程序性细胞死亡受体-1抑制剂治疗中晚期非病毒性相关肝癌的效果及安全性分析
DOI: 10.3969/j.issn.1001-5256.2022.12.015
Effectiveness and safety of programmed cell death-1 inhibitor in the treatment of advanced non-HBV non-HCV-related hepatocellular carcinoma
-
摘要:
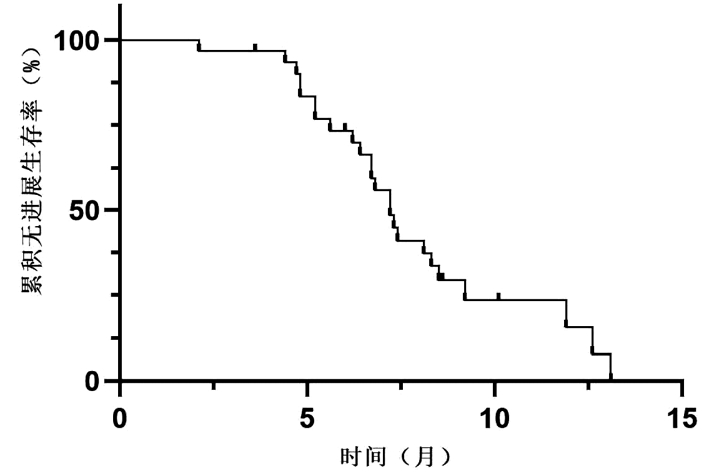
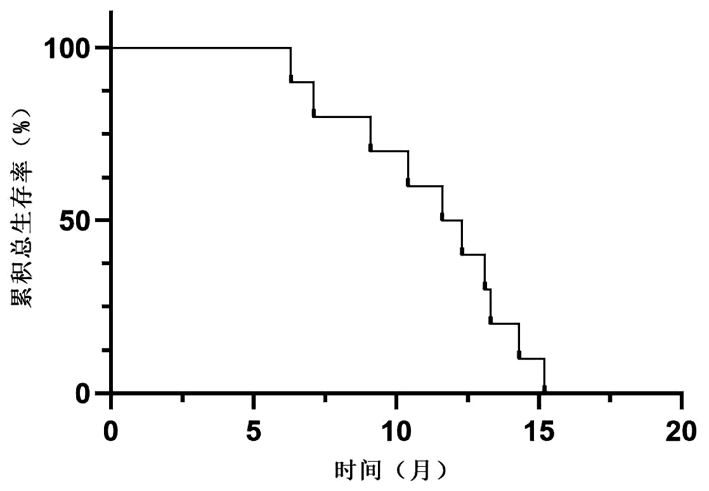
目的 研究国产程序性细胞死亡受体-1(PD-1)抑制剂在中晚期非病毒性相关肝癌(NBNC-HCC)中的临床疗效及不良反应。 方法 选取2019年6月—2022年2月徐州医科大学附属医院收治的使用国产PD-1抑制剂治疗的31例中晚期NBNC-HCC患者。分析患者疾病进展时间、客观缓解率、疾病控制率和不良反应发生情况。采用Kaplan-Meier法绘制生存曲线。 结果 在31例中晚期NBNC-HCC患者中,1例患者疗效评价为完全缓解,4例评价为部分缓解,6例评价为疾病稳定,20例评价为疾病进展,客观缓解率为16.1%,疾病控制率为35.5%,中位疾病进展时间为7.2(6.4~8.0)个月。不良反应发生率为61.30%,常见的不良反应为皮疹(29.03%)和高血压(22.58%),所有患者均无4级不良反应和不良反应相关死亡事件发生。 结论 本研究提示中晚期NBNC-HCC患者对PD-1抑制剂治疗的反应可能较弱,但不良反应可控,未来仍需多中心前瞻性试验进一步验证。 -
关键词:
- 肝肿瘤 /
- 程序性细胞死亡受体1 /
- 实体肿瘤疗效评价标准 /
- 药物相关性副作用和不良反应
Abstract:Objective To investigate the clinical effectiveness and adverse events of domestic programmed cell death -1 (PD-1) inhibitor in the treatment of advanced non-HBV non-HCV-related hepatocellular carcinoma (NBNC-HCC). Methods A totals of 31 patients with advanced NBNC-HCC who received domestic PD-1 inhibitor in the Affiliated Hospital of Xuzhou Medical University from June 2019 to February 2022 were retrospectively enrolled and their clinicopathological data were retrieved from their medical records and analyzed, i.e., the time to disease progression (TTP), disease control rate (DCR), objective response rate (ORR), and adverse events were recorded and statistically analyzed. The Kaplan-Meier method was used for survival analysis. Results Among these 31 patients, only one achieved the complete response and four achieved the partial response, and six had the stable disease, but 20 showed a disease progression, resulting in an ORR of 16.1% and a (DCR of 35.5%. The median TTP was 7.2 months [95% confidence interval: 6.4-8.0) months]. The incidence of adverse events was 61.30% and the common adverse events were skin rash (29.03%) and hypertension (22.58%). However, there was no grade 4 adverse reactions or related death in these patients. Conclusion Advanced NBNC-HCC patients had a relative weak response to the PD-1 inhibitor although the adverse events were controllable. Future multi-center prospective clinical trials are needed to validate the data. -
