnostic value to type IV collagen and hyaluronic acid. Valeric acid with the optimal cut-off value of 141.42 μg/g and caproic acid with the optimal cut-off value of 6.93 μg/g can be used as the auxiliary diagnostic indicators for the early diagnosis of nonalcoholic fatty liver fibrosis. -
Key words:
- Non-alcoholic Fatty Liver Disease /
- Liver Cirrhosis /
- Fatty Acids, Volatile /
- Feces
-
非酒精性脂肪性肝病(NAFLD)是代谢综合征的肝表现,常合并肥胖、血脂异常及胰岛素抵抗(IR),疾病谱包括单纯性脂肪肝(NAFL)、非酒精性脂肪性肝炎(NASH)及其相关肝纤维化、肝硬化和肝细胞癌。NAFLD也是西方国家常见的慢性肝病,预计到2030年NAFLD将成为肝移植最常见的适应证[1-3]。NAFLD可导致肝病残疾和死亡,还与代谢综合征(metabolic syndrome, MetS)、2型糖尿病(T2DM)、动脉硬化性心血管疾病及结直肠肿瘤等密切相关[2]。研究[4]显示从1997年—2014年,NAFLD发病率增加了5倍,其中18~39岁的年轻人增长率最高(7倍),表明NAFLD发病率显著增加且逐渐年轻化。当前认为NAFLD是与遗传-环境-代谢应激相关且无过量饮酒史、肝细胞脂肪变性和脂质贮积为特征的临床病理综合征[5]。至今NAFLD的发病机制仍未完全阐明,经典的“双重打击学说”[6]认为肥胖和IR会造成脂质在肝细胞中堆积,为肝细胞承受的“第一次打击”,继之出现氧化应激、线粒体功能障碍、脂质过氧化等进一步促进肝细胞炎症反应;目前广为认可的“多重打击学说”[7]是在“双重打击学说”的基础上,先天免疫调节紊乱、自噬、营养元素、肠-肝轴、表观遗传学也是影响NAFLD向NASH发展的重要因素。研究[8]证明肠道菌群-代谢物,肠-肝轴之间存在一定程度联系,并且参与NAFLD的发生发展。作为肠-肝轴重要组成部分,肠道菌群通过增加宿主能量摄入、调节胆碱及胆汁酸代谢、激活模式识别受体等机制促发炎症反应,促使NAFLD发生发展;短链脂肪酸(short chain fatty acids, SCFA)是肠道菌群分解碳水化合物或氨基酸的代谢物,包括乙酸、丙酸、丁酸、戊酸、己酸、异丁酸、异戊酸、异己酸等,其中乙酸、丙酸、丁酸的含量较高。正常菌群每日可产生50~100 mmol/L的SCFA,为肠道上皮细胞提供能量,其可通过降低结肠pH、抑制病原体生长、促进水钠吸收等途径参与肠道免疫稳态的调控[9]。多项研究[10-11]表明,SCFA影响NAFLD的进展,具体机制尚不清楚。越来越多证据[12-14]表明,SCFA在维持肠道和代谢方面发挥重要作用。本研究初步探讨SCFA肠道代谢与NAFLD发病机制的关系,为NAFLD的后续防治及临床诊断提供新的理论依据。
1. 材料与方法
1.1 研究对象
通过前瞻性研究方法,选取2020年7月—2021年7月在青海大学附属医院临床诊断为NAFLD的患者共90例,其中NAFL组30例(有病理组织学诊断患者10例)、NASH组30例(有病理组织学诊断患者22例)、非酒精性脂肪性肝纤维化30例(有病理组织学诊断患者30例),90例患者均行Fibroscan检测。分组依据参照《非酒精性脂肪性肝病诊疗指南》[15]中临床分型标准,并选取同期健康体检者40例作为对照组。同时选取NASH组高危患者16例(合并高血压、糖尿病、高脂血症、冠心病、肺气肿、肺心病等)进行随访,脱失病例6例,最终纳入NASH高危患者10例。
纳入标准:(1)居住青海地区10年以上;(2)年龄18~65岁;(3)无饮酒史或过去12个月每周饮用乙醇量男性<210 g,女性<140 g;(4)合并并发症者(高血糖、高血脂、高血压、高尿酸等);(5)肝脏影像学表现符合弥漫性脂肪肝的影像学诊断标准;(6)肝活组织检查组织学改变符合脂肪性肝病的病理学诊断标准。以上符合1~4项和第5或第6中任何一项。排除标准:(1)既往有慢性病毒肝炎感染、有严重的自身免疫性疾病、肝豆状核变性、全胃肠外营养、长期不规律口服药物(抗生素、中药、藏药等)者;(2)既往有大量饮酒史者(过去12个月每周饮用乙醇量男性≥210 g,女性≥140 g);(3)临床病理资料不完整者。
1.2 资料采集
所有研究对象采集病史,编号后记录姓名、年龄、饮酒情况,计算BMI,进一步采集粪便标本,标本编号后置于-80 ℃冰箱保存。Fibroscan检查所有研究对象受控衰减参数(CAP)和肝脏硬度值(LSM)。全自动生化分析仪检测各组标本的ALT、AST、GGT、糖化血红蛋白(HbA1c)、空腹血糖(FPG)、TG、TC、尿酸(UA)、TBil、DBil、IBil、Alb、Glb、ALP、胆汁酸(TBA)、凝血酶原时间(PT)、活化部分凝血活酶时间(APTT)。全自动生化分析仪检测健康组及非酒精性脂肪性肝纤维化组三型前胶原肽(PⅢP N-P)、四型胶原(Ⅳ-C)、透明质酸(HA)、层粘连蛋白(LN),与SCFA联合分析对非酒精性脂肪性肝纤维化诊断价值。
1.3 SCFA水平检测
(1) GC-MS检测方法。色谱条件:色谱柱Agilent HP-INNOWAX毛细管柱(30 m×0.25 mm ID×0.25 μm);分流进样,进样量1 μL,分流比10∶ 1。进样口温度250 ℃;离子源温度230 ℃;传输线温度250 ℃,四极杆温度150 ℃。程序升温起始温度90 ℃;然后以10 ℃/min升温至120 ℃;再以5 ℃/min升温至150 ℃;最后以25 ℃/min升温至250 ℃维持2 min。载气为氦气,载气流速1.0 mL/min。MS条件:电子轰击电离(EI)源,SIM扫描方式,电子能量70 eV。(2)主要试剂。磷酸(国药),乙醚(国药),乙酸(sigma≥ 99.5%),丙酸(sigma>99.0%),丁酸(sigma>99.0%),异丁酸(sigma>99.0%),戊酸(sigma>98.0%),异戊酸(sigma>99.0%),己酸(阿拉丁≥99.5%),异己酸(sigma>98%)。(3)主要仪器平台。Thermo TRACE 1310-ISQ LT气-质联用仪(Thermo,美国);涡旋仪(QL-866);冷冻离心机(湘仪,H1850R)。(4)标准品配置。量取乙酸、丙酸、丁酸、异丁酸、戊酸、异戊酸、己酸纯标准品适量,用乙醚配制成0.02、0.1、0.5、2、5、10、50、100、250、500 μg/mL 10个混合标准浓度梯度。母液及工作标准溶液均保存于0 ℃。(5)样品前处理。取适量样本,加50 μL 15%磷酸,再加125 μg/mL的内标(异己酸)溶液100 μL和乙醚400 μL匀浆1 min,于4 ℃ 12 000 r/min心10 min,取上清上机测试。
1.4 统计学方法
采用SPSS 26.0统计学软件进行统计分析。符合正态分布的计量资料用x±s表示,多组间比较选取单因素方差分析及LSD-t检验多重比较,组内比较采用配对样本t检验;非正态分布的计量资料用M(P25~P75)表示,多组间比较采用Kruskal-Wallis H检验及Bonferroni多重比较法,组内比较采用配对样本的Wilcoxon符号秩和检验;计数资料组间比较采用χ2检验;相关性分析采用Spearman相关分析,诊断性评价采用ROC曲线分析。P<0.05为差异有统计学意义。
2. 结果
2.1 一般资料
入选的90例患者中男56例,女34例,平均年龄(49.82±10.95)岁;健康对照组40例,其中男23例,女17例,平均年龄(49.10±10.85)岁。各组性别、年龄一般基线资料差异无统计学意义(P值均>0.05)(表 1)。
表 1 4组研究对象人口学资料比较Table 1. Comparison of demographic data of four groups of subjects项目 健康组
