血清甲胎蛋白联合碱性磷酸酶评分对可切除肝细胞癌患者预后的预测价值
DOI: 10.3969/j.issn.1001-5256.2023.03.017
Value of a scoring system based on the serum levels of alpha-fetoprotein and alkaline phosphatase in predicting the prognosis of patients with resectable hepatocellular carcinoma
-
摘要:
目的 建立一个基于术前血清甲胎蛋白(AFP)与碱性磷酸酶(ALP)的评分系统,并探讨其在可切除肝细胞癌(HCC)患者中的预后价值。 方法 回顾性纳入2016年1月—2019年8月在天津市第一中心医院以肝切除术作为初始治疗的154例HCC患者。通过受试者工作特征(ROC)曲线确定血清AFP与ALP的最佳临界值。采用Kaplan-Meier曲线和Log-rank检验进行生存分析,以评估AFP联合ALP评分与HCC患者无病生存(DFS)的关系。通过单因素及多因素Cox回归分析确定HCC患者的独立预后因素。符合正态分布的计量资料组间比较采用独立样本t检验;不符合正态分布的计量资料组间比较采用Mann-Whitney U检验。计数资料组间比较采用χ2检验。 结果 ROC曲线显示,血清AFP预测DFS的最佳临界值为250.0 ng/mL,曲线下面积(AUC)为0.674 (95%CI:0.580~0.767);血清ALP的最佳临界值为95.5 U/L, AUC为0.745 (95%CI:0.652~0.838)。生存分析结果展示术前血清高AFP(≥250.0 ng/mL)和高ALP(≥95.5 U/L)均与HCC患者不良预后显著相关(P值均<0.001)。AFP联合ALP评分进一步将HCC患者分为0分(AFP<250.0 ng/mL且ALP<95.5 U/L)、1分(AFP≥250.0 ng/mL,ALP<95.5 U/L或AFP<250.0 ng/mL,ALP≥95.5 U/L)和2分(AFP≥250.0 ng/mL且ALP≥95.5 U/L)共3个研究组。生存曲线展示0分、1分和2分组患者的中位DFS分别为60.0(56.7~67.3)个月、20.0(1.4~36.6)个月和13.0(7.9~18.0)个月,组间生存差异均有统计学意义(P值均<0.05)。血清AFP联合ALP评分(1分vs 0分:HR=4.060, 95%CI: 2.050~8.039,P<0.001;2分vs 0分:HR=4.583, 95%CI: 2.385~8.805,P<0.001)是HCC患者的独立预后因素。 结论 基于血清AFP与ALP的联合评分能够有效识别预后不良的HCC患者,可作为HCC临床治疗中一项简便、可靠的预后评估工具。 Abstract:Objective To establish a scoring system based on the preoperative serum levels of alpha-fetoprotein (AFP) and alkaline phosphatase (ALP), and to investigate its value in predicting the prognosis of patients with resectable hepatocellular carcinoma (HCC). Methods A retrospective analysis was performed for 154 HCC patients who underwent hepatectomy as the initial treatment in Tianjin First Central Hospital from January 2016 to August 2019. The receiver operating characteristic (ROC) curve was used to determine the optimal cut-off values of serum AFP and ALP; the Kaplan-Meier curve and the log-rank test were used for survival analysis to evaluate the relationship between the AFP-ALP score and disease-free survival (DFS); univariate