恶性胆道梗阻经皮胆道引流或支架置入术后感染的病原学特征
DOI: 10.3969/j.issn.1001-5256.2022.06.024
Etiological characteristics of infection after percutaneous biliary drainage or stent implantation for malignant biliary obstruction
-
摘要:
目的 探讨恶性胆道梗阻(MBO)患者经皮胆道引流或支架置入术后感染的病原学特征。 方法 收集2016年1月—2020年12月在苏州大学附属第一医院介入科接受介入治疗后存在或怀疑胆道感染、送检胆汁培养和/或无同期血培养的MBO患者的临床资料。从病原学培养的阳性率、菌群分布、血培养与胆汁培养的一致性、主要致病菌的耐药率方面进行分析。 结果 共纳入患者219例,胆汁病原学培养阳性105例(47.95%),其中革兰氏阴性菌、革兰氏阳性菌、真菌的构成比分别为64.89%、28.24%、6.87%。同期送检血培养者69例,阳性33例(47.82%)。血培养和胆汁培养同为阳性的患者25例,一致性分析结果显示,完全一致占36%(9/25),部分一致占20%(5/25),完全不一致占44%(11/25)。常见致病的革兰氏阴性菌为大肠埃希菌、肺炎克雷伯、阴沟肠杆菌,对其耐药率(<15%)较低的抗生素有头孢哌酮/舒巴坦、阿米卡星、亚胺培南。常见致病的革兰氏阳性菌为屎肠球菌、粪肠球菌,对其耐药率较低的抗生素有万古霉素、利奈唑胺、替考拉宁。 结论 MBO患者经皮胆道引流或支架置入术后感染的常见致病菌为大肠埃希菌、肺炎克雷伯、肠球菌、阴沟肠杆菌。血培养与胆汁培养的一致性较低,均应积极送检。 Abstract:Objective To investigate the etiological characteristics of infection after percutaneous biliary drainage or stent implantation in patients with malignant biliary obstruction (MBO). Methods Clinical data were collected from MBO patients who underwent interventional therapy in Department of Interventional Radiology, The First Affiliated Hospital of Soochow University, from January 2016 to December 2020 and had or were suspected of biliary tract infection, with samples submitted for bile culture and/or simultaneous blood culture. Analysis was performed for the aspects of positive rate of culture, flora distribution, consistency between blood culture and bile culture, and drug resistance rate of major pathogenic bacteria. Results A total