(n=40)NAFL组
(n=30)NASH组
(n=30)非酒精性脂肪性肝纤维化组
(n=30)统计值 P值 性别[例(%)] χ2=4.267 0.234 男 23(57.5) 17(56.7) 23(76.7) 16(53.3) 女 17(42.5) 13(43.3) 7(23.3) 14(46.7) 年龄(岁) 49.10±10.85 53.40±9.00 49.03±11.21 47.03±11.79 F=1.869 0.138 2.2 生化指标比较
NAFL组、NASH组、非酒精性脂肪性肝纤维化组BMI、FPG、TG、DBil、CAP均明显高于健康对照组,IBil、Alb、APTT均明显低于健康对照组(P值均<0.05);非酒精性脂肪性肝纤维化组BMI、HbA1c、ALT、AST、GGT、TBA、LSM明显高于NAFL组(P值均<0.05);非酒精性脂肪性肝纤维化组BMI、TBil、DBil、IBil均低于NASH组(P值均<0.05),Alb、LSM均高于NASH组(P值均<0.05);NASH组BMI、HbA1c、ALT、AST、GGT、LSM均高于NAFL组(P值均<0.05)(表 2)。
表 2 4组研究对象临床生化指标比较Table 2. Comparison of clinical biochemical indexes of four groups项目 健康组(n=40) NAFL组(n=30) NASH组(n=30) 非酒精性脂肪性肝纤维化组(n=30) 统计值 P值 BMI(kg/m2) 20.75±1.62 25.18±2.731) 28.28±3.151)2) 26.70±2.281)2)3) F=62.852 <0.001 HbA1c(%) 5.00(4.43~5.58) 5.30(4.45~6.65) 7.95(7.60~8.33)1)2) 7.85(7.23~9.03)1)2) H=84.220 <0.001 FPG(mmol/L) 4.80(4.40~5.27) 6.90(5.73~8.03)1) 6.75(5.45~7.80)1) 8.20(7.20~9.13)1) H=57.614 <0.001 TG(mmol/L) 1.01(0.70~1.30) 2.17(1.35~3.41)1) 2.16(1.48~3.05)1) 2.90(2.10~4.30)1) H=62.740 <0.001 TC(mmol/L) 4.47±0.74 4.69±0.83 4.52±1.13 4.93±0.73 F=1.831 0.145 ALT(U/L) 25.00(21.00~30.50) 28.50(19.75~33.25) 87.00(50.50~150.00)1)2) 50.00(31.50~73.25)1)2) H=64.890 <0.001 AST(U/L) 21.80(18.00~27.00) 20.50(17.75~23.50) 37.50(27.50~62.25)1)2) 36.50(21.75~63.75)1)2) H=36.106 <0.001 ALP(U/L) 94.00(78.25~119.75) 87.50(74.00~107.00) 101.50(91.50~121.75) 109.00(81.25~137.50) H=6.409 0.093 GGT(U/L) 25.00(19.00~31.75) 30.50(24.75~39.00) 102.00(57.50~268.75)1)2) 78.50(40.50~123.00)1)2) H=69.178 <0.001 TBil(μmol/L) 18.25(16.40~21.78) 13.95(11.23~18.68)1) 17.80(11.10~26.70) 11.90(8.83~17.20)1)3) H=22.698 <0.001 DBil(μmol/L) 2.50(1.90~2.98) 4.15(3.33~5.20)1) 4.90(4.13~8.00)1) 3.75(2.53~5.25)1)3) H=52.403 <0.001 IBil(μmol/L) 16.13±2.93 11.04±5.091) 13.09±7.121) 8.95±4.061)3) F=13.548 <0.001 TBA(μmol/L) 4.74(3.80~5.34) 4.04(2.30~6.87) 4.71(3.73~7.41) 7.52(4.09~9.87)1)2) H=12.455 0.006 Alb(g/L) 48.12±3.51 43.60±3.291) 41.70±4.571) 45.25±3.971)3) F=17.546 <0.001 GLB(g/L) 26.21±6.36 26.32±2.90 26.83±4.40 27.67±5.40 F=0.554 0.646 PT(s) 10.95(9.80~11.60) 9.90(9.68~10.23)1) 10.20(9.68~10.63) 9.75(9.45~10.33)1) H=17.139 0.001 APTT(s) 27.38±2.37 23.47±2.661) 24.97±4.641) 23.33±4.091) F=10.407 <0.001 UA(μmol/L) 301.00(258.50~328.75) 310.00(243.25~440.50) 347.00(292.75~429.75)1) 321.50(286.25~380.50) H=11.066 0.011 CAP(dB/m) 177.66(155.25~198.00) 290.50(258.25~316.50)1) 334.50(295.00~359.75)1) 313.50(262.25~342.50)1) H=81.927 <0.001 LSM(kPa) 4.65(4.13~5.28) 4.00(3.68~4.85) 6.25(5.30~7.38)1)2) 9.60(8.85~10.48)1)2)3) H=89.958 <0.001 注:与健康组比较,1)P<0.05;与NAFL组比较,2)P<0.05;与NASH组比较,3)P<0.05。 2.3 各组SCFA水平测定
非酒精性脂肪性肝纤维化组戊酸、己酸含量显著高于健康对照组,NAFL组戊酸、己酸含量显著低于健康对照组(P值均<0.05);非酒精性脂肪性肝纤维化组戊酸、己酸含量显著高于NAFL组(P值均<0.05);非酒精性脂肪性肝纤维化组戊酸含量显著高于NASH组(P<0.05);NASH组己酸含量显著高于NAFL组(P<0.05)(表 3)。
表 3 4组研究对象SCFA水平测定Table 3. Determination of short chain fatty acids level of four groups of subjects项目 健康组(n=40) NAFL组(n=30) NASH组(n=30) 非酒精性脂肪性肝纤维化组(n=30) 统计值 P值 乙酸(μg/g) 1463.63±408.10 1438.24±539.47 1259.68±467.97 1343.57±436.10 F=1.339 0.265 丙酸(μg/g) 702.74±241.86 747.38±349.46 756.53±421.36 710.30±321.60 F=0.212 0.888 异丁酸(μg/g) 68.74(48.83~104.64) 50.09(16.91~94.80) 66.99(24.30~106.96) 50.40(35.02~83.74) H=2.763 0.430 丁酸(μg/g) 700.38±300.18 567.76±329.82 591.70±282.18 763.35±343.67 F=2.644 0.052 异戊酸(μg/g) 64.60(45.13~102.57) 47.00(16.94~74.37) 65.61(21.95~112.67) 47.64(20.28~77.13) H=4.635 0.201 戊酸(μg/g) 123.18(102.53~159.78) 52.64(7.38~128.77)1) 99.85(31.73~232.23) 204.17(160.05~236.48)1)2)3) H=36.082 <0.001 己酸(μg/g) 4.92(3.81~6.16) 1.33(0.87~2.94)1) 6.39(1.61~28.03)2) 7.96(5.95~10.45)1)2) H=36.414 <0.001 注:与健康组比较,1)P<0.05;与NAFL组比较,2)P<0.05;与NASH组比较,3)P<0.05。 2.4 疾病严重程度与SCFA的相关性