and multivariate Cox regression analyses were used to identify the independent prognostic factors for HCC patients. The independent samples t-test was used for comparison of normally distributed continuous data between groups, and the Mann-Whitney U test was used for comparison of non-normally distributed continuous data between groups; the chi-square test was used for comparison of categorical data between groups. Results The ROC curve analysis showed that serum AFP had an optimal cut-off value of 250.0 ng/mL and an area under the ROC curve (AUC) of 0.674 (95% confidence interval [CI]: 0.580-0.767) in predicting DFS, while serum ALP had an optimal cut-off value of 95.5 U/L and an AUC of 0.745 (95% CI: 0.652-0.838). The survival analysis showed that high preoperative serum levels of AFP (≥250.0 ng/mL) and ALP (≥95.5 U/L) were significantly associated with the poor prognosis of HCC patients (P < 0.001). Based on the AFP-ALP score, all HCC patients were further divided into 0-point group (AFP < 250.0 ng/mL and ALP < 95.5 U/L), 1-point group (AFP≥250.0 ng/mL, ALP < 95.5 U/L; or AFP < 250.0 ng/mL, ALP ≥95.5 U/L), and 2-point group (AFP≥250.0 ng/mL and ALP≥95.5 U/L). The survival curves showed that the 0-, 1-, and 2-point groups had a median DFS of 60.0 (56.7-67.3) months, 20.0 (1.4-36.6) months, and 13.0(7.9-18.0) months, respectively, and there were significant survival differences between the three groups (P < 0.05). Serum AFP-ALP score (1 point vs 0 point: hazard ratio [HR]=4.060, 95% confidence interval [CI]: 2.050-8.039, P < 0.001; 2 points vs 0 point: HR=4.583, 95%CI: 2.385-8.805, P < 0.001) was an independent prognostic factor for HCC patients. Conclusion The scoring system based on the serum levels of AFP and ALP can effectively identify HCC patients with poor prognosis, and therefore, it might be used as a simple and reliable tool for prognostic assessment in the clinical treatment of HCC. -
Key words:
- Carcinoma, Hepatocellular /