of 219 patients were enrolled, among whom 105(47.95%) were positive for bile culture, and the composition ratios of Gram-negative bacteria, Gram-positive bacteria, and fungi were 64.89%, 28.24%, and 6.87%, respectively. A total of 69 patients had samples submitted for blood culture during the same period of time, among whom 33(47.82%) had positive results. Positive results of both bile culture and blood culture were observed in 25 patients, and consistency analysis showed that the patients with complete consistency, partial consistency, and complete inconsistency accounted for 36%(9/25), 20%(5/25), and 44%(11/25), respectively. Common Gram-negative bacteria were Escherichia coli, Klebsiella pneumoniae, and Enterobacter cloacae, with a relatively low level of drug resistance to antibiotics including cefoperazone/sulbactam, amikacin, and imipenem. Common Gram-positive bacteria were Enterococcus faecium and Enterococcus faecalis, with a relatively low level(< 15%) of drug resistance to antibiotics including vancomycin, linezolid, and teicoplanin. Conclusion Common pathogens of infection after percutaneous biliary drainage or stent implantation in MBO patients include Escherichia coli, Klebsiella pneumoniae, Enterococcus, and Enterobacter cloacae. There is a relatively low level of consistency between blood culture and bile culture, and thus samples should be submitted for both tests. -