相关分析显示疾病严重程度与丁酸、戊酸、己酸显著正相关(r值分别为0.238、0.531、0.510,P值分别为0.024、<0.001、<0.001),与乙酸、丙酸、异丁酸、异戊酸无相关性(P值均>0.05)。
2.5 NASH组高危患者治疗前后对比分析
2.5.1 临床指标比较
NASH组高危患者10例治疗后HbA1c、FPG、TG、TC、ALT、AST、GGT、TBA、PT、UA、CAP、LSM均明显低于治疗前(P值均<0.05)(表 4)。
表 4 治疗前后临床指标比较Table 4. Comparison of clinical indexes before and after treatment项目 治疗前 治疗后 Z值 P值 HbA1c(%) 7.75(7.28~8.35) 3.80(3.43~4.80) -2.805 0.005 FPG(mmol/L) 7.30(5.45~7.95) 4.70(4.13~5.33) -2.703 0.007 TG(mmol/L) 2.40(1.45~3.20) 1.42(1.32~1.58) -2.193 0.028 TC(mmol/L) 4.49(3.95~5.43) 3.64(3.15~4.23) -2.599 0.009 ALT(U/L) 151.00(119.50~168.50) 34.00(21.00~42.75) -2.805 0.005 AST(U/L) 51.50(32.75~113.50) 19.50(17.75~21.25) -2.701 0.007 ALP(U/L) 108.50(93.50~121.50) 83.50(73.50~97.50) -1.939 0.052 GGT(U/L) 100.50(67.50~136.25) 24.50(19.00~30.25) -2.803 0.005 TBil(μmol/L) 19.30(16.90~37.25) 20.70(19.30~21.70) -0.459 0.646 DBil(μmol/L) 5.20(4.25~9.15) 4.05(2.35~7.00) -1.580 0.114 IBil(μmol/L) 13.45(8.45~19.40) 17.95(17.18~19.03) -1.172 0.241 TBA(μmol/L) 7.43(4.28~9.14) 3.45(3.05~4.56) -1.988 0.047 Alb(g/L) 45.60(41.15~48.08) 46.50(43.80~52.93) -1.784 0.074 GLB(g/L) 25.30(21.80~27.35) 27.15(23.98~36.63) -1.886 0.059 PT(s) 10.15(9.90~10.75) 7.05(5.83~7.73) -2.807 0.005 APTT(s) 23.10(22.15~26.23) 23.05(21.38~25.38) -0.561 0.575 UA(μmol/L) 427.50(340.00~542.00) 225.50(168.75~298.50) -2.803 0.005 CAP(dB/m) 328.00(287.75~353.00) 159.50(131.25~223.75) -2.803 0.005 LSM(kPa) 5.90(4.80~7.40) 4.00(3.18~4.70) -2.668 0.008 2.5.2 治疗前后SCFA指标比较
NASH组高危患者10例治疗后乙酸、丙酸含量均高于治疗前(P值均<0.05);异丁酸含量低于治疗前(P<0.05)(表 5)。
表 5 治疗前后SCFA水平比较Table 5. Comparison of SCFA level before and after treatment项目 治疗前 治疗后 Z值 P值 乙酸(μg/g) 1052.69(678.66~1621.09) 1412.39(1118.70~1865.72) -2.803 0.005 丙酸(μg/g) 787.81(338.24~1142.04) 955.57(556.52~1273.95) -2.803 0.005 异丁酸(μg/g) 49.82(17.00~106.54) 22.71(9.05~61.05) -2.803 0.005 丁酸(μg/g) 711.04(327.18~852.75) 690.86(549.47~967.25) -1.070 0.285 异戊酸(μg/g) 38.48(16.84~120.40) 41.37(22.29~71.78) -0.866 0.386 戊酸(μg/g) 86.00(16.34~294.74) 40.97(25.31~101.97) -1.784 0.074 己酸(μg/g) 2.41(1.52~13.41) 2.45(1.78~3.32) -0.764 0.445 2.6 SCFA诊断非酒精性脂肪性肝纤维化患者ROC曲线下面积(AUC)
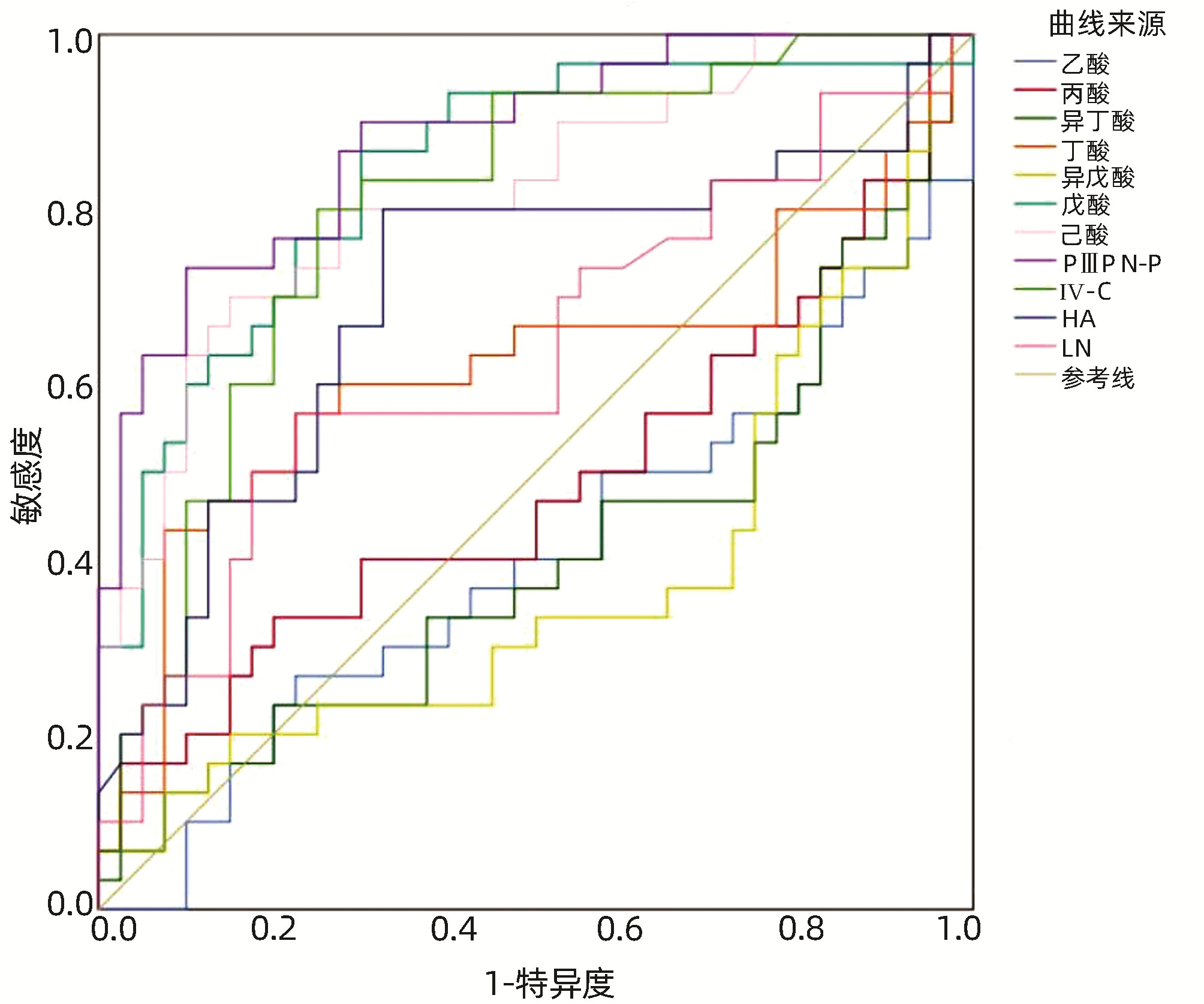
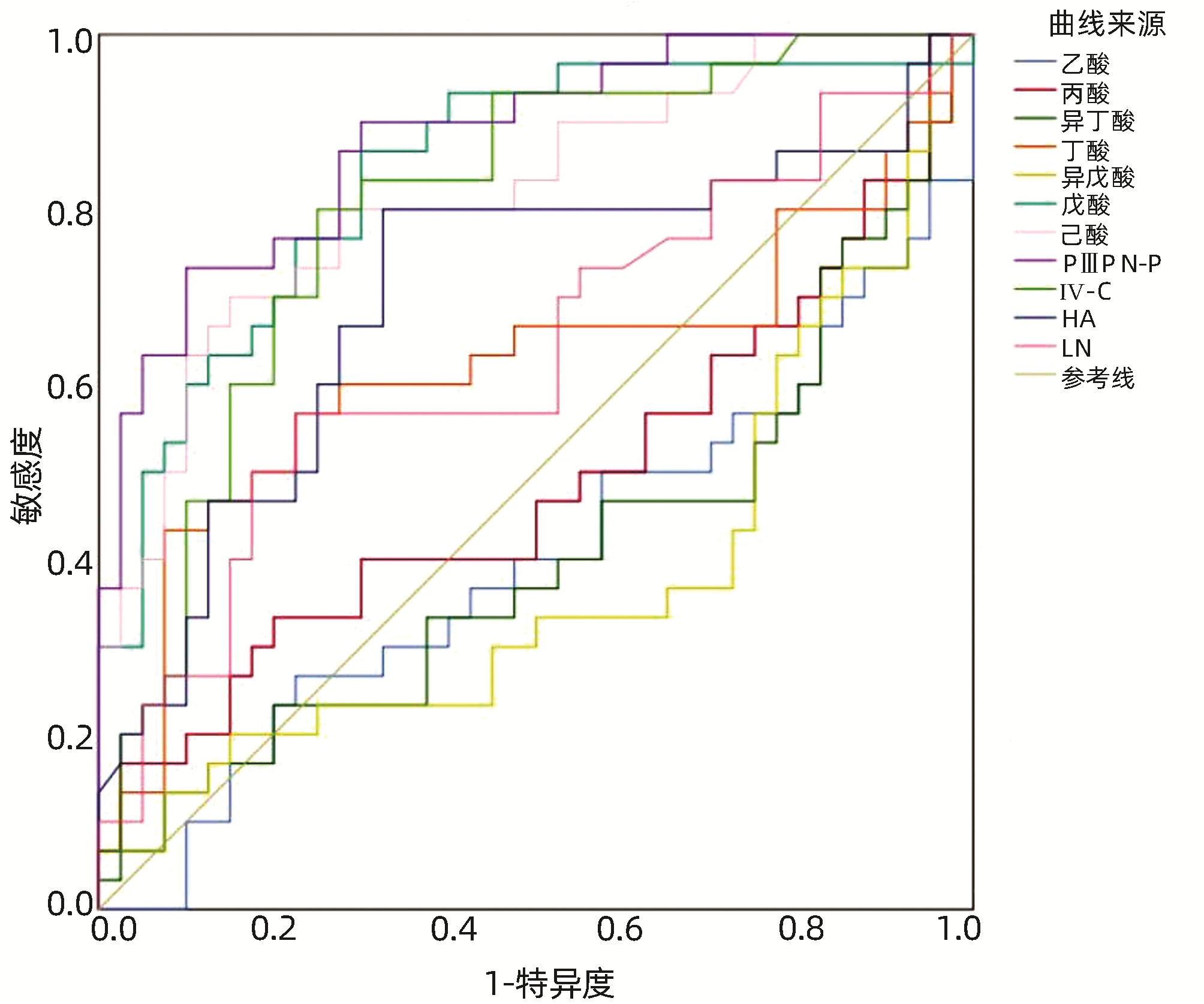
由图 1得出戊酸诊断非酒精性脂肪性肝纤维化的AUC为0.842(95%CI:0.747~0.938),最佳界值为141.42 μg/g,灵敏度86.7%,特异度70%;己酸诊断非酒精性脂肪性肝纤维化的AUC为0.819(95%CI:0.718~0.920),最佳界值为6.93 μg/g,灵敏度70%,特异度85%;PⅢP N-P诊断非酒精性脂肪性肝纤维化的AUC为0.881(95%CI:0.802~0.960),最佳界值为68.06 ng/mL,灵敏度73.3%,特异度90%;Ⅳ-C诊断非酒精性脂肪性肝纤维化的AUC为0.803(95%CI:0.699~0.907),最佳界值为60.12 ng/mL,灵敏度80%,特异度75%;HA诊断非酒精性脂肪性肝纤维化的AUC为0.703(95%CI:0.572~0.834),最佳界值为104.46 ng/mL,灵敏度80%,特异度67.5%。
2.7 肠道内主要微生物数量及其代谢产物
肠道内主要菌属为拟杆菌属、真杆菌属、双歧杆菌属、瘤胃球菌属、消化链球菌属等8种,大部分菌属主要发酵产物为乙酸、丁酸(表 6)。
表 6 肠道内主要微生物数量及其代谢产物Table 6. The number of major microorganisms in the gut and their metabolites菌属 粪便中平均菌数
(log10, CFU/g)主要发酵产物 拟杆菌属 11.3 乙酸、丁酸、琥珀酸 双歧杆菌属 10.2 乙酸、乳酸、甲酸 真杆菌属 10.7 乙酸、丁酸、乳酸 瘤胃球菌属 10.2 乙酸 消化链球菌属 10.1 乙酸、乳酸 梭菌属 9.8 乙酸、丙酸、丁酸、乳酸 乳杆菌属 9.6 乳酸 链球菌属 8.3 乙酸、乳酸 3. 讨论
NAFLD的发病机制与遗传、环境、生活方式、高脂饮食(HFD)、肥胖、血脂代谢紊乱、糖尿病及MetS等多种因素有关[16]。NAFLD也是肝细胞癌的主要危险因素之一,预计将成为全球最常见的慢性肝病[17]。目前在治疗NAFLD的全球流行及其相关代谢和肝脏并发症方面存在巨大挑战,NAFLD的早期诊断及干预治疗至关重要,目前的治疗方法有节制饮食、调整饮食结构并增加运动的生活方式、减重和代谢治疗(内镜和外科手术)、药物治疗、相关并发症的治疗,效果并不显著,并且多数NAFLD患者早期无症状,在进展为肝硬化之前,疾病可能保持沉默。NAFLD患者在初次就诊时发现的最常见症状为肝区轻微疼痛和疲劳,或根据偶然的影像学检查发现脂肪肝,所以目前缺乏行之有效的针对性诊断、治疗方案[18-20]。