乙型肝炎是21世纪全世界共同面临的难题,尽管通过应用乙型肝炎疫苗,我国HBV感染者已减少,但是数据[1]显示,我国现存HBV感染者仍有约7000万,其中2000万~3000万人处于慢性阶段。肝纤维化是疾病从慢性肝炎进展为肝硬化的必经病理过程,因缺乏治疗肝纤维化的化学、生物药物和手段,目前慢性乙型肝炎(CHB)的西医治疗专注于抗病毒。针对病因治疗的抗病毒药物能减轻肝细胞的炎症损伤,从而减少引起肝纤维化的刺激因素,进而一定程度上抑制肝纤维化的形成[2],但是CHB患者的病毒载量与肝纤维化程度不一致,单纯抗肝炎病毒治疗并不能有效抑制肝纤维化向肝硬化发展,部分患者病毒抑制后,肝纤维化仍会持续存在甚至进展[3],因此抗肝纤维化是慢性肝病治疗中的一大难点。由于肝纤维化的形成机制较为复杂,故尚未研制出针对单一作用靶点的有效抗肝纤维化的化学或生物药物。中药虽成分复杂,但有多靶点作用的综合治疗优势,经过几十年来的基础研究和临床实践,目前已有数个经国家食品药品监督管理局批准的治疗肝纤维化的中成药在临床中应用[4]。
中医药治疗可以减轻CHB肝纤维化,已得到证实[5-6]。以西药抗病毒,抑制肝脏炎症、减少肝纤维化的刺激因素为基础,辅以中药抗肝纤维化,可阻止肝星状细胞受炎症刺激的活化和自我活化,减少纤维组织生成和促进纤维组织降解,改善肝脏的微循环,有助于提高抗病毒疗效。两种医疗技术联合应用的优势,有待更多的符合循证医学的临床试验所证实。
1. 资料与方法
1.1 研究对象
选取2011年4月—2013年1月在上海交通大学附属瑞金医院和上海中医药大学附属曙光医院2个中心的乙型肝炎肝纤维化患者。纳入标准:(1)年龄18~65岁,性别不限。(2)CHB病史,HBsAg阳性≥6个月,ALT<5倍正常值上限。(3)肝活检确认显著肝纤维化,Ishak分期≥F3。(4)HBeAg阳性患者,HBV DNA>1.0×105拷贝/ml。HBeAg阴性患者,HBV DNA>1.0×104拷贝/ml。(5)半年内未曾接受抗病毒治疗,或半年前曾服用拉米夫定/替比夫定经耐药位点检测证实无耐药者。(6)观察前1个月内未服用抗肝纤维化药物。(7)有能力理解和签署知情同意书。排除标准:(1)入选前3个月内曾有上消化道大出血者。(2)TBil>2倍正常值上限。(3)AFP>50 μg/L。(4)B超显示肝内明显占位性病变。(5)BMI>30 kg/m2。(6)诊断为CHB重型或有重型肝炎倾向者。(7)失代偿期肝硬化及肝脏肿瘤患者。(8)酒精性、药物性、遗传性、免疫性、其他病毒性和非病毒性慢性肝炎患者。(9)合并心血管、肺、肾、内分泌、神经及血液系统严重疾病者以及精神病患者。(10)活动性消化性溃疡。(11)妊娠及哺乳期妇女。
1.2 随机方案
本研究采用中央随机、双盲、双中心、安慰剂平行对照的研究设计。为保证随机化和双盲,运用中央随机系统(DAS for IWRS),采用动态随机的最小化,通过系统对不同影响因素(纤维化、炎症程度和病毒水平)的不同水平实时计算,达到二组间1:1均衡分配,自动分配随机号。每个随机号,配对一个相应的药物号。
1.3 治疗方法
研究期间,所有患者口服恩替卡韦(entecavir,ETV),1片/次,1次/d;联合组口服扶正化瘀片(FZHY,国药准字:Z20050546),4片/次,3次/d;对照组口服安慰剂片(内含1%扶正化瘀片全浸膏),4片/次,3次/d。治疗48周。FZHY及相应的安慰剂片均0.4 g/片,由上海现代中医药股份有限公司黄海制药有限责任公司生产。ETV(商品名润众)0.5 mg/片,由江苏正大天晴药业股份有限公司生产。
1.4 观察指标、随访、有效性及安全性评估
入组前4周筛选期内行首次肝穿刺检查,48周治疗结束时,行第二次肝穿刺检查。入组后每12周随访1次,分别进行肝功能、血常规、乙型肝炎五项、HBV DNA、AFP、腹部超声等检测。每24周进行FibroScan检查,以及健康状况调查简表(SF-36)评估。研究期间血液生化及病理等检查由各中心实验室进行。所有病理片评分由2位病理专家分别独立双盲打分。
主要疗效评估指标:肝纤维化Ishak分期治疗后比治疗前下降1级及以上的比例。次要疗效评估指标:(1)治疗后HBV DNA阴转率(HBV DNA<50拷贝/ml)和下降幅度;(2)肝脏病理炎症组织学活动指数(HAI)分级治疗后下降1级或以上的比例。
1.5 伦理学审查
本研究方案经由上海交通大学附属瑞金医院和上海中医药大学附属曙光医院伦理委员会审批,批号:(2011)伦审第(20),所有患者入选前均签署知情同意书。本临床试验注册号为ChiCTR-TRC-11001377。
1.6 统计学方法
采用SAS 9.2统计软件进行分析。大部分数据采用全分析集(full analysis set,FAS)进行评估。以完成48周治疗和根据方案进行的第二次肝活检的受试者为研究对象,进行符合方案集(per-protocol set,PPS)分析,以确定主要的测量结果。不良反应发生率采用至少接受一次治疗,且有安全性指标记录的实际数据,进行安全数据集(safety set,SS)分析。符合正态分布的计量资料用x±s表示,两组间比较采用t检验;非正态分布的计量资料用M(P25~P75)表示,两组间比较采用Wilcoxon检验。计数资料两组间比较采用CMH χ2法、χ2检验或Fisher精确概率法。P<0.05为差异有统计学意义。