经皮胆道引流或支架植入术是治疗恶性胆道梗阻(malignant biliary obstruction,MBO)的重要方法[1],与结石所致的良性胆道梗阻不同,MBO通常不合并显性感染。然而,介入操作却可诱发胆管炎、腹膜炎、肝脓肿,甚至脓毒症。文献[2]报道,MBO介入术后轻微脓毒症的发生率为7.7%,而严重脓毒症的发生率达2.5%。在恶性肿瘤的病理状态下,感染将进一步加重肝细胞损伤,甚至导致全身多器官功能紊乱。因此,针对MBO,在有效解除梗阻的基础上,正确使用抗生素也很重要。
病原学分析是正确使用抗生素的基础。目前,关于MBO介入治疗后感染的病原学研究较少,远不如良性胆道梗阻充分,且不同地域或中心的常见致病菌和敏感抗生素也可能存在差异。本研究分析了本中心近5年来219例介入治疗后送检病原学检查的MBO患者的临床资料,报道如下。
1. 资料与方法
1.1 研究对象
收集2016年1月—2020年12月本中心收治的MBO患者的临床资料。纳入标准:(1)针对MBO行介入手术的住院病例,包括经皮胆道引流术、经皮胆道支架置入术、经皮胆道粒子支架置入术、胆道引流管更换术、支架后再狭窄的补救性胆道引流; (2)术后出现或怀疑胆道感染,其判断标准为存在发热、寒战、腹痛,或白细胞、C反应蛋白升高,或胆汁墨绿色、有异味等。(3)送检胆汁培养和/或无同期血培养。排除标准:(1)胆石症或其他原因引起的良性梗阻性黄疸; (2)介入治疗前已存在胆道感染征象或已使用抗生素; (3)合并呼吸道、泌尿道等其他系统感染。
1.2 标本留取
经胆道引流管留取胆汁标本,引流管尾端充分消毒,待胆汁自然溢出10 mL左右,再用无菌标本瓶接取胆汁10 mL,送检验科。胆汁留取过程中避免使用注射器抽吸,以免肠道菌群移位。胆汁培养连续送检3次,间隔24 h。血液标本严格按照无菌操作留取,连续送检2次,间隔24 h。同期血培养是指在胆汁培养前后48 h送检的血培养。同一患者同次住院相同的培养结果,记为1例。
1.3 观察指标
收集患者的性别、年龄、肿瘤类型、介入操作、胆汁培养及药敏结果、血培养及药敏结果等资料。从胆汁培养的阳性率及菌群分布、血培养及其与胆汁培养的一致性、主要致病菌的药敏实验3个方面进行研究。将具有同期血培养的患者分为4类:血培养(+)胆汁培养(+)、血培养(+)胆汁培养(-)、血培养(-)胆汁培养(+)、血培养(-)胆汁培养(-)。对血培养和胆汁培养都为阳性的患者进行一致性研究,分为完全一致(血标本与胆汁标本的病原菌完全相同)、部分一致(血标本病原菌在胆汁标本中为阳性,但胆汁培养中还有其他致病菌)和完全不一致(血标本和胆汁标本的病原菌均不相同)3种类型。对有药敏实验的报告统计抗生素耐药率。
2. 结果
2.1 胆汁培养及菌群分布
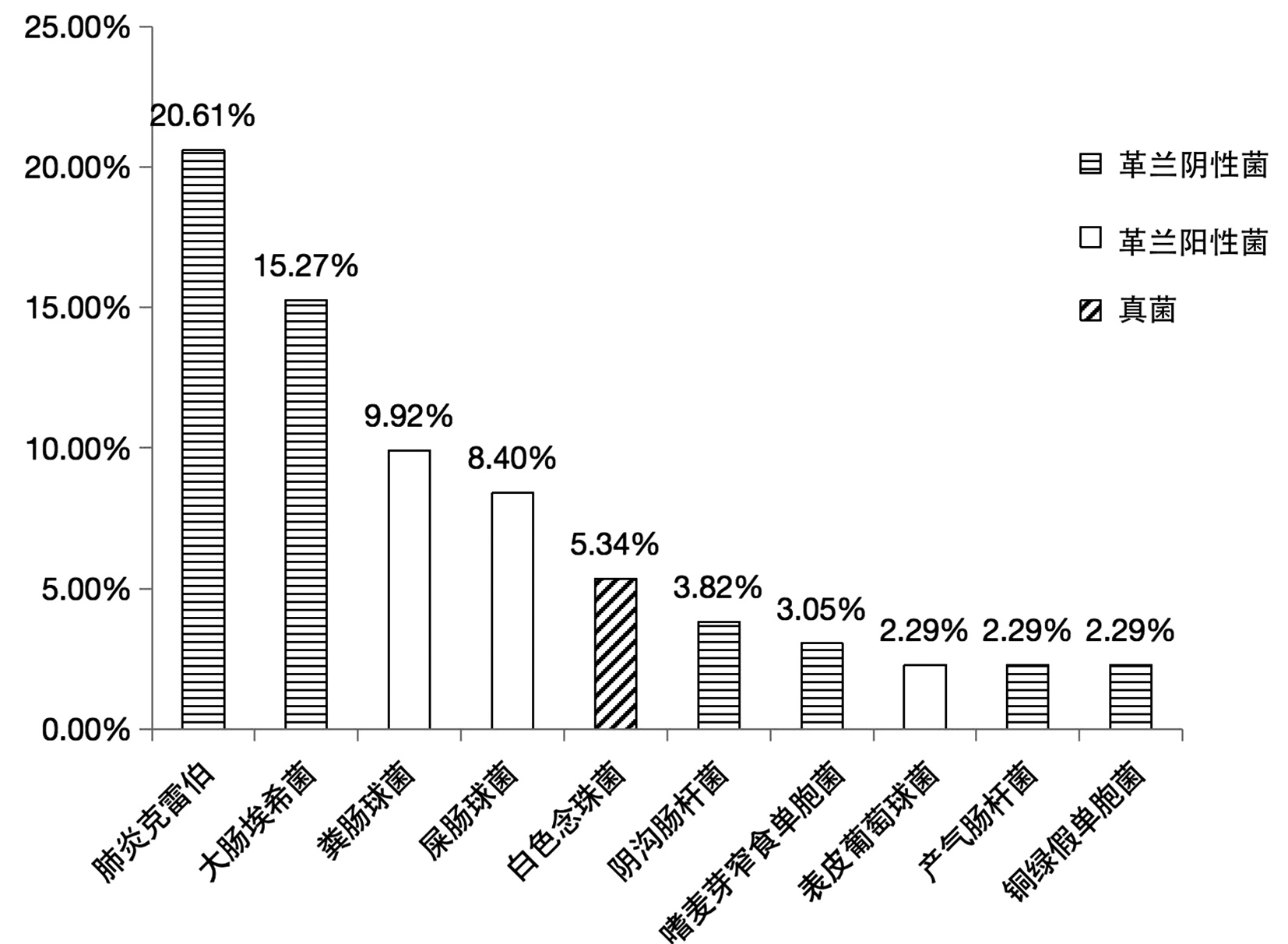
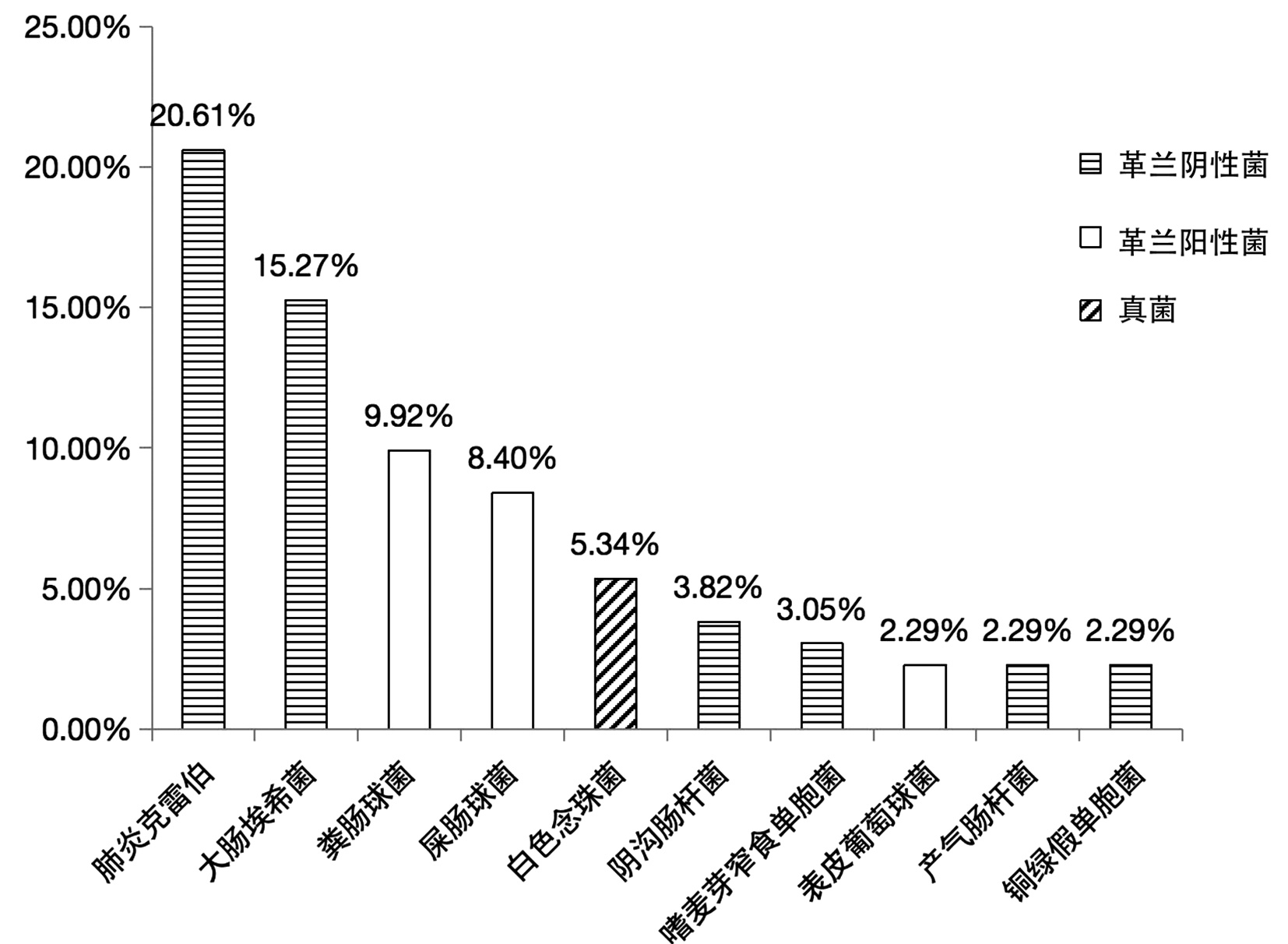
共纳入患者219例,其中男130例,女89例,年龄28~91岁,平均(66.8±11.6)岁。219例胆汁标本中有105例(47.95%)检出致病菌,其中81例培养出1种菌、22例培养出2种菌、2例培养出3种菌; 共培养出37种、131株细菌,其中革兰阴性菌85株、革兰阳性菌37株、真菌9株,构成比分别为64.89%、28.24%、6.87%。
革兰阴性菌有肺炎克雷伯、大肠埃希菌、阴沟肠杆菌、嗜麦芽窄食单胞菌、产气肠杆菌、铜绿假单胞菌、鲍曼不动杆菌、解鸟氨酸拉乌尔菌、琼氏不动杆菌、嗜水气单胞菌、豚鼠气单胞菌、温和气单胞菌、布氏柠檬酸杆菌、产酸克雷伯菌、恶臭假单胞菌、解糖胨普雷沃菌、类志贺邻单胞菌、普通变形杆菌、奇异变形菌、缺陷短波单胞菌、施氏假单胞菌、约式不动杆菌、弗氏柠檬酸杆菌。