- alpha-Fetoproteins /
- Alkaline Phosphatase /
- Prognosis
-
肝细胞癌(HCC)是最常见的原发性肝癌类型,是目前全球发病率与死亡率位列前5位的恶性肿瘤之一[1-2]。尽管根治性肝切除与肝移植是目前治疗HCC的首选方案,但术后复发率依然很高,是导致患者临床预后不良的重要原因[3-5]。因此,探索简单可靠的预测因子对于识别预后不良的HCC患者具有至关重要的临床意义。
目前,已认定的预后预测因素包括巴塞罗那临床肝癌(BCLC)分期、肿瘤直径、微血管侵犯、血清甲胎蛋白(AFP)等[6-9]。作为HCC的传统肿瘤标志物,血清AFP已被广泛用于疾病的早期诊断和术后复发监测[10-11]。既往研究[12]表明,60%~70%的HCC患者血清AFP水平升高,并与肿瘤的侵袭性及恶性程度显著相关。与此同时,一些研究[13-15]发现血清AFP水平可以很好地预测HCC术后患者的预后。不同于其他恶性肿瘤,HCC患者的预后不仅取决于肿瘤负荷,还与肝功能等其他因素显著相关。作为一种广泛存在于肝脏和胆管中的代谢酶,血清碱性磷酸酶(ALP)水平被认为是衡量肝功能与肝细胞损害程度的重要指标之一[16-17]。越来越多的研究证据[18-20]展示较高的ALP水平与HCC患者癌症死亡风险显著相关,说明其可能是一项预测HCC复发与长期生存的新型血清学标志物。最近,有研究[21]发现将血清AFP与ALP结合可以有效预测肝癌破裂患者的预后结局。然而,目前尚未有研究证实结合这两种血清学标志物预测HCC患者的预后价值。因此,本研究旨在建立一个基于血清ALP和AFP水平的评分系统,以评价其对预后不良HCC患者的识别能力。
1. 资料与方法
1.1 研究对象
回顾性纳入2016年1月—2019年8月在天津市第一中心医院肝胆外科接受肝切除术的HCC患者。纳入标准:(1)以肝切除术作为初始治疗,手术切缘为阴性,并经术后病理证实为原发性HCC;(2)年龄≥18岁;(3)术前影像学证实无肺、骨、肾上腺等肝外转移;(4)临床病理资料、实验室检查及随访信息完整。排除标准:(1)复发性肝癌、肝内胆管癌或合并第二原发肿瘤;(2)肝功能为Child-Pugh C级;(3)围手术期内死亡或术后失访时间不足1个月者。
1.2 数据收集
从每位患者的电子病历中收集一般人口学资料、临床病理特征及治疗数据,主要研究变量包括:患者性别、年龄、HBV感染史、是否合并肝硬化、手术切除范围、肿瘤个数及肿瘤的最大直径、有无肝包膜、是否存在微血管侵犯、AJCC第8版TNM分期及肝癌BCLC临床分期等。收集的实验室检查项目主要包括术前血清AFP与ALP水平,所有患者的静脉血样于术前1周内采集并进行检测。
1.3 术后随访
对所有接受肝切除术治疗的HCC患者进行术后定期随访,在每次随访中,对患者进行体格检查、影像学检查(胸部、腹部和盆腔CT平扫+增强)及实验室检查(血常规、肝肾功能及肿瘤标志物等),以确定是否有术后肿瘤复发。对于疑有复发者,应进一步行MRI成像或PET-CT检查,诊断有困难时考虑行穿刺活检。本研究的主要观察结局为无病生存(disease-free survival, DFS),定义为患者自接受肝切除手术之日起至术后肿瘤复发的时间阶段。所有患者的随访数据与生存状态通过查阅患者的医疗记录并结合电话随访等方式获得。
1.4 统计学方法
使用IBM SPSS Statistics 22.0软件进行数据处理与统计分析。符合正态分布的计量资料以x±s表示,组间比较采用独立样本t检验;不符合正态分布的计量资料采用M(P25~P75)表示,组间比较采用Mann-Whitney U检验。计数资料组间比较采用χ2检验。通过受试者工作特征(ROC)曲线确定血清AFP与ALP预测HCC患者DFS的最佳临界值与ROC曲线下面积(AUC)。血清AFP、ALP及AFP联合ALP评分的生存分析采用Kaplan-Meier曲线和Log-rank检验进行。使用单因素Cox回归分析对每一个潜在预后变量进行分析,并将对HCC患者DFS有显著影响的因素引入多变量Cox回归模型中,确定其独立预后意义,结果展示为调整后的风险比(HR)与95%置信区间(95%CI)。P<0.05为差异有统计学意义。
2. 结果
2.1 血清AFP与ALP预测HCC患者预后的最佳临界值及其预后意义
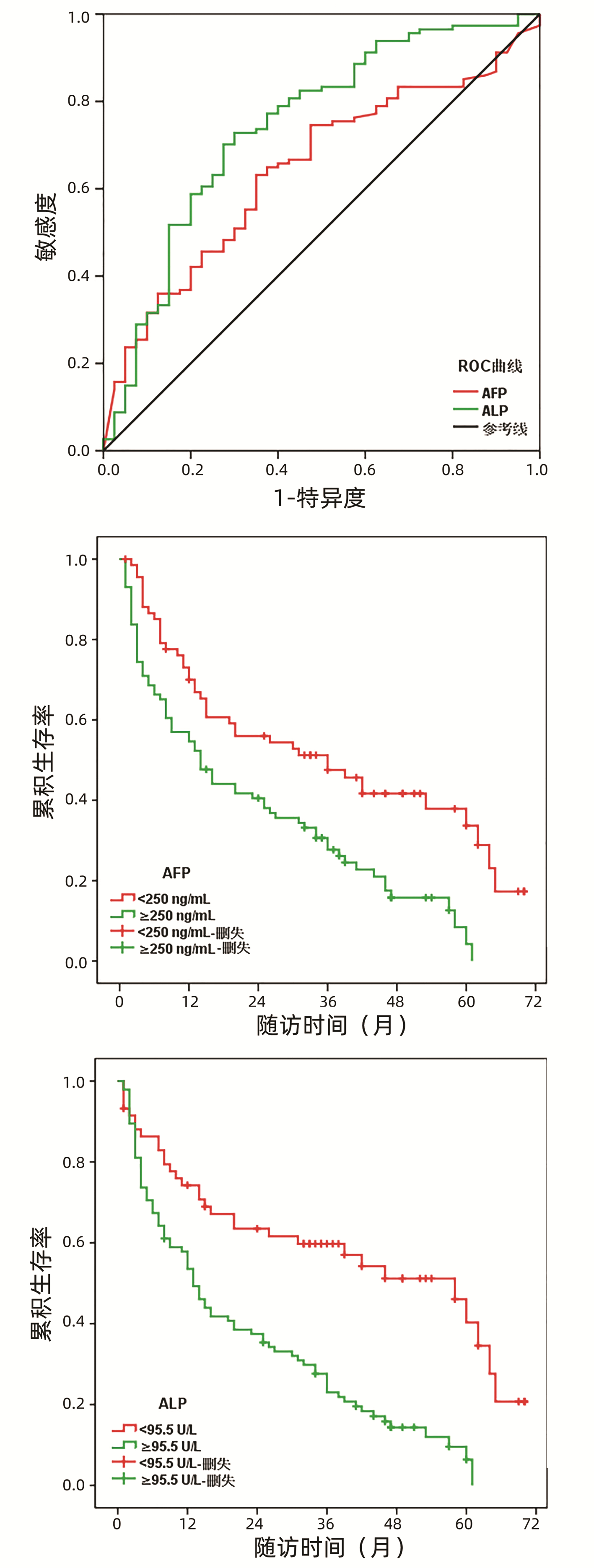
