两样本孟德尔随机化分析原发性硬化性胆管炎与结直肠癌发生风险的关系
DOI: 10.3969/j.issn.1001-5256.2023.03.013
Association between primary sclerosing cholangitis and the risk of colorectal cancer: A two-sample Mendelian randomization study
-
摘要:
目的 运用两样本孟德尔随机化(TSMR)评估原发性硬化性胆管炎(PSC)与结直肠癌(CRC)之间的关联。 方法 PSC与CRC相关的单核苷酸多态性(SNP) 数据分别来自芬兰生物银行及英国生物银行,对基于全基因组关联研究(GWAS)的所有汇总数据进行二次数据分析,选择与PSC密切关联的遗传位点作为工具变量,分别以孟德尔随机化Egger回归法、中位数加权法、IVW随机效应模型、最大似然比法、线性中位数加权法、IVW radial法、IVW固定效应模型七种方法做TSMR,以OR值评价PSC和CRC风险之间的因果关系。 结果 基因预测的PSC对CRC存在正向因果关系,以IVW固定效应模型为例,遗传决定的患PSC患者发生CRC风险增加(OR=1.002 243,95%CI:1.001 319~1.003 167)。TSMR结果不存在异质性(P=0.87),无水平多效性(P=0.95)。本次所选PSC的3个工具变量为强工具变量(F=11.86)。 结论 TSMR发现PSC具有与CRC风险相关的遗传证据。无论是否合并炎症性肠病,在PSC患者中积极进行肠镜筛查或可有利于CRC的早期发现与及时干预。 Abstract:Objective To investigate the association between primary sclerosing cholangitis (PSC) and colorectal cancer (CRC) by using two-sample Mendelian randomization (TSMR). Methods The single nucleotide polymorphism (SNP) data associated with PSC and CRC were obtained from Finland Biobank and UK Biobank, respectively. A secondary data analysis was performed for all pooled data based on genome-wide association studies to select the genetic loci closely associated with PSC as instrumental variables, and TSMR was conducted by seven methods, i.e., Egger regression in Mendelian randomization, weighted median, inverse variance weighted (IVW) random effects model, maximum likelihood, linear weighted median, IVW radial method, and IVW fixed effects model. Odds ratio (OR) value was used to evaluate the causal relationship between PSC and the risk of CRC. Results There was a positive causal relationship between gene predicted PSC and CRC, and with the IVW fixed effects model as an example, genetically determined patients with PSC could increase the risk of CRC (OR=1.002 243, 95% confidence interval: 1.001 319-1.003 167). TSMR results showed no heterogeneity (P=0.87) or horizontal pleiotropy (P=0.95). The three instrumental variables selected for PSC were strong instrumental variables (F=11.86). Conclusion TSMR shows the genetic evidence for the association between PSC and the risk of CRC. Regardless of the presence or absence of inflammatory bowel disease, active enteroscopy screening among patients with PSC may help with the early identification and timely intervention of CRC. -
原发性硬化性胆管炎(PSC)是一种罕见疾病,其以多灶性胆管狭窄和进展期肝病为特征,目前尚缺乏有效治疗的药物,终末期患者必须依靠肝移植(liver transplantation,LT)才能存活[1-3]。有关研究[4]指出,与仅患有炎症性肠病(inflammatory bowel disease,IBD)的患者相比,同时患有PSC的患者患结直肠癌(colorectal cancer,CRC) 的风险增加了近10倍。尽管PSC在我国比较少见,但PSC却是多个欧洲国家的主要移植适应证[5]。研究[6]指出CRC累积发病率最高的LT患者是患有PSC的受者,并建议对LT后CRC进行强化的个体化筛查。荟萃分析[7]也指出,PSC与进展期CRC息息相关, 定期进行CRC筛查可以改善患者的临床结局。但这种相关的机制及因果关系尚不清楚,大多是依靠观察性研究而得出的关联结论。关于因果关系的结论不能仅基于观察设计中存在的关联。观察性研究由于本身的缺陷,受到大量混杂因素与反向因果关系的干扰,并不具备足够的说服力。PSC与CRC风险之间的因果关系也尚未得到证实。孟德尔随机化(mendelian randomization,MR) 已成为使用遗传变异作为工具变量来识别风险因素和疾病之间因果关系的有力方法。基于此,本研究应用了两样本孟德尔随机化(two sample mendelian randomization,TSMR)分析来确定PSC与CRC风险之间的潜在因果关系。
1. 资料与方法
1.1 数据来源与研究设计
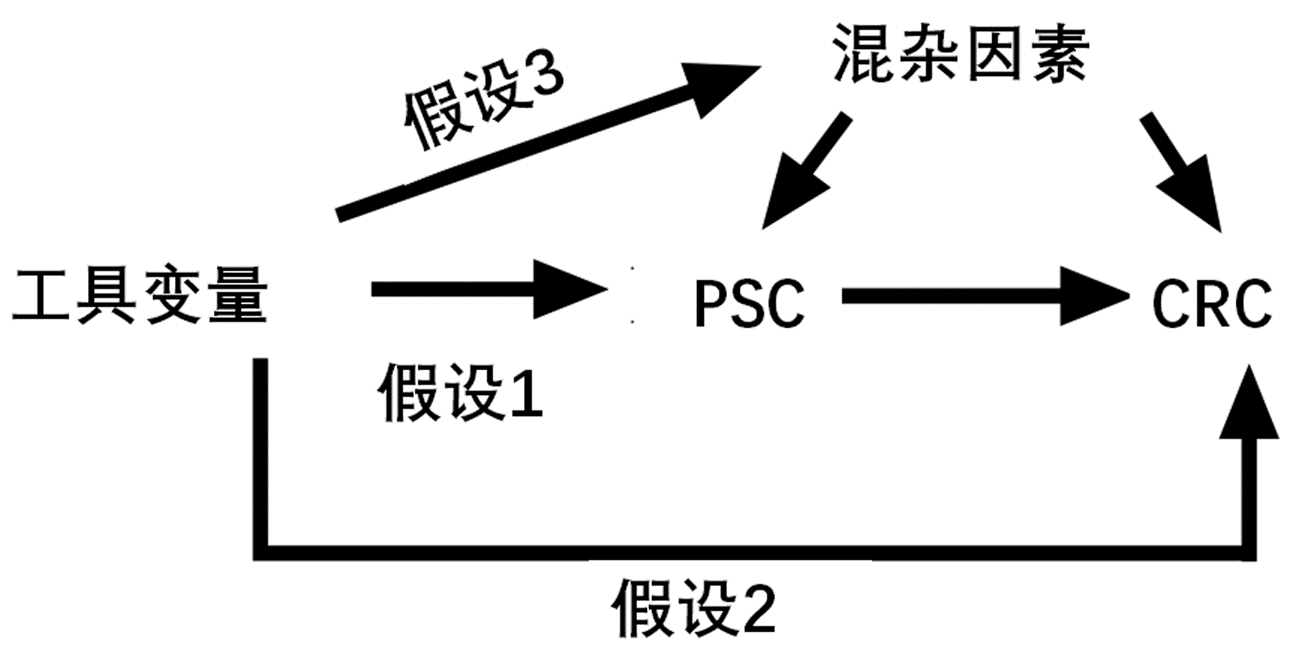
通过搜索全基因组关联研究(GWAS)确认暴露变量为PSC,结局变量为CRC。分别获得PSC与CRC患者的GWAS汇总数据,其中英国生物银行包含377 673个样本(观察组:5657;对照组:372 016),芬兰生物银行195 992个样本(观察组:778;对照组:195 144);具体简要信息如表 1。利用设计的TSMR模型评估PSC对CRC风险的因果影响(图 1)。
表 1 本项TSMR研究中的GWAS数据汇总信息Table 1. Summary of the GWAS included in this two sample mendelian randomization study暴露/结局 数据来源 样本种族来源 样本量 SNP的个数 性别 数据公布年份 PSC 芬兰生物银行 欧洲人群 195 992 16 380 407 男女混合 2021 CRC 英国生物银行 欧洲人群 377 673 11 738 639 男女混合 2021 注:SNP,单核苷酸多态性。 1.2 工具变量的选择