2. 结果
2.1 一般资料
2个中心共52例患者纳入本研究,随机分入联合组和对照组,每组各26例。有1例联合组患者因AFP 228 μg/L,未进入FAS分析;2例联合组患者因工作地点变动,退出研究;1例联合组患者未完成第二次肝穿刺;2例对照组患者未完成第二次肝穿刺。最终,共有46例患者完成了两次肝穿刺,进入PPS分析;所有52例受试者,均进入SS分析。其中联合组入组26例,PPS 22例,FAS 25例,SS 26例;对照组入组26例,PPS 24例,FAS 26例,SS 26例。治疗前两组的年龄、性别、身高、体质量等一般资料比较,差异均无统计学意义(P值均>0.05)(表 1)。两组患者治疗前乙型肝炎病程比较,差异有统计学意义(P<0.05);其他指标如疾病诊断、肝硬化病程、既往治疗史、过敏史、患有其他疾病及用药情况方面差异均无统计学意义(P值均>0.05)。治疗前肝脏炎症各单项评分(界面性炎症,融合性坏死,灶性坏死、凋亡和灶性炎症,汇管区炎症)、肝脏炎症HAI分级、纤维化Ishak分期、肝脏硬度测定(liver stiffness measurement,LSM)值、实验室检测指标等两组比较,差异均无统计学意义(P值均>0.05)(表 1)。两组患者治疗前SF-36评分表中除精神健康评分比较差异有统计学意义[(89.60±20.23)分vs (75.54±19.31)分,t=2.540,P=0.014],其余各项SF-36评分(生理机能、生理职能、躯体疼痛、一般健康状况、精力、社会功能、情感职能和健康变化)差异均无统计学意义(P值均>0.05)。两组依从性均良好,研究期间在合并其他用药方面差异无统计学意义(P值均>0.05)。
表 1 两组患者主要基线资料比较项目 联合组(n=25) 对照组(n=26) 统计值 P值 年龄(岁) 44.48±10.31 42.46±11.39 t=0.663 0.511 男性[例(%)] 15(60.0) 19(73.1) χ2=0.981 0.322 身高(cm) 165.44±7.81 168.27±7.70 t=1.302 0.199 体质量(kg) 65.26±11.15 64.94±9.51 t=0.110 0.913 诊断 χ2=0.024 0.877 乙型肝炎[例(%)] 11(44.0) 12(46.2) 乙型肝炎肝硬化[例(%)] 14(56.0) 14(53.8) 病程 乙型肝炎病程(年) 11.00(4.75~14.25) 15.50(10.34~21.13) χ2=3.896 0.048 肝硬化病程(月) 22.20(3.36~63.88) 6.45(1.03~29.69) χ2=1.218 0.270 实验室检查指标 HBV DNA (log拷贝/ml) 5.94±0.92 5.87±1.23 t=0.222 0.826 HBeAg阳性[例(%)] 15(60.0) 14(53.8) χ2=0.197 0.662 HBeAg阴性[例(%)] 10(40.0) 12(46.2) ALT(U/L) 42.24±22.23 43.35±24.93 t=0.167 0.868 AST(U/L) 34.60±16.32 36.88±16.17 t=0.502 0.618 TBil(μmol/L) 18.12±5.18 16.15±5.46 t=1.320 0.193 ALP(U/L) 72.24±24.19 67.96±15.29 t=0.752 0.457 白蛋白(g/L) 44.30±2.99 44.12±3.72 t=0.187 0.853 球蛋白(g/L) 30.62±3.68 31.38±3.62 t=0.740 0.463 PT(s) 13.19±1.40 13.71±1.23 t=1.062 0.298 肝脏炎症HAI分级[例(%)] χ2=0.008 0.928 1级 2(8.0) 0 2级 11(44.0) 17(65.4) 3级 11(44.0) 7(26.9) 4级 1(4.0) 2(7.7) 肝纤维化Ishak分期[例(%)] χ2=0.034 0.854 3期 8(32.0) 5(19.2) 4期 4(16.0) 9(34.6) 5期 6(24.0) 8(30.8) 6期 7(28.0) 4(15.4) LSM(kPa) 13.80±8.94 16.24±13.11 t=0.774 0.442 2.2 药物安全性
用药后两组受试者生命体征均平稳,尿常规、血常规、肾功能、心电图等均未见异常,联合组和对照组各有1例出现不良事件,不良事件发生率均为3.8%,联合组发生1例严重不良事件(肝癌死亡)。两组均无不良反应发生。两组不良事件发生率、严重不良事件发生率、不良反应发生率、生命体征安全性分析、实验室安全性指标等比较,差异均无统计学意义(P值均>0.05)。
2.3 疗效