肠道菌群失调在NAFLD及相关代谢紊乱中发挥重要作用。肝脏与胃肠道同为消化系统重要组成部分,由于肝脏与肠道特殊的解剖生理关系,肠道微生物通过肝-肠循环,在肝损伤、慢性纤维化、炎症及肿瘤发生发展中发挥重要作用[21-22]。SCFA是具有1~6个碳原子的有机脂肪酸,由肠道内微生物发酵膳食纤维产生,产生SCFA的肠道微生物主要包括厌氧类杆菌、真杆菌、链球菌、双歧杆菌和乳酸杆菌,受发酵底物、细菌种类等因素的影响,产生SCFA的种类、数量不同,在肠道内发挥的作用也不相同[23-24]。由此可见SCFA的形成与上述肠道微生物密切相关,进一步的研究[25]表明SCFA是饮食和肠道菌群在肠道微环境中相互作用的结果,是介导饮食、肠道菌群、宿主之间的信号因子,在机体免疫系统、代谢系统及内分泌系统等方面发挥重要作用。有研究[26]表明,SCFA与肠易激综合征、肝癌、结直肠癌、NAFLD等消化系统疾病有着密切联系。
众多研究[27-28]表明NAFLD促进了T2DM及其并发症的发展,而T2DM加剧了NAFLD的严重程度及脂代谢紊乱,已被越来越多的人所认识。另外有研究[29]显示超重/肥胖也与NAFLD息息相关,即肥胖与NAFL、NASH、相关肝硬化和肝细胞癌相关。一项基于健康人群NAFLD患病率及危险因素调查结果[30]显示NAFLD组超重或肥胖、T2DM、血脂异常、尿酸升高、血黏度异常、肝损伤的发生率均高于对照组,存在显著差异。本研究结果显示NAFL组、NASH组、非酒精性脂肪性肝纤维化组BMI、FPG、TG、DB、CAP均高于健康组,IBil、Alb、APTT低于健康组,差异有统计学意义(P<0.05),与众多研究结果基本一致。此外本研究还表明非酒精性脂肪性肝纤维化组及NASH组相关肝功能指标较NAFL组升高,可以推测两组患者可能存在更加严重的肝损伤;另外,非酒精性脂肪性肝纤维化组的LSM较NAFL组、NASH组均高,说明LSM具有广泛的应用价值,在评估肝纤维化程度方面表现良好,与Eddowes、Vuppalanchi等[31-32]研究结果一致。基于之前的实验结果,NAFLD患者确实存在不同程度的肠道菌群紊乱,假设NAFLD患者的肠道菌群代谢产物也因此受到影响,进一步利用GC-MS进行代谢组学分析。本研究选择粪便SCFA进行检测,发现非酒精性脂肪性肝纤维化组戊酸、己酸含量显著高于对照组,NAFL组戊酸、己酸含量显著低于对照组(P值均<0.05);非酒精性脂肪性肝纤维化组戊酸、己酸含量显著高于NAFL组,非酒精性脂肪性肝纤维化组戊酸含量显著高于NASH组(P值均<0.05);NASH组己酸含量显著高于NAFL组(P值均<0.05)。各组间乙酸、丙酸、异丁酸、丁酸、异戊酸含量无显著性差异(P值均>0.05)。据此可以推测戊酸、己酸可能促进NAFLD病程进展,机制可能是通过肝-肠循环,促进肝损伤、慢性纤维化、炎症的发生发展,但本研究中NAFL组戊酸、己酸含量却低于健康对照组,推测可能与疾病初步进展,机体戊酸、己酸含量尚未产生明显变化有关,另外程靖等[33]利用HFD小鼠诱导NAFLD模型并检测其SCFA的变化,结果显示实验组粪便乙酸、丙酸和总SCFA水平明显降低,与本研究实验结果不一致,推测可能与实验检测误差及样本量偏小相关。本研究进一步分析疾病严重程度与SCFA的相关性,发现疾病严重程度与丁酸、戊酸、己酸显著正相关,与前文研究结果相对应。
目前临床针对NAFLD患者缺乏特异性药物,本研究基于临床现状,选取NASH组高危患者10例治疗干预(服水飞蓟宾胶囊105 mg/次、3次/d及每日晨起进行有氧运动1 h)3个月后随访并检测血清学指标及粪便SCFA含量的差异。本研究结果显示NASH患者治疗后HbA1c、FPG、TG、TC、ALT、AST、GGT、TBA、PT、UA、CAP、LSM均明显低于治疗前(P值均<0.05),治疗前后ALP、TBil、DBil、IBil、Alb、Glb、APTT差异无统计学意义(P值均>0.05)。NASH患者10例治疗后乙酸、丙酸含量均高于治疗前(P值均<0.05),治疗后异丁酸含量低于治疗前(P<0.05),治疗前后丁酸、异戊酸、戊酸、己酸差异无统计学意义(P值均>0.05),Pingitore等[11]研究表明乙酸盐可减轻NASH小鼠的肝脏脂肪变性和炎性浸润,并降低血清中TG、游离脂肪酸和胆固醇的水平;丙酸可通过抑制β细胞凋亡而增强葡萄糖刺激的胰岛素释放,与本研究结果一致,推测乙酸、丙酸可能通过参与脂肪代谢相关基因的表达,通过肠-肝轴减少肠道黏膜通透性,减少内毒素的移位,可能对NAFLD患者肝脏存在一定程度的保护作用,此外,本研究结果显示异丁酸含量低于治疗前,推测异丁酸可能参与NASH的发生发展,目前异丁酸对NAFLD研究较少,缺乏相应文献支持,期待更进一步的研究。另有研究[34]表明,丁酸产生的益生菌能纠正HFD引起的小鼠肠肝免疫失调和减轻脂肪性肝炎,本研究结果显示治疗前后丁酸无统计学差异,需要更进一步的研究证实。此外,本研究结果中HbA1c、FPG、TG、TC、ALT、AST、GGT、TBA、PT、UA、CAP、LSM等临床指标治疗后均明显低于治疗前,乙酸、丙酸含量均高于治疗前,推测乙酸、丙酸对肝脏的保护作用可能进一步导致血清学相关指标的降低。
目前研究表明,在进展至非酒精性脂肪性肝纤维化之前,NAFL与NASH是可逆的,一旦进展为肝硬化则不可逆,可能进一步发展为肝癌,目前临床对于非酒精性脂肪性肝纤维化的诊断依赖于影像学检查,如腹部彩超、CT、MRI等,肝穿刺活检仍然是目前确诊非酒精性脂肪性肝纤维化的金标准。超声作为筛查手段,具有无创、简易、价格低等优点,但只能检测出>30%的脂肪变性,且不能做出定量评估,存在局限性;CT可以精确检测出脂肪变,但此方法针对轻度脂肪变较为敏感,加之具有电离辐射,具有一定局限性;MRI虽无辐射,但其对脂肪肝诊断尚缺乏大量临床试验,且价格昂贵,不宜多次重复检测;肝穿刺活检为有创性检查且脂肪变不均匀会有取材误差,还存在禁忌证等限制;临床常用血清肝纤维化四项检查一般耗时较长等特点,临床亟需一种无创、简便、价格低廉的方法。
本研究基于SCFA及PⅢP N-P、Ⅳ-C、HA、LN对非酒精性脂肪性肝纤维化诊断绘制ROC曲线,可以看出戊酸、己酸、PⅢP N-P、Ⅳ-C、HA对非酒精性脂肪性肝纤维化诊断价值较高,血清肝纤四项作为评估肝纤维化程度的可靠指标在临床广泛应用,本研究得出PⅢP N-P、Ⅳ-C、HA三者诊断价值较高,但LN诊断能力有限,推测可能PⅢP N-P、Ⅳ-C、HA早期明显升高,HA后期才显著增高有关,还可能与样本量偏少,代表性不足有关,期待后续更大样本量的研究。本研究进一步对比戊酸、己酸与PⅢP N-P、Ⅳ-C、HA对非酒精性脂肪性肝纤维化诊断价值,可以看出戊酸、己酸诊断价值劣于PⅢP N-P,但优于Ⅳ-C、HA,推测戊酸、己酸可以作为非酒精性脂肪性肝纤维化的辅助诊断指标,进一步根据约登指数最大值对应的检验变量值(戊酸为141.42 μg/g、己酸为6.93 μg/g、PⅢP N-P为68.06 ng/ml、Ⅳ-C为60.12 ng/mL、HA为104.46 ng/mL)作为诊断临界值,期待可以为临床非酒精性脂肪性肝纤维化的诊断提供一定的参考价值。
综上所述,NAFLD患者存在生化指标及肠道菌群代谢产物SCFA的异常,说明SCFA代谢状态的改变可以影响疾病进程。肠道菌群代谢产物SCFA在NAFLD早期诊断和治疗中具有重要的作用和应用前景。但是由于本实验样本量偏少,后续还需要进一步开展更大样本的研究,进一步探讨SCFA肠道代谢与NAFLD发病机制的关系。
-
表 1 4组研究对象人口学资料比较
Table 1. Comparison of demographic data of four groups of subjects
项目 健康组
(n=40)NAFL组
(n=30)NASH组
(n=30)非酒精性脂肪性肝纤维化组
(n=30)统计值 P值 性别[例(%)] χ2=4.267 0.234 男 23(57.5) 17(56.7) 23(76.7) 16(53.3) 女 17(42.5) 13(43.3) 7(23.3) 14(46.7) 年龄(岁) 49.10±10.85 53.40±9.00 49.03±11.21 47.03±11.79 F=1.869 0.138 表 2 4组研究对象临床生化指标比较