共154例患者纳入研究,其中男101例,女53例,平均(60.3±10.8)岁,肿瘤平均最大直径为(5.8±2.9)cm。ROC曲线显示,血清AFP预测DFS的最佳临界值为250.0 ng/mL,AUC=0.674 (95%CI:0.580~0.767),敏感度与特异度分别为65.6%和63.6%;血清ALP的最佳临界值为95.5 U/L,AUC=0.745 (95%CI:0.652~0.838),敏感度与特异度为73.8%和68.2%(图 1a)。根据两者的最佳临界值将所有患者分为高AF组P(≥250.0 ng/mL, n=86) 与低AFP组(<250.0 ng/mL, n=68)、高ALP组(≥95.5 U/L, n=95)与低ALP组(<95.5 U/L, n=59)。
研究队列的中位随访时间为24.0(11.0~44.0)个月,总体复发率为61.0%(94/154), 中位DFS为20.0(11.2~28.8)个月。Kaplan-Meier曲线展示高、低AFP组中位DFS分别为14.0(8.3~19.7)个月和36.0(17.1~54.8)个月,差异有统计学意义(χ2=13.383, P=0.001)(图 1b)。同样地,高ALP组患者的中位DFS明显短于低ALP组,分别为13.0(10.1~15.9)个月和58.0(38.0~77.9)个月,差异有统计学意义(χ2=21.844, P<0.001)(图 1c)。
2.2 血清AFP联合ALP评分对HCC患者预后的预测价值
根据血清AFP与ALP的最佳临界值,将所有HCC患者分为0分(血清AFP<250.0 ng/mL且ALP<95.5 U/L)、1分(血清AFP≥250.0 ng/mL,ALP<95.5 U/L或AFP<250.0 ng/mL,ALP≥95.5 U/L)和2分组(血清AFP≥250.0 ng/mL且ALP≥95.5 U/L)。从分布比例上可以看出,AFP联合ALP评分越高,多发肿瘤(χ2=6.116,P=0.047)和BCLC分期C期(χ2=11.279,P=0.024)的比例越大,发生微血管侵犯(χ2=11.631,P=0.003)的风险越高(表 1)。
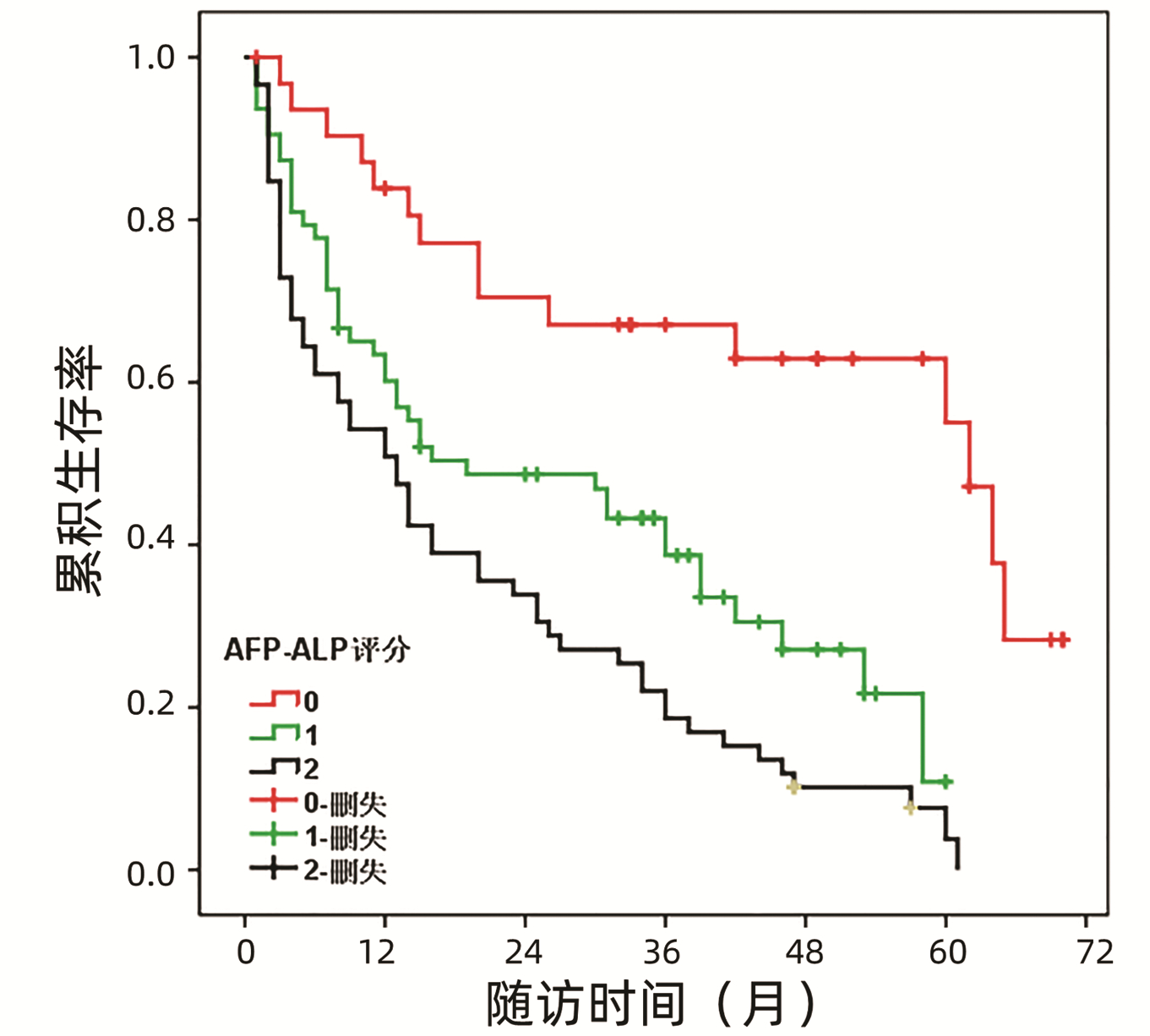
表 1 AFP联合ALP不同评分的基线特征比较Table 1. Comparison of baseline characteristics between different groups based on the serum AFP and ALP levels临床变量 0分(n=32) 1分(n=63) 2分(n=59) χ2值 P值 年龄[例(%)] 2.088 0.352 <60岁 17 (53.1) 33 (52.4) 24 (40.7) ≥60岁 15 (46.9) 30 (47.6) 35 (59.3) 性别[例(%)] 1.463 0.481 女 9 (28.1) 25 (39.7) 19 (32.2) 男 23 (71.9) 38 (60.3) 40 (67.8) HBV感染[例(%)] 4.971 0.083 无 12 (37.5) 14 (22.2) 10 (16.9) 有 20 (62.5) 49 (77.8) 49 (83.1) 肝硬化[例(%)] 0.069 0.966 无 7 (21.9) 14 (22.2) 12 (20.3) 有 25 (78.1) 49 (77.8) 47 (79.7) 肿瘤个数[例(%)] 6.116 0.047 单发 29 (90.6) 49 (77.8) 40 (67.8) 多发 3 (9.4) 14 (22.2) 19 (32.2) 肿瘤直径[例(%)] 2.724 0.256 ≤5 cm 15 (46.9) 35 (55.6) 24 (40.7) >5 cm 17 (53.1) 28 (44.4) 35 (59.3) 肝包膜[例(%)] 1.577 0.454 无 22 (68.8) 36 (57.1) 33 (55.9) 有 10 (31.2) 27 (42.9) 26 (44.1) TNM分期[例(%)] 4.802 0.091 Ⅰ~Ⅱ 11 (34.4) 12 (19.0) 9 (15.3) Ⅲ 21 (65.6) 51 (81.0) 50 (84.7) BCLC分期[例(%)] 11.279 0.024 A 9 (28.1) 7 (11.1) 7 (11.9) B 17 (53.1) 33 (52.4) 23 (39.0) C 6 (18.8) 23 (36.5) 29 (49.2) 微血管侵犯[例(%)] 11.631 0.003 否 21 (65.6) 44 (69.8) 24 (40.7) 是 11 (34.4) 19 (30.2) 35 (59.3) 基于AFP联合ALP评分的生存曲线如图 2所示,0分、1分和2分组患者的中位DFS分别为60.0(56.7~67.3)个月、20.0(1.4~36.6)个月和13.0(7.9~18.0)个月,组间生存差异均有统计学意义。总的来说,2分组患者的DFS明显短于1分组(χ2=5.144, P=0.023),而1分组患者的DFS明显短于0分组(χ2=10.576, P=0.001)。