革兰阳性菌有粪肠球菌、屎肠球菌、表皮葡萄球菌、头状葡萄球菌、尿肠球菌、巴氏葡萄球菌、发酵乳杆菌、松鼠葡萄球菌、唾液链球菌、沃式葡萄球菌、咽峡炎链球菌。
真菌有白色念珠菌、白假丝酵母、热带念珠菌。
胆汁培养出的37种菌中,最常见的10种及其构成比见图 1。
2.2 血培养及其与胆汁培养的一致性
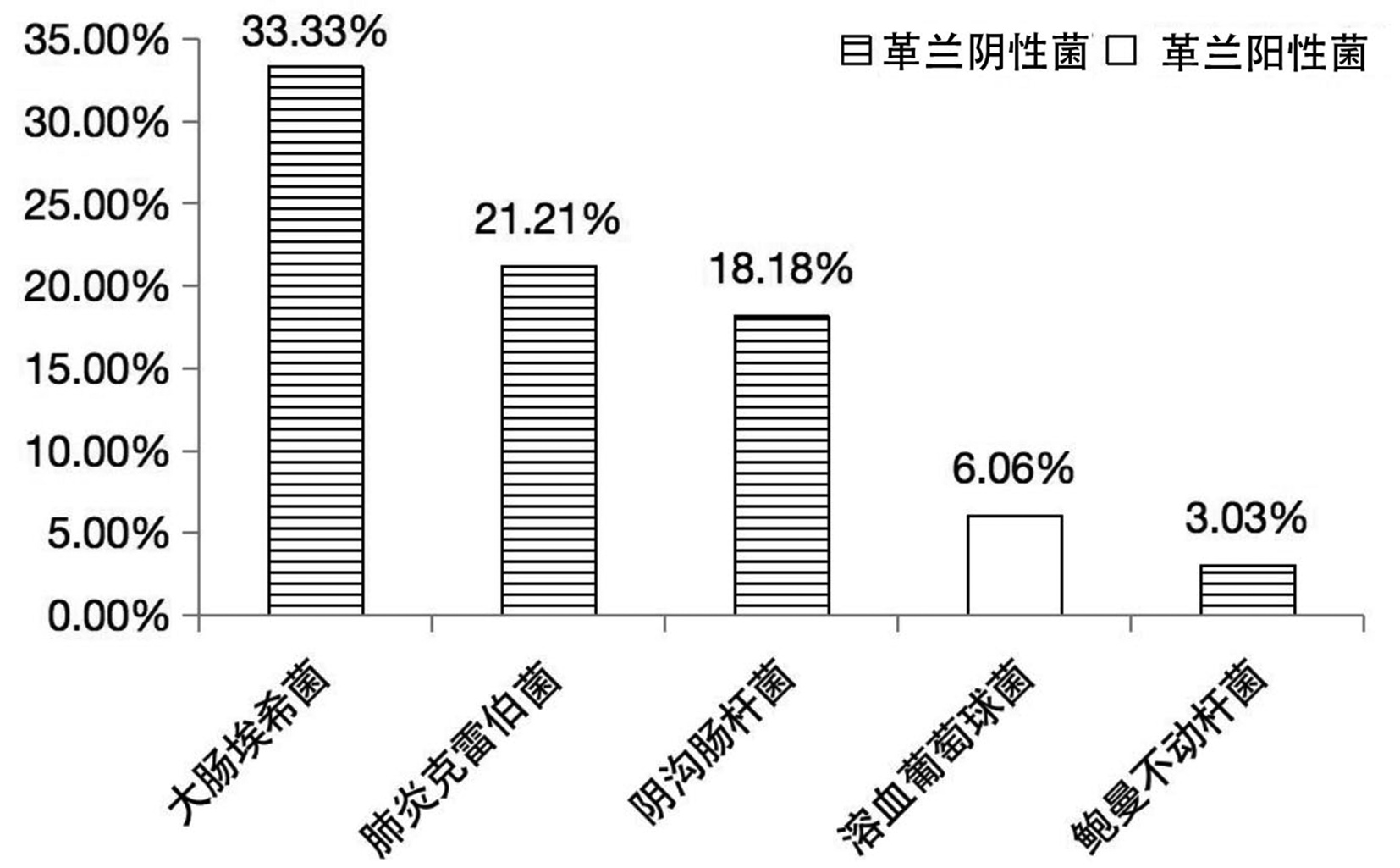
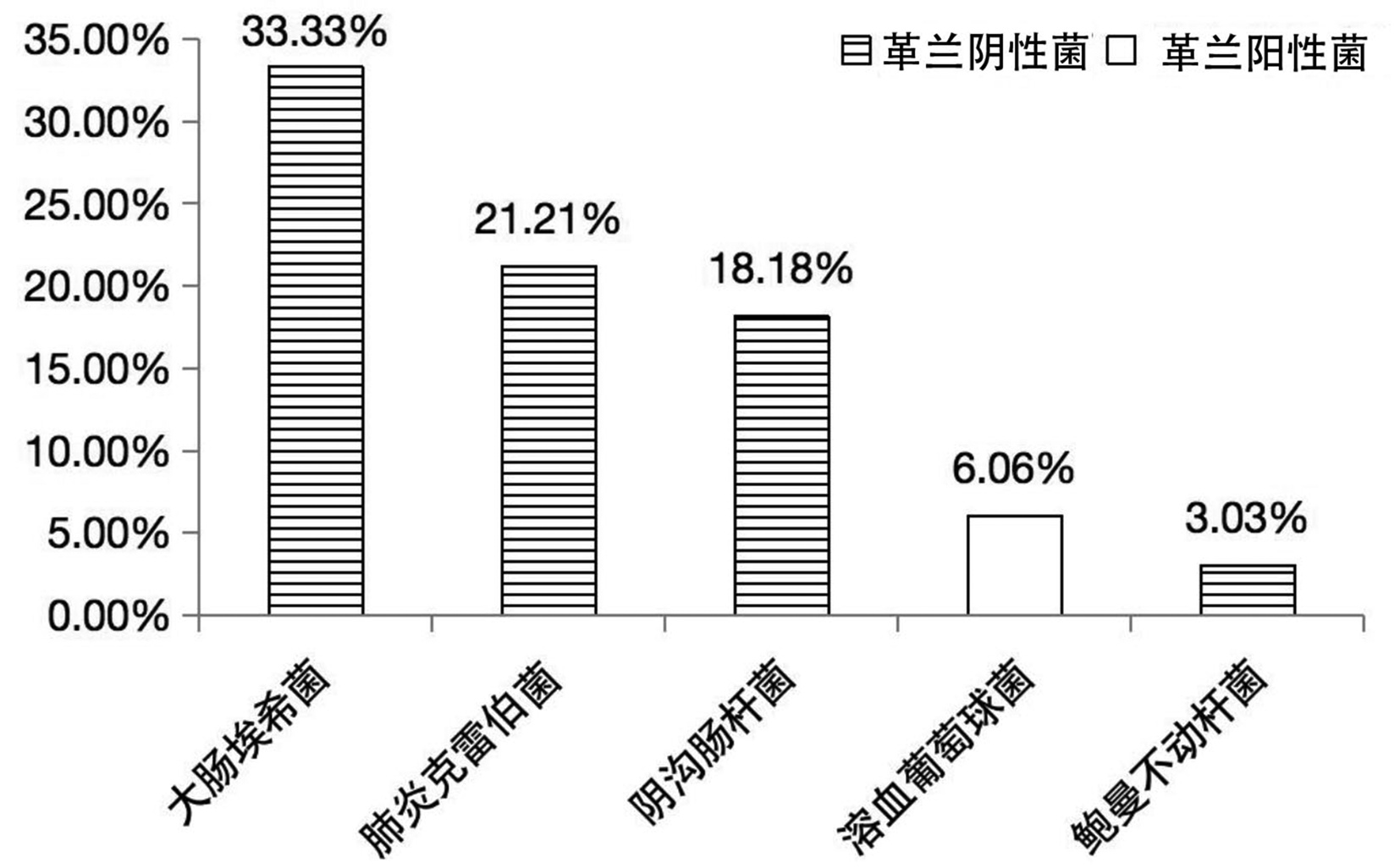
219例患者中,同期送检血培养者69例,其中阳性33例(47.82%)。血培养结果皆为单一细菌,共11种、33株,分别为大肠埃希菌占33.33%(11/33)、肺炎克雷伯菌占21.21%(7/33)、阴沟肠杆菌占18.18%(6/33)、溶血葡萄球菌占6.06%(2/33)、鲍曼不动杆菌占3.03%(1/33)、棒状杆菌占3.03%(1/33)、摩氏摩根菌占3.03%(1/33)、人葡萄球菌占3.03%(1/33)、屎肠球菌占3.03%(1/33)、嗜麦芽窄食单胞菌占3.03%(1/33)、铜绿假单胞菌占3.03%(1/33)。其中,最常见的5种菌及其构成比见图 2。
根据培养结果,该69例患者可分为:血培养(+)胆汁培养(+)25例、血培养(+)胆汁培养(-)8例、血培养(-)胆汁培养(+)22例、血培养(-)胆汁培养(-)14例。血培养和胆汁培养同为阳性的患者一致性分析:完全一致占36%(9/25)、部分一致占20%(5/25)、完全不一致占44%(11/25)。
2.3 主要致病菌的耐药情况
胆汁培养和血培养中主要革兰氏阴性菌的耐药率见表 1。对主要革兰阴性菌(大肠埃希菌、肺炎克雷伯、阴沟肠杆菌)耐药率<35%的抗生素有头孢哌酮/舒巴坦、阿米卡星、亚胺培南。胆汁培养中主要革兰阳性菌的耐药率见表 2。对主要革兰阳性菌(屎肠球菌、粪肠球菌)耐药率<15%的抗生素有万古霉素、利奈唑胺、替考拉宁。
表 1 胆汁培养及血培养主要革兰阴性菌耐药率Table 1. Drug resistance rate of major gram-negative bacteria in bile and blood cultures药品 胆汁培养(%) 血培养(%) 大肠埃希菌
(n=18)肺炎克雷伯
(n=23)阴沟肠杆菌
(n=3)大肠埃希菌
(n=9)肺炎克雷伯
(n=5)阴沟肠杆菌
(n=5)头孢曲松 88.89 65.22 66.67 66.67 60.00 60.00 头孢唑啉 72.22 56.52 66.67 66.67 60.00 80.00 环丙沙星 72.22 60.87 33.33 55.56 40.00 20.00 复方新诺明 72.22 56.52 66.67 44.44 40.00 60.00 哌拉西林 66.67 56.52 33.33 66.67 60.00 60.00 氨苄西林 66.67 65.22 66.67 77.78 100.00 60.00 头孢他啶 44.44 43.48 33.33 33.33 40.00 60.00 头孢吡肟 38.89 30.43 33.33 33.33 60.00 20.00 亚胺培南 33.33 34.78 33.33 0 0 20.00 头孢哌酮/舒巴坦 5.56 13.04 33.33 0 0 20.00 阿米卡星 0 4.35 0 11.11 0 0 表 2 胆汁培养主要革兰阳性菌耐药率Table 2. Drug resistance rate of major gram-positive bacteria in bile cultures药品 屎肠球菌(%)