选择与PSC具有全基因组显著性(P<5×10-8)的遗传变异SNP位点进行汇集。设置连锁不平衡参数(r2) 阈值为0.1,遗传距离为1000 kb以选择SNP,从而确保其独立性,并排除连锁不平衡(linkage disequilibrium,LD)对结果的影响。通过人类基因型-表型关联数据库检索剩余的SNP有关的表型,重点排除其对应表型与IBD具有相关意义的SNP,剩余同时满足假设1、2、3的SNP。通过GWAS提取结局CRC的信息,从结局中获取满足假设1、2、3的SNP与结局的关系。合并暴露与结局数据集,该合并数据集包含以上工具变量同时与结局、暴露的关系,并删除回文SNP。最后剩余SNP即为指代暴露的最终工具变量。
1.3 TSMR方案
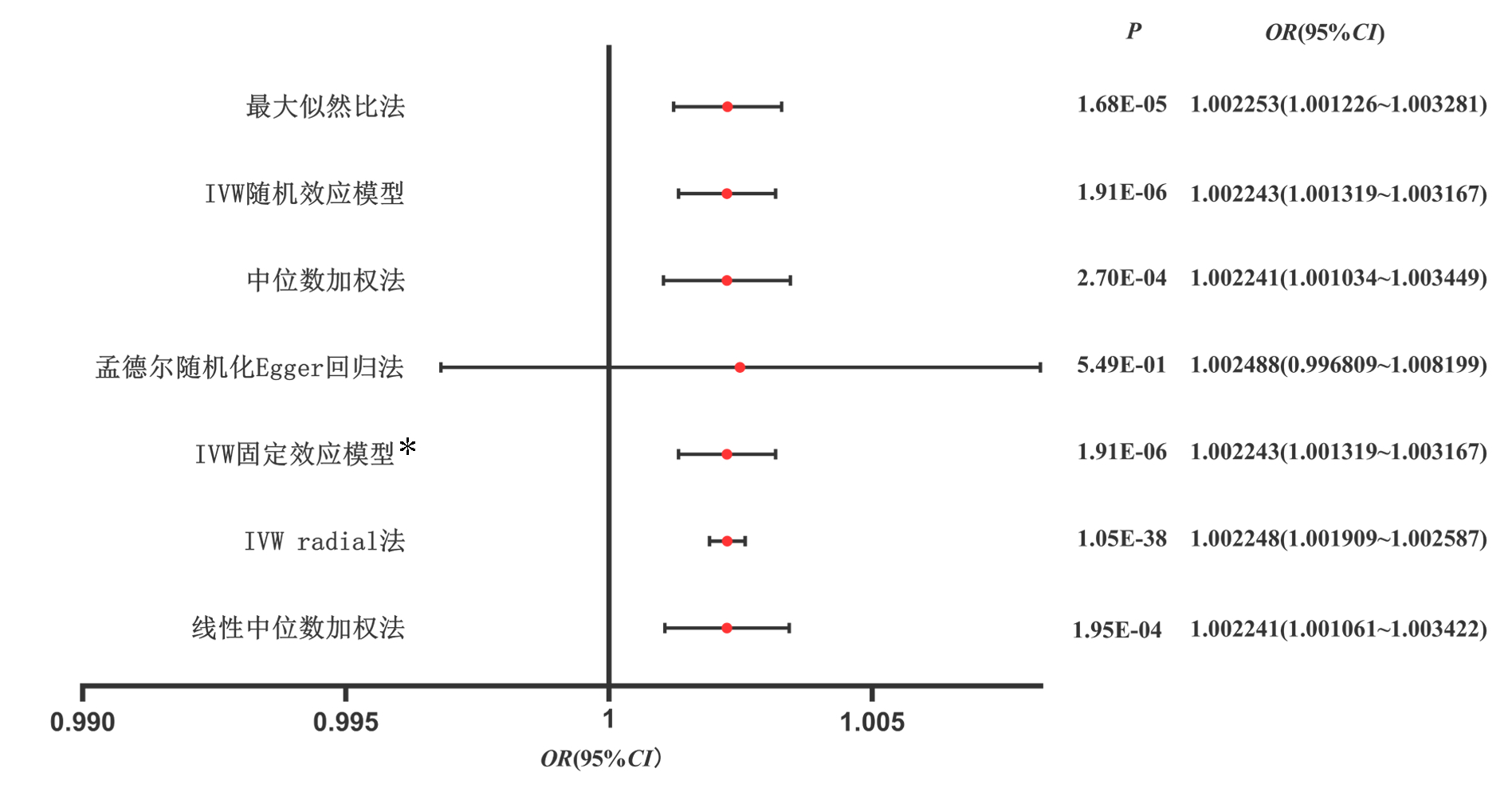
分别应用孟德尔随机化Egger回归法、中位数加权法、IVW随机效应模型、最大似然比法、线性中位数加权法、IVW radial法、IVW固定效应模型等7种方法开展TSMR,并可视化以上7种方法的OR值及95%CI。
1.4 敏感性分析
使用Racial孟德尔随机化R包对符合3个假设的SNP进行Cochran’s Q检验,用于评估个体遗传变异之间的异质性。如果Cochran’s Q检验的P<0.05,结果则存在异质性,代表暴露与结局的关系受不同年龄、不同性别等因素的影响,则孟德尔随机化的最终结果参考IVW随机效应模型作为金标准;否则,使用IVW固定效应模型作为金标准,以森林图可视化异质性检验的结果。还使用孟德尔随机化多效性检验Egger-intercept法和孟德尔随机化-PRESSO检验检查是否存在由于水平多效性而违反孟德尔随机化假设。对于水平多效性的Egger-intercept法,其中截断值估计了遗传变异是否显著通过暴露以外的途径影响结局,P<0.05代表存在水平多效性,即所挑选的工具变量显著通过暴露以外的途径影响结局,则违反了假设2、3。P>0.05即为暴露并不显著通过暴露以外的途径影响结局变量。以留一法检验作为敏感性分析,可推断最终SNP的任意一个是否为离群值。还通过观察漏斗图中的不对称性来检查结果是否稳定。再以孟德尔随机化-PRESSO法识别离群值,并评估离群值对结果的影响。
1.5 工具变量的评价
利用计算公式:R2=2×(1-MAF)(MAF)x(βSD)2。其中,MAF为暴露的次要等位基因频率,β为暴露的等位基因效应值,SD为标准差。注意,参与F值计算的R2为所有SNP的R2相加之和,表示最终工具变量指代了暴露的百分之几。再利用计算公式:F=R2(N−K−1)(1−R2)K,其中N为暴露的总样本数,K为SNP个数,R2同上,F>10即为强工具变量,F<10为弱工具变量。以上所有方法学及可视化图形部分采用R 4.1.2版本及GraphPad Prism 9.0获得。
2. 结果
2.1 参与TSMR的最终工具变量
利用R软件选择与PSC具有全基因组显著性(P<5×10-8)的遗传变异SNP位点进行汇集。设置连锁不平衡参数(r2)阈值为0.1,遗传距离为1000 kb,即筛选出同时满足假设1、2、3的SNP,剩余4个SNP分别为rs9268127、rs3094662、rs281863378、rs241438。利用人类基因型-表型关联数据库检索4个SNP有关的表型,主要排除其对应表型与IBD具有相关意义的SNP(n=0),通过GWAS提取结局CRC的信息,从结局中获取以上4个SNP与结局的关系,由于英国生物银行在CRC中未测定rs281863378的位点,结果剩余3个SNP为rs9268127、rs3094662、rs241438。合并暴露与结局数据集,该合并数据集包含以上3个工具变量同时与结局、暴露的关系,并删除回文SNP(n=0)。最后剩余的SNP即为指代暴露的最终工具变量(表 2)。
表 2 最终工具变量的信息Table 2. The information for final tool variablesSNP CHR POS EA OA EAF β值 SE P值 rs3094662 6 31 121 945 C A 0.243 585 0.000 829 149 0.000 325 454 0.010 999 90 rs9268127 6 32 253 559 C T 0.190 381 0.001 158 570 0.000 355 312 0.001 099 99 rs241438 6 32 797 620 T C 0.358 179 0.000 703 693 0.000 291 436 0.016 000 00 注:CHR,染色体; POS,SNP在染色体上的位置;EA,效应等位基因; OA,非效应等位基因; EAF,效应等位基因频率; β,等位基因效应值; SE,β的标准误。 2.2 7种方法的孟德尔随机化结果