采用FAS进行生化、病毒学和血清学比较。两组48周生化终点无明显差异。联合组和对照组48周时较0周ALT改变分别为-4.00(-23.00~2.63) U/L和-5.00(-14.83~8.00) U/L,AST改变分别为-1.00(-13.45~4.33) U/L和-2.83(-13.64~1.00) U/L,TBil改变分别为0.05(-3.75~3.30) μmol/L和0.70(-2.98~5.40) μmol/L,白蛋白改变分别为-1.00(-3.00~1.00) g/L和-1.37(-3.52~0) g/L,球蛋白改变分别为-1.40(-3.00~1.00) g/L和0(-3.00~2.00) g/L,PT改变分别为0.15(-0.70~1.10) s和-0.25(-0.85~0.70) s,各项指标两组间均无统计学意义(P值均>0.05)。
在48周内,联合组和对照组中分别有91%(21/23)、92%(23/25)的患者实现了完全病毒应答(HBV DNA<1×103拷贝/ml);联合组有30.8%(4/13),对照组有35.7%(5/14)在48周时发生HBeAg血清阴转;联合组有7.7%(1/13),对照组有28.6%(4/14)发生HBeAg血清转化成抗-HBe阳性,两组间HBeAg血清转化方面均无统计学意义(P值均>0.05)。两组均无HBsAg血清转换发生。
对SF-36评分表进行FAS历时性分析,两组治疗前后在生理机能、生理职能、躯体疼痛、一般健康状况、精力、社会功能、情感职能等方面均无明显差异。在精神健康方面,联合组评分较治疗前改变幅度为-8.00(-16.00~4.00)分,对照组改变幅度为8.00(-12.00~16.00)分,两组间差异有统计学意义(Z=2.043,P=0.047)。
对PPS进行Ishak分期治疗前后比较,联合组22例患者中,较治疗前Ishak分期下降≥1期的有18例,对照组24例患者中仅有13例下降,两组比较差异有统计学意义(P<0.05);对照组有4例发生Ishak分期较治疗前升高,联合组无Ishak分期加重情况发生(表 2)。
表 2 治疗前后肝纤维化Ishak分期和肝脏炎症HAI分级改变情况项目 联合组
(n=22)对照组
(n=24)χ2值 P值 Ishak分期改变[例(%)] 5.297 0.021 增加≥1期 0 4(16.7) 不变 4(18.2) 7(29.2) 下降≥1期 18(81.8) 13(54.2) HAI分级改变[例(%)] 6.822 0.009 增加≥1级 0 3(12.5) 不变 9(40.9) 15(62.5) 下降≥1级 13(59.1) 6(25.0) 对PPS进行HAI分级治疗前后比较,联合组22例患者中,有13例在48周内较基线改善了HAI分级,对照组24例患者中仅有6例下降,两组在HAI分级改变上治疗前后差异有统计学意义(P<0.05);对照组有3例发生HAI分级较治疗前升高,联合组无坏死性炎症恶化情况发生(表 2)。
3. 讨论
抗病毒治疗可以持续抑制HBV复制,降低肝细胞癌(HCC)的发病率,然而有研究[7]显示,接受ETV等抗病毒药物治疗的CHB患者一旦已经发生肝硬化,仍有较高的HCC患病风险。虽然近半个世纪以来中医药专家总结前人经验,通过探索,在抗肝纤维化治疗方面取得了很多进展,但是中医药抗肝纤维化的疗效,尚未被国际广泛承认,有待依靠更多的大样本随机对照、以肝穿刺病理为诊断和评判疗效“金标准”的临床多中心研究来加以验证和支持。
《肝纤维化中西医结合诊疗指南(2019年版)》[4]的发布,提高了业界对中医药抗肝纤维化的认识。FZHY是一种抗肝纤维化的中成药,由丹参、发酵虫草菌粉、绞股蓝、桃仁、松花粉、五味子(制)组成,以CHB肝纤维化为适应证,作用于肝纤维化病理过程的11个环节,达到消除或减轻肝脏炎症,减轻或去除肝纤维化,防治肝硬化的目的。既往动物和临床研究均未发现该药物有明显的毒副作用。经过多项基础和临床试验也已证实其在抗肝纤维化方面有一定的疗效[8]。国内Ⅱ期临床研究[5]证明其肝纤维化组织学逆转率达52%。一项回顾性研究[9]显示,服用FZHY的肝硬化患者中位生存时间为351.6周,对照组(服用和络舒肝胶囊)为112.1周,两组5年生存率差异显著(P<0.001)。然而,此前尚无通过治疗前后肝组织活检来评判比较联合应用FZHY、核苷和核苷酸类药物在减轻肝纤维化方面疗效的随机对照双盲临床试验。
在CHB的治疗中,组织病理学结果仍然是疗效评估的一个重要方面,有助于确定疗程。尽管存在肝穿刺样本误差和观察者间的差异,但肝活检仍是评估慢性肝炎分级和肝纤维化分期的最佳标准。本研究获得肝纤维化改善的结果是建立在组织病理学客观基础之上,而不是单纯依靠对肝纤维化的无创评估。本研究表明,与ETV单药治疗相比,FZHY和ETV 48周联合治疗在坏死性炎症活动度和纤维化方面有明显改善。治疗后HAI分级降低的患者比例,联合组(59.1%)是对照组(25.0%)的2.36倍(P<0.05),且对照组有12.5%的患者HAI分级较治疗前升高,联合组则为0;治疗后Ishak分期下降的患者比例,联合组为81.8%,对照组为54.2%,联合用药较单用ETV提高肝纤维化的逆转率27.6%(P<0.05),且对照组亦有16.7%的患者Ishak分期较治疗前升高,而联合组为0。
最近有荟萃分析[10]显示,相比传统乙型肝炎肝硬化的疗法,FZHY能更有效改善肝功能(ALT、AST、TBil等),在降低Child-Pugh评分、提高HBV DNA阴转率等方面更有效,且无严重不良反应。本研究观察到,在第24周和第48周,联合组与对照组在生化、病毒学和血清学应答等方面的改善程度差异并无统计学意义。本研究与荟萃分析结果不同的原因可能有:一是本研究入组患者中,肝硬化只占半数左右;二是荟萃分析纳入的研究中,FZHY的剂量每日0.6~4.5 g不等,疗程6~12个月不等。不可否认的是,在生化、病毒学和血清学反应等方面获得近期疗效,抗病毒药物可能发挥了主要作用[2, 11-12]。