单变量分析结果表明,肿瘤直径、TNM分期、BCLC分期、微血管侵犯和血清AFP联合ALP评分与患者低DFS具有显著相关性。通过多因素Cox回归分析发现血清AFP联合ALP评分(1分vs 0分:HR=4.060, 95%CI: 2.050~8.039,P<0.001;2分vs 0分:HR=4.583, 95%CI: 2.385~8.805,P<0.001)仍然是HCC患者预后不良的独立预测因素。此外,肿瘤直径、BCLC分期和微血管侵犯也被证实可以独立预测HCC患者预后(表 2)。
表 2 HCC患者预后因素的单因素及多因素Cox回归分析Table 2. Univariate and multivariate Cox regression analysis of prognostic factors for HCC patients临床变量 单因素分析 多因素分析 HR(95%CI) P值 HR(95%CI) P值 年龄(≥60岁vs<60岁) 1.027 (0.710~1.485) 0.888 性别(男vs女) 1.175 (0.796~1.736) 0.416 HBV感染(有vs无) 1.206 (0.777~1.871) 0.403 肝硬化(有vs无) 1.119 (0.714~1.753) 0.624 肿瘤个数(多发vs单发) 1.313 (0.854~2.018) 0.215 肿瘤直径(>5 cm vs ≤5 cm) 1.719 (1.181~2.502) 0.005 1.681 (1.138~2.484) 0.009 肝包膜(有vs无) 1.263 (0.868~1.836) 0.222 TNM分期(Ⅲ vs Ⅰ~Ⅱ) 3.322 (1.821~6.060) <0.001 1.811 (0.953~3.442) 0.066 BCLC分期(C vs A~B) 2.500 (1.719~3.636) <0.001 2.037 (1.379~3.010) <0.001 微血管侵犯(是vs否) 2.110 (1.456~3.057) <0.001 2.142 (1.442~3.182) <0.001 AFP联合ALP评分 1分vs 0分 3.029 (1.567~5.853) 0.001 4.060 (2.050~8.039) <0.001 2分vs 0分 4.813 (2.541~9.117) <0.001 4.583 (2.385~8.805) <0.001 3. 讨论
本研究基于术前血清AFP与ALP水平建立了一个简单的评分系统,并探讨了其在接受肝切除手术的HCC患者中的预后价值。结果发现,AFP联合ALP评分越高,肿瘤侵袭性较强。更为重要的是,基于血清AFP与ALP的评分对预后不良的HCC患者显示出了良好的识别能力,AFP联合ALP评分越高,患者预后越差。单因素及多因素Cox回归分析进一步证实血清AFP联合ALP评分是HCC患者的独立预后因子。这些数据证明,基于术前血清AFP与ALP的评分系统可以作为预测行肝切除手术的HCC患者预后的有效工具。
血清AFP是临床诊断HCC最常用的生物学标志物之一,被广泛用于肿瘤早期筛查、治疗效果评价和随访监测[10, 22]。此外,一些研究还表明,术前血清AFP在HCC患者预后评估方面亦贡献着重要的预测价值。Yang等[23]的研究证实术前血清AFP升高与HCC患者预后不良显著相关,是术后复发和低总生存期的独立风险因素。在Tsilimigras等[24]的一项回顾性分析中,研究者发现以AFP>400 ng/mL作为临界值,术前高AFP与低AFP组患者的5年的总生存率分别为48.5%和66.1%,且血清AFP越高,HCC患者预后越差。然而,不同研究术前血清AFP对评估HCC预后有着不同的预测范围,其最佳临界值因HCC的亚型和临床分期而异。本研究中,通过ROC曲线将AFP≥ 250.0 ng/mL作为最佳临界值,结果再次证实了其对HCC患者预后的预测价值。