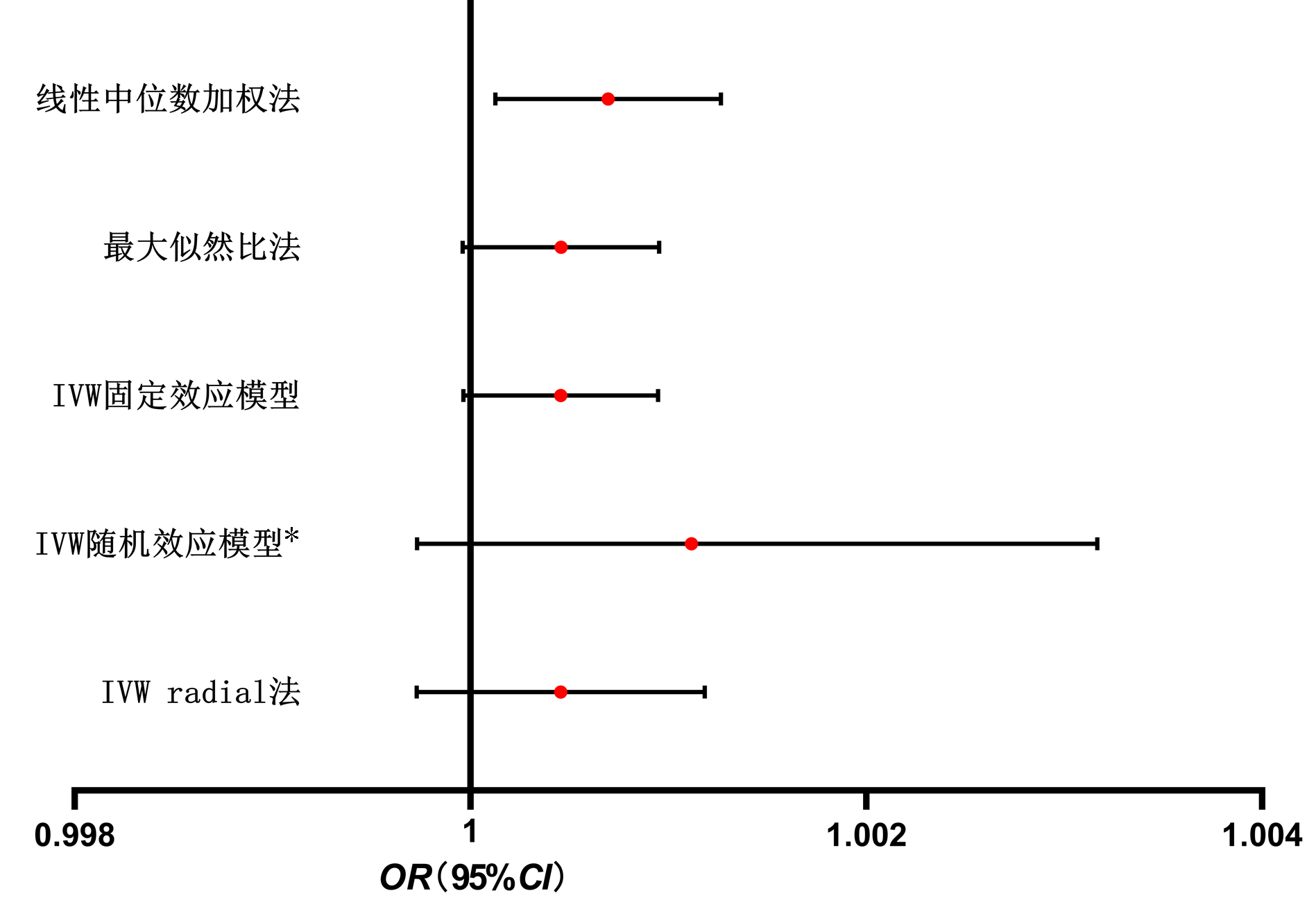
以孟德尔随机化Egger回归法、中位数加权法、IVW随机效应模型、最大似然比法、线性中位数加权法、IVW radial法、IVW固定效应模型做TSMR,除孟德尔随机化Egger回归法外, 皆显示OR值及95%CI均>1,具有统计学意义。以IVW固定效应模型为例(OR=1.002 243, 95%CI:1.001 319~1.003 167,P=1.91×10-6), 可知PSC对CRC具有正向因果关系(图 2)。
2.3 异质性及水平多效性
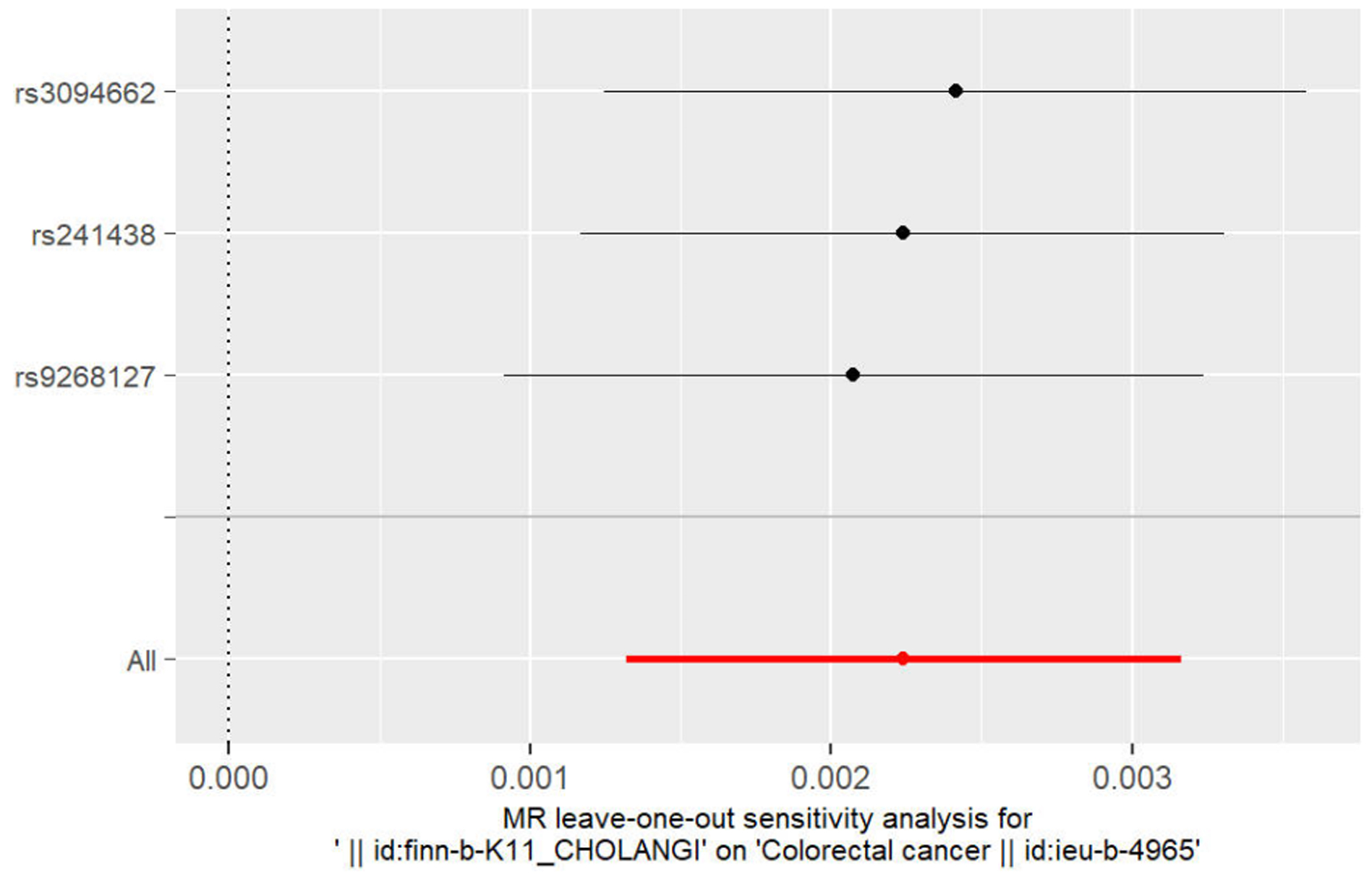
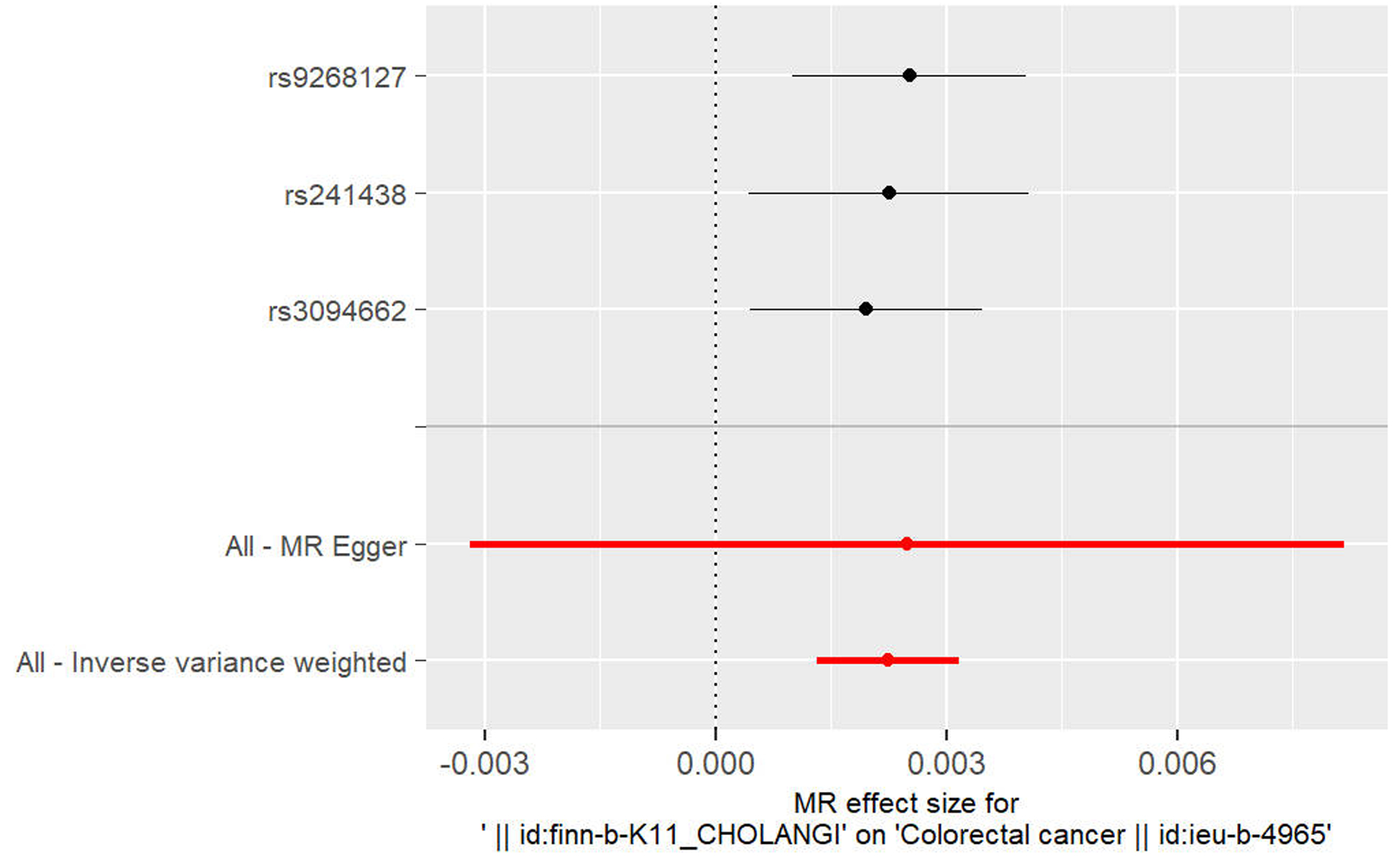
以IVW法计算PSC至CRC的孟德尔随机化结果的异质性P=0.87>0.05,未表现出明显异质性。水平多效性检验结果以Egger-intercept法求出P=0.95>0.05,说明工具变量并不显著通过暴露以外的途径影响结局。留一法检验(敏感性分析)示结果很稳定。工具变量森林图及留一法检验结果的森林图见3、4。
2.4 识别离群值及工具变量的评价
最后,以Radial包识别离群值,所筛选3个工具变量并无真正离群值,这与留一法检验的结果是一致的。利用R2及F值的计算公式,分别计算出总R2值为0.000 18,F值为11.86,证明本次研究所选取3个工具变量为强工具变量。
3. 讨论
在欧洲,PSC患者发生CRC的风险很高几乎已成为共识,被认为主要与其多伴发IBD相关,而其伴发炎症性肠病的概率约为70%,这似乎意味着正因为其伴发IBD,所以才好发CRC[8]。已经有学者[9]借助TSMR的方法探究了PSC与IBD的因果关系,并证实两者的正因果关系。然而,PSC本身与CRC的因果关系仍不清楚,本研究基于此,从遗传学的角度避开混杂因素,明确了仅仅两者之间的正因果关系。最近的一项匹配队列研究[10]中,也再次强调了PSC与各项癌症的关联。作为欧洲多国LT的主要适应证,这无疑为改善患者预后起到了指导性作用。研究[11]指出,约一半的PSC患者死于癌症。在中国,LT的适应证大多为乙型肝炎所引发的肝硬化,PSC的患者极为罕见,且对PSC的认识稍有不足,这项研究能提高对于该病的认识,并为PSC所致的结直肠癌变提供证据。以往,有学者对LT本身是否导致癌症产生了兴趣。然而,2019年一项对8115患者的多中心研究[12]显示,单独的LT与CRC发生风险的增加无关,而PSC合并溃疡性结肠炎的LT人群的CRC发生风险才显著高于普通人群,且诊断时晚期的比例很高,患者存活率明显降低。两项荟萃分析[5, 13]同时指出,即使已经接受了LT,因PSC而LT的患者产生CRC的风险仍旧很高且持续存在。这项研究无疑为荟萃分析的理论提供了依据,并加深了合理运用结肠镜筛查对部分LT患者以及PSC患者预后管理的认识。研究[14-16]指出,对于PSC患者每1~2年进行一次结肠镜检查是合适的。
在以往的一些队列研究中估算的OR值或HR值似乎因混杂因素影响而有所夸大,尤其是PSC合并IBD患者[5, 17-18]。本研究的OR值很微弱,尽管以稳定的结果证明了这种因果关系。这也是本研究的不足之处,因为未能对于PSC与IBD的患者进行分层,探讨单纯性IBD、PSC合并IBD分别对结肠癌、直肠癌的因果关系。令人遗憾的是,其实笔者已经进行了尝试,从GWAS提取数据时,未找到与之相对应的满意的数据集、与暴露相关的工具变量,或者对于结局分层展现了阴性结果等。所以,对于PSC导致CRC的机制还需要进一步的研究与探索。如Adamowicz等[19]即发现了PSC患者的结肠中microRNA-155为关键的调控节点,这为后续治疗PSC的药物研发提供了方向。包括PSC影响肠道菌群的有关研究,还值得进一步的探索与努力,而不仅仅是熊去氧胆酸治疗与LT[20-21]。