Table 2. Comparison of clinical biochemical indexes of four groups
项目 健康组(n=40) NAFL组(n=30) NASH组(n=30) 非酒精性脂肪性肝纤维化组(n=30) 统计值 P值 BMI(kg/m2) 20.75±1.62 25.18±2.731) 28.28±3.151)2) 26.70±2.281)2)3) F=62.852 <0.001 HbA1c(%) 5.00(4.43~5.58) 5.30(4.45~6.65) 7.95(7.60~8.33)1)2) 7.85(7.23~9.03)1)2) H=84.220 <0.001 FPG(mmol/L) 4.80(4.40~5.27) 6.90(5.73~8.03)1) 6.75(5.45~7.80)1) 8.20(7.20~9.13)1) H=57.614 <0.001 TG(mmol/L) 1.01(0.70~1.30) 2.17(1.35~3.41)1) 2.16(1.48~3.05)1) 2.90(2.10~4.30)1) H=62.740 <0.001 TC(mmol/L) 4.47±0.74 4.69±0.83 4.52±1.13 4.93±0.73 F=1.831 0.145 ALT(U/L) 25.00(21.00~30.50) 28.50(19.75~33.25) 87.00(50.50~150.00)1)2) 50.00(31.50~73.25)1)2) H=64.890 <0.001 AST(U/L) 21.80(18.00~27.00) 20.50(17.75~23.50) 37.50(27.50~62.25)1)2) 36.50(21.75~63.75)1)2) H=36.106 <0.001 ALP(U/L) 94.00(78.25~119.75) 87.50(74.00~107.00) 101.50(91.50~121.75) 109.00(81.25~137.50) H=6.409 0.093 GGT(U/L) 25.00(19.00~31.75) 30.50(24.75~39.00) 102.00(57.50~268.75)1)2) 78.50(40.50~123.00)1)2) H=69.178 <0.001 TBil(μmol/L) 18.25(16.40~21.78) 13.95(11.23~18.68)1) 17.80(11.10~26.70) 11.90(8.83~17.20)1)3) H=22.698 <0.001 DBil(μmol/L) 2.50(1.90~2.98) 4.15(3.33~5.20)1) 4.90(4.13~8.00)1) 3.75(2.53~5.25)1)3) H=52.403 <0.001 IBil(μmol/L) 16.13±2.93 11.04±5.091) 13.09±7.121) 8.95±4.061)3) F=13.548 <0.001 TBA(μmol/L) 4.74(3.80~5.34) 4.04(2.30~6.87) 4.71(3.73~7.41) 7.52(4.09~9.87)1)2) H=12.455 0.006 Alb(g/L) 48.12±3.51 43.60±3.291) 41.70±4.571) 45.25±3.971)3) F=17.546 <0.001 GLB(g/L) 26.21±6.36 26.32±2.90 26.83±4.40 27.67±5.40 F=0.554 0.646 PT(s) 10.95(9.80~11.60) 9.90(9.68~10.23)1) 10.20(9.68~10.63) 9.75(9.45~10.33)1) H=17.139 0.001 APTT(s) 27.38±2.37 23.47±2.661) 24.97±4.641) 23.33±4.091) F=10.407 <0.001 UA(μmol/L) 301.00(258.50~328.75) 310.00(243.25~440.50) 347.00(292.75~429.75)1) 321.50(286.25~380.50) H=11.066 0.011 CAP(dB/m) 177.66(155.25~198.00) 290.50(258.25~316.50)1) 334.50(295.00~359.75)1) 313.50(262.25~342.50)1) H=81.927 <0.001 LSM(kPa) 4.65(4.13~5.28) 4.00(3.68~4.85) 6.25(5.30~7.38)1)2) 9.60(8.85~10.48)1)2)3) H=89.958 <0.001 注:与健康组比较,1)P<0.05;与NAFL组比较,2)P<0.05;与NASH组比较,3)P<0.05。 表 3 4组研究对象SCFA水平测定
Table 3. Determination of short chain fatty acids level of four groups of subjects
项目 健康组(n=40) NAFL组(n=30) NASH组(n=30) 非酒精性脂肪性肝纤维化组(n=30) 统计值 P值 乙酸(μg/g) 1463.63±408.10 1438.24±539.47 1259.68±467.97 1343.57±436.10 F=1.339 0.265 丙酸(μg/g) 702.74±241.86 747.38±349.46 756.53±421.36 710.30±321.60 F=0.212 0.888 异丁酸(μg/g) 68.74(48.83~104.64) 50.09(16.91~94.80) 66.99(24.30~106.96) 50.40(35.02~83.74) H=2.763 0.430 丁酸(μg/g) 700.38±300.18 567.76±329.82 591.70±282.18 763.35±343.67 F=2.644 0.052 异戊酸(μg/g) 64.60(45.13~102.57) 47.00(16.94~74.37) 65.61(21.95~112.67) 47.64(20.28~77.13) H=4.635 0.201 戊酸(μg/g) 123.18(102.53~159.78) 52.64(7.38~128.77)1) 99.85(31.73~232.23) 204.17(160.05~236.48)1)2)3) H=36.082 <0.001 己酸(μg/g) 4.92(3.81~6.16) 1.33(0.87~2.94)1) 6.39(1.61~28.03)2) 7.96(5.95~10.45)1)2) H=36.414 <0.001 注:与健康组比较,1)P<0.05;与NAFL组比较,2)P<0.05;与NASH组比较,3)P<0.05。 表 4 治疗前后临床指标比较
Table 4. Comparison of clinical indexes before and after treatment