本研究以治疗前后的肝穿刺病理评定结果作为临床抗纤维化疗效评价指标,相对以理化检查等其他指标评价纤维化改变更为客观,但本研究最大的缺陷在于样本量相对较小。为获得更详实可靠的临床研究结果,上海中医药大学肝病研究所后续将开展列为国家科技部“十二·五”重点项目的一项多中心随机双盲对照大样本临床试验(NCT02241590),预期此研究结果能为中医药抗肝纤维化疗效提供强有力的循证医学依据。本研究为上述大样本临床试验提供了可靠的预初试验结果,并提供了计算样本量的统计依据。
综上所述,本研究表明,与ETV单药治疗相比,FZHY联合ETV治疗方案对于有明显纤维化/肝硬化的CHB患者是一种较好的治疗方案。入组的大多数CHB患者在48周的治疗中获得持续的生化应答、病毒学抑制和肝脏组织学改善等方面的疗效。为推广应用抗病毒和抗肝纤维化的“双抗疗法”提供了可靠证据。
-
表 1 入组患者的临床资料
Table 1. Clinical information of enrolled patients
项目 数值 年龄(岁) 57.13±11.55 性别[例(%)] 男 20(64.5) 女 11(35.5) 病因[例(%)] NAFLD 24(77.4) 酒精性肝病 6(19.4) 自身免疫性肝病 1(3.2) 肿瘤大小[例(%)] <5 cm 13(41.9) ≥5 cm 18(58.1) 门静脉浸润[例(%)] 有 22(71.0) 无 9(29.0) 肝外转移[例(%)] 有 13(41.9) 无 18(58.1) Child-Pugh分级[例(%)] A 23(74.2) B 8(25.8) BCLC分期[例(%)] B 5(16.1) C 26(83.9) ECOG评分[例(%)] 0 3(9.7) 1 22(71.0) 2 6(19.4) 既往治疗方式[例(%)] TACE 19(61.3) 手术 6(19.4) 射频消融 4(12.9) 仑伐替尼 13(41.9) 索拉非尼 9(29.0) 阿帕替尼 6(19.4) 沙利度胺 8(25.8) 无 3(9.7) PD-1抑制剂[例(%)] 卡瑞利珠单抗 18(58.1) 信迪利单抗 9(29.0) 特瑞普利单抗 4(12.9) 注:NAFLD,非酒精性脂肪性肝病;TACE,经导管动脉化疗栓塞术。 表 2 入组患者的实验室资料
Table 2. Laboratory information of enrolled patients
项目 数值 AFP[例(%)] <400 ng/mL 13(42.0) ≥400 ng/mL 18(58.0) AST(U/L) 36.87±14.64 ALT(U/L) 27.74±13.32 Alb(g/L) 39.55±5.01 TBil(μmol/L) 17.57±8.76 PT(s) 12.04±1.05 表 3 入组患者肿瘤应答情况
Table 3. Tumor response of enrolled patients
项目 数值 CR(例) 1 PR(例) 4 SD(例) 6 PD(例) 20 ORR(%) 16.1(5/31) DCR(%) 35.5(11/31) 表 4 所有患者的不良反应情况
Table 4. Incidence of adverse reactions in all patients
项目 3级不良反应 总不良反应 皮疹[例(%)] 2(6.45) 9(29.03) 腹泻[例(%)] 1(3.23) 5(16.13) 疲劳[例(%)] 1(3.23) 6(19.35) 恶心[例(%)] 0 4(12.90) 蛋白尿[例(%)] 1(3.23) 5(16.13) 甲状腺炎[例(%)] 1(3.23) 3(9.68) 感觉异常[例(%)] 0 2(6.45) 高血压[例(%)] 3(9.68) 7(22.58) 口腔溃疡[例(%)] 0 4(12.90) 胆红素升高[例(%)] 0 2(6.45) 反应性皮肤毛细血管增生症[例(%)] 0 2(6.45) ALT升高[例(%)] 0 4(12.90) AST升高[例(%)] 1(3.23) 2(6.45) 肾上腺皮质功能减退[例(%)] 0 2(6.45) -
[1] SUNG H, FERLAY J, SIEGEL RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021, 71(3): 209-249. DOI: 10.3322/caac.21660. [2] NENU I, BREABAN I, PASCALAU S, et al. The future is now: beyond first line systemic therapy in hepatocellular carcinoma[J]. Transl Cancer Res, 2019, 8(Suppl 3): S261-S274. DOI: 10.21037/tcr.2018.11.23. [3] XUE X, LIAO W, XING Y. Comparison