本次研究存在一些局限性。首先,由于数据集的限制,研究初衷必须要求基于两个不同的具有高度可信度的生物银行展开TSMR研究,这对笔者带来了限制,特别是单纯性IBD、PSC合并IBD的暴露因素分层与大肠癌的结局分层的关联没有被探索。第二,数据集包括欧洲人群,这限制了结果对非欧洲人群的适用性。未来需要更多的研究来验证这些结果在其他人群和其他种族中的适用性。对于亚洲人群,PSC的全基因组研究还是空白,本研究结果是否可以作为亚洲人群的适用参考还有待考证;第三,PSC是罕见病,本研究暴露变量的数据集样本量不足,统计效能较低,虽然研究结果与观察性研究相符合。但利用如前所述的方法学,笔者在更换暴露变量数据集,也即2017年所公布的目前为止样本量最大的关于PSC的GWAS数据集[22]中呈现了阴性结果(Cochran’s Q检验示P<0.05,水平多效性P>0.05,以IVW随机效应模型为金标准,PMID:27992413)(图 5)。基于数据集不同而产生的不同结果,可能有如下原因:(1)不同数据集筛选入组的PSC患者可能有种族差异,欧洲人群大多为混合人种,这为结果带来了偏倚,具有明显的人群效应可能;(2)不同数据集的纳入排除标准不一致,本研究主要采用2021年芬兰生物银行公布的数据,无法明确该数据集的纳入排除标准与2017年数据集是否一致;(3)样本量不足,尽管2017年公布数据集为目前为止样本量最大的数据集,病例组有2871例,但基于PSC的罕见性,样本量不足以获得较高的统计学效能。综上,还有待数据集的扩充与进一步的研究予以明确PSC与CRC的因果关系。
但毫无疑问的是,本研究在一定程度上证实了PSC与CRC的因果关系,为PSC患者或部分LT患者的结肠镜筛查的合理性提供了证据。通过TSMR,证明了PSC本身与CRC存在正向因果关系。因此笔者谨慎地建议无论是否合并IBD,都应加强对PSC患者的肠镜筛查。例如,对单纯PSC患者每年进行1次肠镜筛查,而对PSC合并IBD的患者进行每年2次肠镜筛查。
志谢 感谢英国生物银行及芬兰生物银行的公开数据集及有关工作人员的努力。
-
表 1 本项TSMR研究中的GWAS数据汇总信息
Table 1. Summary of the GWAS included in this two sample mendelian randomization study
暴露/结局 数据来源 样本种族来源 样本量 SNP的个数 性别 数据公布年份 PSC 芬兰生物银行 欧洲人群 195 992 16 380 407 男女混合 2021 CRC 英国生物银行 欧洲人群 377 673 11 738 639 男女混合 2021 注:SNP,单核苷酸多态性。 表 2 最终工具变量的信息
Table 2. The information for final tool variables
SNP CHR POS EA OA EAF β值 SE P值 rs3094662 6 31 121 945 C A 0.243 585 0.000 829 149 0.000 325 454 0.010 999 90 rs9268127 6 32 253 559 C T 0.190 381 0.001 158 570 0.000 355 312 0.001 099 99 rs241438 6 32 797 620 T C 0.358 179 0.000 703 693 0.000 291 436 0.016 000 00 注:CHR,染色体; POS,SNP在染色体上的位置;EA,效应等位基因; OA,非效应等位基因; EAF,效应等位基因频率; β,等位基因效应值; SE,β的标准误。 -