尽管目前指南推荐将AFP作为HCC患者的预后标志物,但其对识别HCC高危人群的敏感度与特异度并不令人满意[11, 25]。在本研究中,AFP预测HCC患者预后的敏感度与特异度仅为65.6%和63.6%,这进一步强调了需要联合AFP与其他参数进行预后评估的必要性。本研究结果展示,将血清AFP与ALP联合使用,可以更为有效预测HCC患者的临床结局。ALP是一种常用的肝功能检测指标,可由正常肝脏、骨骼和小肠组织分泌[26]。多项研究表明,血清ALP水平在HCC等恶性肿瘤中显著升高,并在促进癌细胞增殖、血管侵袭和转移等方面发挥重要作用[27-28]。本研究发现术前高ALP组(≥95.5 U/L)患者与低ALP组(<95.5 U/L)具有明显不同的预后结局,表明血清ALP可能是HCC患者的潜在预后标志物。这些发现也得到了一些研究的支持,Huang等[29]的研究表明术前ALP变化是接受肝切除术HCC患者的一项可靠预后因子,可能反映了肝脏的损伤程度。最近,Chicco等[30]使用人工智能算法对HCC患者的生存数据进行挖掘与统计分析,结果发现血清ALP、AFP和血红蛋白水平是与患者预后最相关的3个临床变量,这为血清AFP与ALP联合预测HCC患者预后提供了支持。本研究的结果证实,AFP联合ALP评分可以进一步分层HCC患者的预后,或许可作为一项有前景的预测工具。
然而,本研究为单中心回顾性设计,存在样本量不足、选择性偏倚等局限性,亦无法从根本上消除一些可能存在的混杂因素,这或许会影响目前的分析结果。因此,本研究的结论仍需谨慎解释,并需要进一步开展大型前瞻性、多中心临床研究验证AFP联合ALP评分的预后价值,并推动其在临床中的应用。
总之,本研究综合评估了术前血清AFP与ALP对接受肝切除手术的HCC患者的预后价值,并建立了一个基于两者的评分系统。结果表明,AFP联合ALP评分可以有效识别高危HCC患者,且评分越高,患者预后越差,可作为HCC临床治疗中一项简便、可靠的预后评估工具。
-
表 1 AFP联合ALP不同评分的基线特征比较
Table 1. Comparison of baseline characteristics between different groups based on the serum AFP and ALP levels
临床变量 0分(n=32) 1分(n=63) 2分(n=59) χ2值 P值 年龄[例(%)] 2.088 0.352 <60岁 17 (53.1) 33 (52.4) 24 (40.7) ≥60岁 15 (46.9) 30 (47.6) 35 (59.3) 性别[例(%)] 1.463 0.481 女 9 (28.1) 25 (39.7) 19 (32.2) 男 23 (71.9) 38 (60.3) 40 (67.8) HBV感染[例(%)] 4.971 0.083 无 12 (37.5) 14 (22.2) 10 (16.9) 有 20 (62.5) 49 (77.8) 49 (83.1) 肝硬化[例(%)] 0.069 0.966 无 7 (21.9) 14 (22.2) 12 (20.3) 有 25 (78.1) 49 (77.8) 47 (79.7) 肿瘤个数[例(%)] 6.116 0.047 单发 29 (90.6) 49 (77.8) 40 (67.8) 多发 3 (9.4) 14 (22.2) 19 (32.2) 肿瘤直径[例(%)] 2.724 0.256 ≤5 cm 15 (46.9) 35 (55.6) 24 (40.7) >5 cm 17 (53.1) 28 (44.4) 35 (59.3) 肝包膜[例(%)] 1.577 0.454 无 22 (68.8) 36 (57.1) 33 (55.9) 有 10 (31.2) 27 (42.9) 26 (44.1) TNM分期[例(%)] 4.802 0.091 Ⅰ~Ⅱ 11 (34.4) 12 (19.0) 9 (15.3) Ⅲ 21 (65.6) 51 (81.0) 50 (84.7) BCLC分期[例(%)] 11.279 0.024 A 9 (28.1) 7 (11.1) 7 (11.9) B 17 (53.1) 33 (52.4) 23 (39.0) C 6 (18.8) 23 (36.5) 29 (49.2) 微血管侵犯[例(%)] 11.631 0.003 否 21 (65.6) 44 (69.8) 24 (40.7) 是 11 (34.4) 19 (30.2) 35 (59.3) 表 2 HCC患者预后因素的单因素及多因素Cox回归分析
Table 2. Univariate and multivariate Cox regression analysis of prognostic factors for HCC patients