(n=8)粪肠球菌(%)
(n=7)环丙沙星 75.00 0 青霉素 62.50 0 氨苄西林 62.50 0 四环素 50.00 71.43 利奈唑胺 12.50 0 万古霉素 0 0 替考拉宁 0 0 3. 讨论
传统的观点认为,生理状态下胆道是无菌的。然而,随着宏基因组技术的发展,学者们在胆道系统中发现了丰富的细菌群落。而胆道菌群种类和数量的异常可能参与了胆道结石、自身免疫性胆管病、胆管癌的发生[3]。本研究共送检219份胆汁标本,采用普通的培养法,阳性率达47.95%,略低于既往报道的49.03%~75.3%[4-6]。这可能与笔者判断胆道感染的标准较宽有关。一些胆汁颜色、气味异常,而无明确感染症状的患者也送检了胆汁培养。
正向流动的胆汁具有强大的抗菌作用[3]。胆汁中的免疫球蛋白A、肿瘤坏死因子α、白三烯等及其代谢物可刺激肠道的免疫系统,维持菌群平衡。水脂两亲的胆汁酸可结合并溶解膜脂质成分,从而裂解细菌。而胆汁酸进入细菌后,酸化细胞质产生硫化氢等物质,杀伤细菌。此外,胆汁还能通过DNA损伤、氧化应激、渗透作用等杀灭细菌。
在MBO情况下,胆汁的正向流动停止,胆道压力升高,以上抗菌机制逐渐消失。经皮经肝穿刺、胆管内注射造影剂、导丝导管进出胆管等介入操作将不可避免地造成胆汁与血液的交通。支架或内外引流管的放置破坏了Oddi括约肌的功能,为肠液反流、肠道菌群移位提供了通路。因此,经皮胆道引流或支架置入术后并发感染的可能性较大[7-8]。我国《抗菌药物临床应用指导原则(2015年版)》推荐此类手术前预防性使用抗生素[9]。
病原学分析是正确使用抗生素的关键。本中心胆汁培养结果以革兰阴性菌为主,构成比为64.89%,常见的菌种为大肠埃希菌、肺炎克雷伯、阴沟肠杆菌等。革兰阳性菌的构成比为28.24%,常见菌种为屎肠球菌、粪肠球菌。这与我国细菌耐药监测网的报告基本吻合[10-11],但常见病原菌顺序不同。本研究结果显示,对革兰阴性菌耐药较少的抗生素有头孢哌酮/舒巴坦、阿米卡星、亚胺培南,对革兰阳性菌耐药较少的抗生素有万古霉素、利奈唑胺、替考拉宁。这对今后经验性选用抗生素有一定的参考价值。此外,本研究中血培养结果与胆汁培养结果的一致性较差,在两者均为阳性的25例患者中,完全不一致率高达44%,提示在送检胆汁培养的同时,应积极送检血培养。参考两者的结果,更好地选用/调整抗生素。
本研究为回顾性分析,样本量相对较少,送检胆汁培养和血培养的标准较为宽泛,存在一定的选择偏倚。这可能会影响阳性率、一致率的精确性,但不干扰常见菌及药敏结果。今后还需进行严格的前瞻性研究,以弥补本研究的缺点和不足。此外,患者年龄、原发肿瘤、胆道梗阻类型、介入操作方法和时间均可能影响致病菌分布,今后还需积累样本量,做进一步研究。
综上所述,本中心MBO患者经皮胆道引流或支架置入术后感染的常见致病菌以革兰阴性菌为主,也有革兰阳性菌,常见的菌种为大肠埃希菌、肺炎克雷伯、肠球菌、阴沟肠杆菌。血培养与胆汁培养的一致性较低,均应积极送检。
-
表 1 胆汁培养及血培养主要革兰阴性菌耐药率
Table 1. Drug resistance rate of major gram-negative bacteria in bile and blood cultures
药品 胆汁培养(%) 血培养(%) 大肠埃希菌
(n=18)肺炎克雷伯
(n=23)阴沟肠杆菌