[1] KARLSEN TH, FOLSERAAS T, THORBURN D, et al. Primary sclerosing cholangitis - a comprehensive review[J]. J Hepatol, 2017, 67(6): 1298-1323. DOI: 10.1016/j.jhep.2017.07.022. [2] VESTERHUS M, KARLSEN TH. Emerging therapies in primary sclerosing cholangitis: pathophysiological basis and clinical opportunities[J]. J Gastroenterol, 2020, 55(6): 588-614. DOI: 10.1007/s00535-020-01681-z. [3] HENSON JB, HELZBERG JH, MUIR AJ. Patient-predicted outcomes are associated with quality of life in patients with primary sclerosing cholangitis[J]. Dig Dis Sci, 2022, 67(12): 5483-5492. DOI: 10.1007/s10620-022-07482-z. [4] de KRIJGER M, CARVALHO B, RAUSCH C, et al. Genetic profiling of colorectal carcinomas of patients with primary sclerosing cholangitis and inflammatory bowel disease[J]. Inflamm Bowel Dis, 2022, 28(9): 1309-1320. DOI: 10.1093/ibd/izac087. [5] ABBAS N, QURAISHI MN, TRIVEDI P. Emerging drugs for the treatment of primary sclerosing cholangitis[J]. Curr Opin Pharmacol, 2022, 62: 23-35. DOI: 10.1016/j.coph.2021.11.003. [6] NASSER-GHODSI N, MARA K, WATT KD. De novo colorectal and pancreatic cancer in liver-transplant recipients: identifying the higher-risk populations[J]. Hepatology, 2021, 74(2): 1003-1013. DOI: 10.1002/hep.31731. [7] SINGH S, EDAKKANAMBETH VARAYIL J, LOFTUS EV Jr, et al. Incidence of colorectal cancer after liver transplantation for primary sclerosing cholangitis: a systematic review and meta-analysis[J]. Liver Transpl, 2013, 19(12): 1361-1369. DOI: 10.1002/lt.23741. [8] BRADEN B, HALLIDAY J, ARYASINGHA S, et al. Risk for colorectal neoplasia in patients with colonic Crohn's disease and concomitant primary sclerosing cholangitis[J]. Clin Gastroenterol Hepatol, 2012, 10(3): 303-308. DOI: 10.1016/j.cgh.2011.10.020. [9] XIE Y, CHEN X, DENG M, et al. Causal linkage between inflammatory bowel disease and primary sclerosing cholangitis: a two-sample mendelian randomization analysis[J]. Front