项目 治疗前 治疗后 Z值 P值 HbA1c(%) 7.75(7.28~8.35) 3.80(3.43~4.80) -2.805 0.005 FPG(mmol/L) 7.30(5.45~7.95) 4.70(4.13~5.33) -2.703 0.007 TG(mmol/L) 2.40(1.45~3.20) 1.42(1.32~1.58) -2.193 0.028 TC(mmol/L) 4.49(3.95~5.43) 3.64(3.15~4.23) -2.599 0.009 ALT(U/L) 151.00(119.50~168.50) 34.00(21.00~42.75) -2.805 0.005 AST(U/L) 51.50(32.75~113.50) 19.50(17.75~21.25) -2.701 0.007 ALP(U/L) 108.50(93.50~121.50) 83.50(73.50~97.50) -1.939 0.052 GGT(U/L) 100.50(67.50~136.25) 24.50(19.00~30.25) -2.803 0.005 TBil(μmol/L) 19.30(16.90~37.25) 20.70(19.30~21.70) -0.459 0.646 DBil(μmol/L) 5.20(4.25~9.15) 4.05(2.35~7.00) -1.580 0.114 IBil(μmol/L) 13.45(8.45~19.40) 17.95(17.18~19.03) -1.172 0.241 TBA(μmol/L) 7.43(4.28~9.14) 3.45(3.05~4.56) -1.988 0.047 Alb(g/L) 45.60(41.15~48.08) 46.50(43.80~52.93) -1.784 0.074 GLB(g/L) 25.30(21.80~27.35) 27.15(23.98~36.63) -1.886 0.059 PT(s) 10.15(9.90~10.75) 7.05(5.83~7.73) -2.807 0.005 APTT(s) 23.10(22.15~26.23) 23.05(21.38~25.38) -0.561 0.575 UA(μmol/L) 427.50(340.00~542.00) 225.50(168.75~298.50) -2.803 0.005 CAP(dB/m) 328.00(287.75~353.00) 159.50(131.25~223.75) -2.803 0.005 LSM(kPa) 5.90(4.80~7.40) 4.00(3.18~4.70) -2.668 0.008 表 5 治疗前后SCFA水平比较
Table 5. Comparison of SCFA level before and after treatment
项目 治疗前 治疗后 Z值 P值 乙酸(μg/g) 1052.69(678.66~1621.09) 1412.39(1118.70~1865.72) -2.803 0.005 丙酸(μg/g) 787.81(338.24~1142.04) 955.57(556.52~1273.95) -2.803 0.005 异丁酸(μg/g) 49.82(17.00~106.54) 22.71(9.05~61.05) -2.803 0.005 丁酸(μg/g) 711.04(327.18~852.75) 690.86(549.47~967.25) -1.070 0.285 异戊酸(μg/g) 38.48(16.84~120.40) 41.37(22.29~71.78) -0.866 0.386 戊酸(μg/g) 86.00(16.34~294.74) 40.97(25.31~101.97) -1.784 0.074 己酸(μg/g) 2.41(1.52~13.41) 2.45(1.78~3.32) -0.764 0.445 表 6 肠道内主要微生物数量及其代谢产物
Table 6. The number of major microorganisms in the gut and their metabolites
菌属 粪便中平均菌数
(log10, CFU/g)主要发酵产物 拟杆菌属 11.3 乙酸、丁酸、琥珀酸 双歧杆菌属 10.2 乙酸、乳酸、甲酸 真杆菌属 10.7 乙酸、丁酸、乳酸 瘤胃球菌属 10.2 乙酸 消化链球菌属 10.1 乙酸、乳酸 梭菌属 9.8 乙酸、丙酸、丁酸、乳酸 乳杆菌属 9.6 乳酸 链球菌属 8.3 乙酸、乳酸 -
[1] RINELLA ME, SANYAL AJ. NAFLD in 2014: Genetics, diagnostics and therapeutic advances in NAFLD[J]. Nat Rev Gastroenterol Hepatol, 2015, 12(2): 65-66. DOI: 10.1038/nrgastro.2014.232. [2] National Workshop on Fatty Liver and Alcoholic Liver Disease, Chinese Society of Hepatology, Chinese Medical Association; Fatty Liver Expert Committee, Chinese Medical Doctor Association. Guidelines of prevention and treatment for nonalcoholic fatty liver disease: A 2018 update[J]. J Clin Hepatol, 2018, 34(5): 947-957. DOI: 10.3969/j.issn.1001-5256.2018.05.007.中华医学会肝病学分会脂肪肝和酒精性肝病学组, 中国医师协会脂肪性肝病专家委员会. 非酒精性脂肪性肝病防治指南(2018年更新版)[J]. 临床肝胆病杂志, 2018, 34(5): 947-957. DOI: 10.3969/j.issn.1001-5256.2018.05.007. [3] JIAN J, ZHU X. Biological mechanism of intestinal flora in the occurrence and development of nonalcoholic fatty liver disease[J]. Chin J Biochem Mol Biol, 2020, 36(8): 888-894. DOI: 10.13865/j.cnki.cjbmb.2020.05.1061.简捷, 朱萱. 肠道菌群在非酒精性脂肪性肝病发生发展中的生物学机制[J]. 中国生物化学与分子生物学报, 2020, 36(8): 888-894. DOI: 10.13865/j.cnki.cjbmb.2020.05.1061. [4] MURAG S, AHMED A, KIM D. Recent epidemiology of nonalcoholic fatty liver disease[J]. Gut Liver, 2021, 15(2): 206-216. DOI: 10.5009/gnl20127 [5] MICHELOTTI GA, MACHADO MV, DIEHL AM. NAFLD, NASH and liver cancer[J]. Nat Rev Gastroenterol Hepatol, 2013, 10(11): 656-665. DOI: 10.1038/nrgastro.2013.183. [6] FRIEDMAN SL, NEUSCHWANDER-TETRI BA, RINELLA M, et al. Mechanisms of NAFLD development and therapeutic strategies[J]. Nat Med, 2018, 24(7): 908-922. DOI: 10.1038/s41591-018-0104-9. [7] BUZZETTI E, PINZANI M, TSOCHATZIS EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD)[J]. Metabolism, 2016, 65(8): 1038-1048. DOI: 10.1016/j.metabol.2015.12.012. [8] HUI DC, SUN MY. Association between nonalcoholic fatty liver disease and gut microbiota based on the theory of gut-liver axis[J]. J Clin Hepatol, 2020, 36(7): 1627-1630. DOI: 10.3969/j.issn.1001-5256.2020.07.039.惠登城, 孙明瑜. 