of clinical features and outcomes between HBV-related and non-B non-C hepatocellular carcinoma[J]. Infect Agent Cancer, 2020, 15: 11. DOI: 10.1186/s13027-020-0273-2. [4] BSISU I, RMILAH AA. Global elimination of chronic hepatitis[J]. N Engl J Med, 2019, 381(6): 589. DOI: 10.1056/NEJMc1908197. [5] SEKO Y, YAMAGUCHI K, ITOH Y. The genetic backgrounds in nonalcoholic fatty liver disease[J]. Clin J Gastroenterol, 2018, 11(2): 97-102. DOI: 10.1007/s12328-018-0841-9. [6] MALUCCIO M, COVEY A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma[J]. CA Cancer J Clin, 2012, 62(6): 394-399. DOI: 10.3322/caac.21161. [7] CHUNG SY, KIM KJ, SEONG J. Biomarkers for locally advanced hepatocellular carcinoma patients treated with liver-directed combined radiotherapy[J]. Liver Cancer, 2022, 11(3): 247-255. DOI: 10.1159/000522000. [8] FINN RS, QIN S, IKEDA M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma[J]. N Engl J Med, 2020, 382(20): 1894-1905. DOI: 10.1056/NEJMoa1915745. [9] EL-KHOUEIRY AB, SANGRO B, YAU T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial[J]. Lancet, 2017, 389(10088): 2492-2502. DOI: 10.1016/S0140-6736(17)31046-2. [10] ZHU AX, FINN RS, EDELINE J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial[J]. Lancet Oncol, 2018, 19(7): 940-952. DOI: 10.1016/S1470-2045(18)30351-6. [11] PFISTER D, NÚÑEZ NG, PINYOL R, et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC[J]. Nature, 2021, 592(7854): 450-456. DOI: 10.1038/s41586-021-03362-0. [12] JI F, NGUYEN MH. Cabozantinib plus atezolizumab in advanced hepatocellular carcinoma and the role of adjuvant antiviral therapy[J]. Lancet Oncol, 2022, 23(8): 962-963. DOI: 10.1016/S1470-2045(22)00383-7. [13] KELLEY RK, RIMASSA L, CHENG AL, et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): a multicentre, open-label, randomised, phase 3 trial[J]. Lancet Oncol, 2022, 23(8): 995-1008. DOI: 10.1016/S1470-2045(22)00326-6. [14] LENCIONI R, LLOVET JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma[J]. Semin Liver Dis, 2010, 30(1): 52-60. DOI: 10.1055/s-0030-1247132. [15] European Association for the Study of the Liver. Corrigendum to "EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma"[J Hepatol 69 (2018) 182-236][J]. J Hepatol, 2019, 70(4): 817. DOI: 