Genet, 2021, 12: 649376. DOI: 10.3389/fgene.2021.649376. [10] LUNDBERG BÅVE A, BERGQUIST A, BOTTAI M, et al. Increased risk of cancer in patients with primary sclerosing cholangitis[J]. Hepatol Int, 2021, 15(5): 1174-1182. DOI: 10.1007/s12072-021-10214-6. [11] FUNG BM, LINDOR KD, TABIBIAN JH. Cancer risk in primary sclerosing cholangitis: Epidemiology, prevention, and surveillance strategies[J]. World J Gastroenterol, 2019, 25(6): 659-671. DOI: 10.3748/wjg.v25.i6.659. [12] ROMPIANESI G, RAVIKUMAR R, JOSE S, et al. Incidence and outcome of colorectal cancer in liver transplant recipients: A national, multicentre analysis on 8115 patients[J]. Liver Int, 2019, 39(2): 353-360. DOI: 10.1111/liv.13947. [13] KOMAKI Y, KOMAKI F, MICIC D, et al. Risk of colorectal cancer in chronic liver diseases: a systematic review and meta-analysis[J]. Gastrointest Endosc, 2017, 86(1): 93-104.e5. DOI: 10.1016/j.gie.2016.12.009. [14] GOSSARD AA, GORES GJ. Primary sclerosing cholangitis: what the gastroenterologist and hepatologist needs to know[J]. Clin Liver Dis, 2017, 21(4): 725-737. DOI: 10.1016/j.cld.2017.06.004. [15] SHAH SC, TEN HOVE JR, CASTANEDA D, et al. High risk of advanced colorectal neoplasia in patients with primary sclerosing cholangitis associated with inflammatory bowel disease[J]. Clin Gastroenterol Hepatol, 2018, 16(7): 1106-1113.e3. DOI: 10.1016/j.cgh.2018.01.023. [16] HORSLEY-SILVA JL, RODRIGUEZ EA, FRANCO DL, et al. An update on cancer risk and surveillance in primary sclerosing cholangitis[J]. Liver Int, 2017, 37(8): 1103-1109. DOI: 10.1111/liv.13354. [17] TRIVEDI PJ, CROTHERS H, MYTTON J, et al. Effects of primary sclerosing cholangitis on risks of cancer and death in people with inflammatory bowel disease, based on sex, race, and