基于肠-肝轴理论探讨非酒精性脂肪性肝病和肠道菌群的关系[J]. 临床肝胆病杂志, 2020, 36(7): 1627-1630. DOI: 10.3969/j.issn.1001-5256.2020.07.039. [9] MENG Q, DUAN XP, WANG CY, et al. Alisol B 23-acetate protects against non-alcoholic steatohepatitis in mice via farnesoid X receptor activation[J]. Acta Pharmacol Sin, 2017, 38(1): 69-79. DOI: 10.1038/aps.2016.119. [10] PINGITORE A, CHAMBERS ES, HILL T, et al. The diet-derived short chain fatty acid propionate improves beta-cell function in humans and stimulates insulin secretion from human islets in vitro[J]. Diabetes Obes Metab, 2017, 19(2): 257-265. DOI: 10.1111/dom.12811. [11] JIN CJ, SELLMANN C, ENGSTLER AJ, et al. Supplementation of sodium butyrate protects mice from the development of non-alcoholic steatohepatitis (NASH)[J]. Br J Nutr, 2015, 114(11): 1745-1755. DOI: 10.1017/S0007114515003621. [12] PERRY RJ, PENG L, BARRY NA, et al. Acetate mediates a microbiome-brain-β-cell axis to promote metabolic syndrome[J]. Nature, 2016, 534(7606): 213-217. DOI: 10.1038/nature18309. [13] ZHOU D, FAN JG. Microbial metabolites in non-alcoholic fatty liver disease[J]. World J Gastroenterol, 2019, 25(17): 2019-2028. DOI: 10.3748/wjg.v25.i17.2019. [14] CUI Y, WANG Q, CHANG R, et al. Intestinal barrier function-non-alcoholic fatty liver disease interactions and possible role of gut microbiota[J]. J Agric Food Chem, 2019, 67(10): 2754-2762. DOI: 10.1021/acs.jafc.9b00080. [15] Fatty liver and alcoholic liver disease group, Hepatology branch, Chinese Medical Association. Guidelines for the diagnosis and treatment of nonalcoholic fatty liver disease[J]. Chin Hepatol, 2006, 11(1): 68-70. DOI: 10.3969/j.issn.1008-1704.2006.01.032.中华医学会肝脏病学分会脂肪肝和酒精性肝病学组. 非酒精性脂肪性肝病诊疗指南[J]. 肝脏, 2006, 11(1): 68-70. DOI: 10.3969/j.issn.1008-1704.2006.01.032. [16] LIKHITSUP A, DUNDULIS J, ANSARI S, et al. Prevalence of non-alcoholic fatty liver disease on computed tomography in patients with inflammatory bowel disease visiting an emergency department[J]. Ann Gastroenterol, 2019, 32(3): 283-286. DOI: 10.20524/aog.2019.0371. [17] YOUNOSSI ZM, MARCHESINI G, PINTO-CORTEZ H, et al. Epidemiology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: Implications for liver transplantation[J]. Transplantation, 2019, 103(1): 22-27. DOI: 10.1097/TP.0000000000002484. [18] LOOMBA R, FRIEDMAN SL, SHULMAN GI. Mechanisms and disease consequences of nonalcoholic fatty liver disease[J]. Cell, 2021, 184(10): 2537-2564. DOI: 10.1016/j.cell.2021.04.015. [19] THANAPIROM K, TSOCHATZIS EA. Non-alcoholic fatty liver disease (NAFLD) and the quest for effective treatments[J]. Hepatobiliary Surg Nutr, 2019, 8(1): 77-79. DOI: 10.21037/hbsn.2018.11.06. [20] DOU KF, YANG XS. Surgeons should attach importance to the understanding of metabolic associated fatty liver disease[J]. Chin J Dig Surg, 2021, 1(1): 40-45. DOI: 10.3760/cma.j.cn115610-20201214-00780.窦科峰, 杨西胜. 外科医师应重视对代谢相关脂肪性肝病的认识[J]. 中华消化外科杂志, 2021, 1(1): 40-45. DOI: 10.3760/cma.j.cn115610-20201214-00780. [21] LIAN XX, GUO XX. Research progress of gut liver axis theory[J]. Chin J Integr Tradit West Med Liver Dis, 2017, 27(4): 251-254. DOI: 10.3969/j.issn.1005-0264.2017.04.023.廉晓晓, 郭晓霞. 肠-肝轴学说的研究进展[J]. 中西医结合肝病杂志, 2017, 27(4): 251-254. DOI: 10.3969/j.issn.1005-0264.2017.04.023. [22] ZHANG YN, LIU YT. Correlation between the degree of liver fibrosis and chronic kidney disease in patients with nonalcoholic fatty liver disease[J]. Chin J Gerontol, 2021, 41(19): 4214-4218. DOI: 10.3969/j.issn.1005-9202.2021.19.018.章雅南, 刘奕婷. 非酒精性脂肪性肝病患者肝纤维化程度与慢性肾病的相关性[J]. 