临床变量 单因素分析 多因素分析 HR(95%CI) P值 HR(95%CI) P值 年龄(≥60岁vs<60岁) 1.027 (0.710~1.485) 0.888 性别(男vs女) 1.175 (0.796~1.736) 0.416 HBV感染(有vs无) 1.206 (0.777~1.871) 0.403 肝硬化(有vs无) 1.119 (0.714~1.753) 0.624 肿瘤个数(多发vs单发) 1.313 (0.854~2.018) 0.215 肿瘤直径(>5 cm vs ≤5 cm) 1.719 (1.181~2.502) 0.005 1.681 (1.138~2.484) 0.009 肝包膜(有vs无) 1.263 (0.868~1.836) 0.222 TNM分期(Ⅲ vs Ⅰ~Ⅱ) 3.322 (1.821~6.060) <0.001 1.811 (0.953~3.442) 0.066 BCLC分期(C vs A~B) 2.500 (1.719~3.636) <0.001 2.037 (1.379~3.010) <0.001 微血管侵犯(是vs否) 2.110 (1.456~3.057) <0.001 2.142 (1.442~3.182) <0.001 AFP联合ALP评分 1分vs 0分 3.029 (1.567~5.853) 0.001 4.060 (2.050~8.039) <0.001 2分vs 0分 4.813 (2.541~9.117) <0.001 4.583 (2.385~8.805) <0.001 -
[1] SUNG H, FERLAY J, SIEGEL RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021, 71(3): 209-249. DOI: 10.3322/caac.21660. [2] SIEGEL RL, MILLER KD, FUCHS HE, et al. Cancer statistics, 2021[J]. CA Cancer J Clin, 2021, 71(1): 7-33. DOI: 10.3322/caac.21654. [3] FORNER A, REIG M, BRUIX J. Hepatocellular carcinoma[J]. Lancet, 2018, 391(10127): 1301-1314. DOI: 10.1016/S0140-6736(18)30010-2. [4] YANG JD, HAINAUT P, GORES GJ, et al. A global view of hepatocellular carcinoma: trends, risk, prevention and management[J]. Nat Rev Gastroenterol Hepatol, 2019, 16(10): 589-604. DOI: 10.1038/s41575-019-0186-y. [5] European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma[J]. J Hepatol, 2018, 69(1): 182-236. DOI: 10.1016/j.jhep.2018.03.019. [6] HEINRICH S, SPRINZL M, SCHMIDTMANN I, et al. Validation of prognostic accuracy of MESH, HKLC, and BCLC classifications in a large German cohort of hepatocellular carcinoma patients[J]. United European Gastroenterol J, 2020, 8(4): 444-452. DOI: 10.1177/2050640620904524. [7] XIANG YJ, WANG K, ZHENG YT, et al. Prognostic value of microvascular invasion in eight existing staging systems for hepatocellular carcinoma: A Bi-Centeric retrospective cohort study[J]. Front Oncol, 2021, 11: 726569. DOI: 10.3389/fonc.2021.726569. [8] YANG A, XIAO W, CHEN D, et al. The power of tumor