10.1016/j.jhep.2019.01.020. [16] KUDO M, MATILLA A, SANTORO A, et al. CheckMate 040 cohort 5: A phase Ⅰ/Ⅱ study of nivolumab in patients with advanced hepatocellular carcinoma and Child-Pugh B cirrhosis[J]. J Hepatol, 2021, 75(3): 600-609. DOI: 10.1016/j.jhep.2021.04.047. [17] WANG F, QIN S, SUN X, et al. Reactive cutaneous capillary endothelial proliferation in advanced hepatocellular carcinoma patients treated with camrelizumab: data derived from a multicenter phase 2 trial[J]. J Hematol Oncol, 2020, 13(1): 47. DOI: 10.1186/s13045-020-00886-2. [18] ONZI G, MORETTI F, BALBINOT S S, et al. Hepatocellular carcinoma in non-alcoholic fatty liver disease with and without cirrhosis[J]. Hepato Res, 2019, 5: 7. DOI: 10.20517/2394-5079.2018.114. [19] DEGASPERI E, COLOMBO M. Distinctive features of hepatocellular carcinoma in non-alcoholic fatty liver disease[J]. Lancet Gastroenterol Hepatol, 2016, 1(2): 156-164. DOI: 10.1016/S2468-1253(16)30018-8. [20] XU W, ZHANG X, WU JL, et al. O-GlcNAc transferase promotes fatty liver-associated liver cancer through inducing palmitic acid and activating endoplasmic reticulum stress[J]. J Hepatol, 2017, 67(2): 310-320. DOI: 10.1016/j.jhep.2017.03.017. [21] SHEN ZQ, CHEN YF, CHEN JR, et al. CISD2 haploinsufficiency disrupts calcium homeostasis, causes nonalcoholic fatty liver disease, and promotes hepatocellular carcinoma[J]. Cell Rep, 2017, 21(8): 2198-2211. DOI: 10.1016/j.celrep.2017.10.099. [22] SCHUSTER S, CABRERA D, ARRESE M, et al. Triggering and resolution of inflammation in NASH[J]. Nat Rev Gastroenterol Hepatol, 2018, 15(6): 349-364. DOI: 10.1038/s41575-018-0009-6. [23] BOLAND P, WU J. Systemic therapy for hepatocellular carcinoma: beyond sorafenib[J]. Chin Clin Oncol, 2018, 7(5): 50. DOI: 10.21037/cco.2018.10.10. [24] BENSON AB, D'ANGELICA MI, ABBOTT DE, et al. Hepatobiliary cancers, version 2.2021, NCCN clinical practice guidelines in oncology[J]. J Natl Compr Canc Netw, 2021, 19(5): 541-565. DOI: 10.6004/jnccn.2021.0022. [25] General Office of National Health Commission. Standard for diagnosis and treatment of primary liver cancer (2022 edition)[J]. J Clin Hepatol, 2022, 38(2): 288-303. DOI: 10.3969/j.issn.1001-5256.2022.02.009.国家卫生健康委办公厅. 原发性肝癌诊疗指南(2022年版)[J]. 