(n=3)大肠埃希菌
(n=9)肺炎克雷伯
(n=5)阴沟肠杆菌
(n=5)头孢曲松 88.89 65.22 66.67 66.67 60.00 60.00 头孢唑啉 72.22 56.52 66.67 66.67 60.00 80.00 环丙沙星 72.22 60.87 33.33 55.56 40.00 20.00 复方新诺明 72.22 56.52 66.67 44.44 40.00 60.00 哌拉西林 66.67 56.52 33.33 66.67 60.00 60.00 氨苄西林 66.67 65.22 66.67 77.78 100.00 60.00 头孢他啶 44.44 43.48 33.33 33.33 40.00 60.00 头孢吡肟 38.89 30.43 33.33 33.33 60.00 20.00 亚胺培南 33.33 34.78 33.33 0 0 20.00 头孢哌酮/舒巴坦 5.56 13.04 33.33 0 0 20.00 阿米卡星 0 4.35 0 11.11 0 0 表 2 胆汁培养主要革兰阳性菌耐药率
Table 2. Drug resistance rate of major gram-positive bacteria in bile cultures
药品 屎肠球菌(%)
(n=8)粪肠球菌(%)
(n=7)环丙沙星 75.00 0 青霉素 62.50 0 氨苄西林 62.50 0 四环素 50.00 71.43 利奈唑胺 12.50 0 万古霉素 0 0 替考拉宁 0 0 -
[1] JIN L, ZOU YH. Expert consensus of percutaneous transhepatic biliary drainage and stent implantation in treatment of obstructive jaundice (2018 Edition)[J]. Chin J Interv Imaging Ther, 2019, 16(1): 2-7. DOI: 10.13929/j.1672-8475.201810014.金龙, 邹英华. 梗阻性黄疸经皮肝穿刺胆道引流及支架植入术专家共识(2018)[J]. 中国介入影像与治疗学, 2019, 16(1): 2-7. DOI: 10.13929/j.1672-8475.201810014. [2] UBEROI R, DAS N, MOSS J, et al. British Society of Interventional Radiology: Biliary drainage and stenting registry (BDSR)[J]. Cardiovasc Intervent Radiol, 2012, 35(1): 127-138. DOI: 10.1007/s00270-011-0103-4. [3] NICOLETTI A, PONZIANI FR, NARDELLA E, et al. Biliary tract microbiota: A new kid on the block of liver diseases?[J]. Eur Rev Med Pharmacol Sci, 2020, 24(5): 2750-2775. DOI: 10.26355/eurrev_202003_20548. [4] LIU J, LIU B, LI HF, et al. Etiological analysis of biliary tract infection[J]. Chin J Nosocomiol, 2018, 28(20): 3111-3114. DOI: 10.11816/cn.ni.2018-182836.