中国老年学杂志, 2021, 41(19): 4214-4218. DOI: 10.3969/j.issn.1005-9202.2021.19.018. [23] GANAPATHY V, THANGARAJU M, PRASAD PD, et al. Transporters and receptors for short-chain fatty acids as the molecular link between colonic bacteria and the host[J]. Curr Opin Pharmacol, 2013, 13(6): 869-874. DOI: 10.1016/j.coph.2013.08.006. [24] LIU SZ, ZHANG Y, ZHANG MW, et al. Research progress on the production mechanism and physiological function of intestinal short chain fatty acids[J]. Guangdong Agricultural Science, 2013, 40(11): 99-103. DOI: 10.3969/j.issn.1004-874x.2013.11.029.刘松珍, 张雁, 张名位, 等. 肠道短链脂肪酸产生机制及生理功能的研究进展[J]. 广东农业科学, 2013, 40(11): 99-103. DOI: 10.3969/j.issn.1004-874X.2013.11.029. [25] BOETS E, GOMAND SV, DEROOVER L, et al. Systemic availability and metabolism of colonic-derived short-chain fatty acids in healthy subjects: A stable isotope study[J]. J Physiol, 2017, 595(2): 541-555. DOI: 10.1113/JP272613. [26] CUI LH. Relationship between gut microbiota and digestive disease[J] Acad J Chinese PLA Postgrad Med Sch, 2015, 36 (10): 965-969. DOI: 10.3969/j.issn.2095-5227.2015.10.001.崔立红. 肠道菌群与消化系疾病的关系[J]. 解放军医学院学报, 2015, 36(10): 965-969. DOI: 10.3969/j.issn.2095-5227.2015.10.001. [27] TILG H, MOSCHEN AR, RODEN M. NAFLD and diabetes mellitus[J]. Nat Rev Gastroenterol Hepatol, 2017, 14(1): 32-42. DOI: 10.1038/nrgastro.2016.147. [28] KHNEIZER G, RIZVI S, GAWRIEH S. Non-alcoholic fatty liver disease and diabetes mellitus[J]. Adv Exp Med Biol, 2021, 1307: 417-440. DOI: 10.1007/5584_2020_532. [29] POLYZOS SA, KOUNTOURAS J, MANTZOROS CS. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutic[J]. Metabolism, 2019, 92: 82-97. DOI: 10.1016/j.metabol.2018.11.014. [30] YIN JM. Investigation on NAFLD prevalence and risk factors based on physical examination population[D]. Tianjin: Tianjin Medical University, 2012.殷珺妹. 基于健康体检人群的NAFLD患病率及危险因素调查[D]. 天津: 天津医科大学, 2012. [31] EDDOWES PJ, SASSO M, ALLISON M, et al. Accuracy of fibroScan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease[J]. Gastroenterology, 2019, 156(6): 1717-1730. DOI: 10.1053/j.gastro.2019.01.042. [32] VUPPALANCHI R, SIDDIQUI MS, VAN NATTA ML, et al. Performance characteristics of vibration-controlled transient elastography for evaluation of nonalcoholic fatty liver disease[J]. Hepatology, 2018, 67(1): 134-144. DOI: 10.1002/hep.29489. [33] CHENG J, LI FL, ZHANG B, et al. Effect of high-fat diet on intestinal short chain fatty acids in rats with nonalcoholic fatty liver disease[J]. Chin J Clin Nutr, 2016, 24(4): 236-240. DOI: 10.3760/cma.j.issn.1674-635x.2016.04.009.程靖, 李枫林, 张宝, 等. 高脂饮食对非酒精性脂肪肝病模型大鼠肠道短链脂肪酸的影响[J]. 中华临床营养杂志, 2016, 24(4): 236-240. DOI: 10.3760/cma.j.issn.1674-635X.2016.04.009. [34] LIANG Y, LIN C, ZHANG Y, et al. Probiotic mixture of Lactobacillus and Bifidobacterium alleviates systemic adiposity and inflammation in non-alcoholic fatty liver disease rats through Gpr109a and the commensal metabolite butyrate[J]. Inflammopharmacology, 2018, 26(4): 1051-1055. DOI: 10.1007/s10787-018-0479-8. 期刊类型引用(4)
1. 程之梁,张钰龙,杨涵,哈惠,王潆笛,陈菲菲,刘飞,焦月华. 益生菌及其代谢产物调控胰高血糖素样肽-1缓解2型糖尿病的研究进展. 食品科学. 2024(12): 292-303 .  百度学术
百度学术2. 邓静文,黄紫橙,王庆怡,黄宇旭,倪知全,徐江韬,黄小流. 基于代谢组学研究马齿苋多糖对老年大鼠粪便代谢物的影响. 井冈山大学学报(自然科学版). 2024(04): 66-75 .  百度学术
百度学术3. 曹婷,刘长青,海荣,杜小琴. 肠道菌群多样性及其代谢产物与慢性心力衰竭患者心功能的相关性分析. 实用心脑肺血管病杂志. 2024(09): 46-50+55 .  百度学术
百度学术4. 廖小妹,陈美丽,张露,王玺舜,唐博翔,何文智. 基于肠肝轴探讨加味消脂利肝方调控肠道菌群治疗非酒精性脂肪肝病的作用机制. 中国微生态学杂志. 2023(08): 876-884 .  百度学术
百度学术其他类型引用(2)
-




 PDF下载 ( 2410 KB)
PDF下载 ( 2410 KB)

 下载:
下载:

 下载:
下载:
 百度学术
百度学术