临床肝胆病杂志, 2022, 38(2): 288-303. DOI: 10.3969/j.issn.1001-5256.2022.02.009. [26] RODERBURG C, WREE A, DEMIR M, et al. The role of the innate immune system in the development and treatment of hepatocellular carcinoma[J]. Hepat Oncol, 2020, 7(1): HEP17. DOI: 10.2217/hep-2019-0007. [27] QIN S, REN Z, MENG Z, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial[J]. Lancet Oncol, 2020, 21(4): 571-580. DOI: 10.1016/S1470-2045(20)30011-5. [28] CHEN J, HU X, LI Q, et al. Effectiveness and safety of toripalimab, camrelizumab, and sintilimab in a real-world cohort of hepatitis B virus associated hepatocellular carcinoma patients[J]. Ann Transl Med, 2020, 8(18): 1187. DOI: 10.21037/atm-20-6063. 期刊类型引用(18)
1. 何平,徐婧怡,张雪雪,陈晓龙. 丙酚替诺福韦联合扶正化淤片对乙肝肝硬化代偿期肝纤维化的影响. 中国实用医刊. 2024(02): 118-120 .  百度学术
百度学术2. 杨世达. 扶正化瘀胶囊联合富马酸替诺福韦二吡呋酯治疗慢性乙型肝炎肝纤维化的临床效果. 临床合理用药. 2024(13): 71-74 .  百度学术
百度学术3. 王协揆,王拱辰,张明香. 扶正化瘀片联合恩替卡韦片治疗乙型肝炎肝硬化伴食管胃底静脉曲张的临床效果. 临床合理用药. 2024(17): 61-64 .  百度学术
百度学术4. 王春艳,纪冬,陈艳,周光德,董政,王建军,陈国凤,杨永平. 慢性乙型肝炎患者经恩替卡韦治疗后获得显著组织学应答的影响因素及列线图模型构建. 解放军医学杂志. 2023(02): 143-150 .  百度学术
百度学术5. 袁媛,杨阿妹. 扶正化瘀片联合聚乙二醇干扰素α-2b治疗慢性乙型肝炎肝纤维化的疗效. 临床合理用药. 2023(12): 88-91 .  百度学术
百度学术6. 吴健,麦盛始,赵气孟. 磁共振定量成像技术在肝纤维化分期诊断及评估中的应用. 中国CT和MRI杂志. 2023(08): 103-105 .  百度学术
百度学术7. 程宁,李吉彦,李梅,李薇. 乙肝相关肝纤维化的中西医治疗进展与思考. 中国中医药现代远程教育. 2023(24): 197-199 .  百度学术
百度学术8. 吴萌萌. 扶正化瘀片联合恩替卡韦治疗慢性乙肝肝硬化的效果观察. 罕少疾病杂志. 2023(12): 62-63+66 .  百度学术
百度学术9. 钟镇康,周晓玲,王月明,吴腾,陆世银,沈新辉. 济生肾气汤加三七、鳖甲对CCl_4诱导肝纤维化大鼠模型的干预作用及机制. 海南医学院学报. 2022(10): 742-749 .  百度学术
百度学术10. 王晗笑,陈欣菊,赵晴,赵文霞. 中医药在核苷(酸)类似物治疗慢性乙型肝炎中的作用. 临床肝胆病杂志. 2022(08): 1886-1891 .  本站查看
本站查看11. 冯建超,周进学,李庆军. Wnt/β-catenin通路相关蛋白在乙型肝炎肝纤维化进程中的临床意义. 中西医结合肝病杂志. 2022(09): 794-798 .  百度学术
百度学术12. 孙宁宁,孙凤霞. 抗病毒联合中医药治疗肝炎后肝硬化的意义. 中西医结合肝病杂志. 2022(09): 849-851 .  百度学术
百度学术13. 丁小平,邱彬,施海斌. 复方鳖甲软肝片治疗慢性乙型肝炎肝纤维化临床研究. 新中医. 2022(22): 72-75 .  百度学术
百度学术14. 王刚. 恩替卡韦联合硫普罗宁对慢性乙型肝炎患者肝功能的影响观察. 现代诊断与治疗. 2022(18): 2792-2794 .  百度学术
百度学术15. 李姣,杜景海. 异甘草酸镁联合恩替卡韦治疗慢性乙型肝炎有效性与安全性分析. 中国药物滥用防治杂志. 2021(03): 327-331 .  百度学术
百度学术16. 安祯祥,何远利,唐东昕,黄丹,王敏,王芳. 扶脾柔肝颗粒对肝纤维化模型大鼠的改善作用机制研究. 中国药房. 2021(21): 2587-2592 .  百度学术
百度学术17. 尹常健. 中医药治疗肝硬化的若干理论与临床细节. 中西医结合肝病杂志. 2021(11): 978-982 .  百度学术
百度学术18. 任孟群,谢雅. 恩替卡韦对慢性乙型肝炎临床疗效观察. 深圳中西医结合杂志. 2021(20): 141-143 .  百度学术
百度学术其他类型引用(13)
-




 PDF下载 ( 1945 KB)
PDF下载 ( 1945 KB)

 下载:
下载:
 下载:
下载:
 百度学术
百度学术