刘娟, 刘波, 李惠芬, 等. 胆道感染病原学分析[J]. 中华医院感染学杂志, 2018, 28(20): 3111-3114. DOI: 10.11816/cn.ni.2018-182836. [5] CAI YL, LIU YG, ZHANG L, et al. Distribution of pathogenic bacteria and clinical characteristics in patients with biliary tract infections[J]. Chin J Nosocomiol, 2016, 26(8): 1801-1803. DOI: 10.11816/cn.ni.2016-152999.蔡轶伦, 刘玉国, 张磊, 等. 胆道感染患者病原菌分布与临床特征分析[J]. 中华医院感染学杂志, 2016, 26(8): 1801-1803. DOI: 10.11816/cn.ni.2016-152999. [6] WANG ZG, PAN LY, XU JL, et al. An analysis of distribution and drug resistance of pathogens of patients with biliary tract infection[J]. Chin J Exp Surg, 2015, 32(8): 1973-1975. DOI: 10.3760/cma.j.issn.1001-9030.2015.08.067.王志刚, 潘乐玉, 徐金莲, 等. 胆道感染致病菌分布及耐药性的研究[J]. 中华实验外科杂志, 2015, 32(8): 1973-1975. DOI: 10.3760/cma.j.issn.1001-9030.2015.08.067. [7] SAAD WE, WALLACE MJ, WOJAK JC, et al. Quality improvement guidelines for percutaneous transhepatic cholangiography, biliary drainage, and percutaneous cholecystostomy[J]. J Vasc Interv Radiol, 2010, 21(6): 789-795. DOI: 10.1016/j.jvir.2010.01.012. [8] PAN ZB, WANG YH, KONG Z, et al. Effects of different preoperative biliary drainage methods on bile bacterial culture and drug resistence of malignant obstructive jaundice[J]. Chin J Dig Surg, 2021, 20(11): 1191-1200. DOI: 10.3760/cma.j.cn115610-20210916-00467.潘孜博, 王宇宏, 孔哲, 等. 不同术前胆道引流方式对恶性梗阻性黄疸胆汁细菌培养及耐药性的影响[J]. 