age[J]. Gastroenterology, 2020, 159(3): 915-928. DOI: 10.1053/j.gastro.2020.05.049. [18] LOFTUS EV, SANDBORN WJ, TREMAINE WJ, et al. Risk of colorectal neoplasia in patients with primary sclerosing cholangitis[J]. Gastroenterology, 1996, 110(2): 432-440. DOI: 10.1053/gast.1996.v110.pm8566590. [19] ADAMOWICZ M, STUKAN I, MILKIEWICZ P, et al. Modulation of mismatch repair and the SOCS1/p53 axis by microRNA-155 in the colon of patients with primary sclerosing cholangitis[J]. Int J Mol Sci, 2022, 23(9): 4905. DOI: 10.3390/ijms23094905. [20] VESTERHUS M, KARLSEN TH. Emerging therapies in primary sclerosing cholangitis: pathophysiological basis and clinical opportunities[J]. J Gastroenterol, 2020, 55(6): 588-614. DOI: 10.1007/s00535-020-01681-z. [21] SAFFIOTI F, GURUSAMY KS, HAWKINS N, et al. Pharmacological interventions for primary sclerosing cholangitis: an attempted network meta-analysis[J]. Cochrane Database Syst Rev, 2017, 3(3): CD011343. DOI: 10.1002/14651858.CD011343.pub2. [22] JI SG, JURAN BD, MUCHA S, et al. Genome-wide association study of primary sclerosing cholangitis identifies new risk loci and quantifies the genetic relationship with inflammatory bowel disease[J]. Nat Genet, 2017, 49(2): 269-273. DOI: 10.1038/ng.3745. 期刊类型引用(5)
1. 汤熙然,陈伟坚,姜涛,谭羡允,刘文刚. 脂肪酸种类及含量与膝骨关节炎的发病风险. 中国组织工程研究. 2025(17): 3724-3731 .  百度学术
百度学术2. 从云,陈挺松,袁计红,王昊,姚文亿. ~(125)I放射性粒子治疗结直肠癌肝转移临床效果观察. 临床军医杂志. 2024(04): 393-395+399 .  百度学术
百度学术3. 陈川,杨玉洁,郭怡鲲,杨旭豪,郑旭威,陈海明,卢传坚. 基于孟德尔随机化方法探讨支气管哮喘与湿疹发病风险的因果关系. 国际呼吸杂志. 2024(06): 699-705 .  百度学术
百度学术4. 郭怡鲲,晏军. 饮食因素与慢性阻塞性肺疾病的两样本孟德尔随机化分析. 国际呼吸杂志. 2023(10): 1167-1173 .  百度学术
百度学术5. 尚文茹,魏莉莉,卢存存. 受教育程度对系统性红斑狼疮的因果效应:孟德尔随机化研究. 华西医学. 2023(12): 1880-1884 .  百度学术
百度学术其他类型引用(0)
-




 PDF下载 ( 2338 KB)
PDF下载 ( 2338 KB)

 下载:
下载:





 下载:
下载:
 百度学术
百度学术