中华消化外科杂志, 2021, 20(11): 1191-1200. DOI: 10.3760/cma.j.cn115610-20210916-00467. [9] Revision working group of guidelines for the clinical application of antimicrobial drugs. Guidelines for the clinical application of antimicrobial drugs(2015 Edition)[EB/OL]. http://www.gov.cn/xinwen/2015-08/27/content_2920799.htm.《抗菌药物临床应用指导原则》修订工作组. 抗菌药物临床应用指导原则(2015年版)[EB/OL]. http://www.gov.cn/xinwen/2015-08/27/content_2920799.htm. [10] Biliary Surgery Group of Surgery Branch of Chinese Medical Association. Guidelines for diagnosis and treatment of acute biliary tract infections(2021)[J]. Chin J Surg, 2021, 59(6): 422-429. DOI: 10.3760/cma.j.cn112139-20210421-00180.中华医学会外科学分会胆道外科学组. 急性胆道系统感染的诊断和治疗指南(2021版)[J]. 中华外科杂志, 2021, 59(6): 422-429. DOI: 10.3760/cma.j.cn112139-20210421-00180. [11] China Antimicrobial Resistance Surveillance System. Antimicrobial resistance of bacteria: Surveillance report from China Antimicrobial Resistance Surveillance System in 2014-2019[J]. Chin J Infect Control, 2021, 20(1): 15-30. DOI: 10.3760/cma.j.cn112139-20210421-00180.全国细菌耐药监测网. 全国细菌耐药监测网2014—2019年细菌耐药性监测报告[J]. 中国感染控制杂志, 2021, 20(1): 15-30. DOI: 10.3760/cma.j.cn112139-20210421-00180. 期刊类型引用(3)
1. 袁世轩,吕子彦,杨勇,陈璐,张丽娟. 模型引导下的万古霉素血药浓度监测临床研究. 中国临床药理学与治疗学. 2024(12): 1344-1352 .  百度学术
百度学术2. 林华骏,冯哲文,辛城霖,管成剑,张小东,闵逸洋,顾晓哲,郭伟,汪栋. 术前胆道引流对胰十二指肠切除术后手术相关并发症发生的影响因素分析. 中华消化外科杂志. 2023(07): 909-915 .  百度学术
百度学术3. 黄燕茹,何南凤,郑巧梅. 安全预警管理干预联合抗菌药物在儿童胆总管囊肿术后的应用观察. 北方药学. 2022(07): 186-188 .  百度学术
百度学术其他类型引用(1)
-




 PDF下载 ( 2071 KB)
PDF下载 ( 2071 KB)


 下载:
下载:

 下载:
下载:
 百度学术
百度学术