sizes in predicting the survival of solitary hepatocellular carcinoma patients[J]. Cancer Med, 2018, 7(12): 6040-6050. DOI: 10.1002/cam4.1873. [9] LIANG L, WANG MD, ZHANG YM, et al. Association of postoperative biomarker response with recurrence and survival in patients with hepatocellular carcinoma and high alpha-fetoprotein expressions (> 400 ng/ml)[J]. J Hepatocell Carcinoma, 2021, 8: 103-118. DOI: 10.2147/JHC.S289840. [10] ZHENG Y, ZHU M, LI M. Effects of alpha-fetoprotein on the occurrence and progression of hepatocellular carcinoma[J]. J Cancer Res Clin Oncol, 2020, 146(10): 2439-2446. DOI: 10.1007/s00432-020-03331-6. [11] HANIF H, ALI MJ, SUSHEELA AT, et al. Update on the applications and limitations of alpha-fetoprotein for hepatocellular carcinoma[J]. World J Gastroenterol, 2022, 28(2): 216-229. DOI: 10.3748/wjg.v28.i2.216. [12] GALLE PR, FOERSTER F, KUDO M, et al. Biology and significance of alpha-fetoprotein in hepatocellular carcinoma[J]. Liver Int, 2019, 39(12): 2214-2229. DOI: 10.1111/liv.14223. [13] SUN LY, CEN WJ, TANG WT, et al. Alpha-fetoprotein ratio predicts alpha-fetoprotein positive hepatocellular cancer patient prognosis after hepatectomy[J]. Dis Markers, 2022, 2022: 7640560. DOI: 10.1155/2022/7640560. [14] SHE WH, CHAN MY, MA KW, et al. Alpha-fetoprotein in predicting survival of patients with ruptured hepatocellular carcinoma after resection[J]. J Invest Surg, 2022, 35(5): 1091-1097. DOI: 10.1080/08941939.2021.2012615. [15] TSILIMIGRAS DI, MORIS D, HYER JM, et al. Serum α-fetoprotein levels at time of recurrence predict post-recurrence outcomes following resection of hepatocellular carcinoma[J]. Ann Surg Oncol, 2021, 28(12): 7673-7683. DOI: 10.1245/s10434-021-09977-x. [16] HEINRICH D, BRULAND Ø, GUISE TA, et al. Alkaline phosphatase in metastatic castration-resistant prostate cancer: reassessment of an older biomarker[J]. Future Oncol, 2018, 14(24): 2543-2556. DOI: 10.2217/fon-2018-0087. [17] CAI X, CHEN Z, CHEN J, et al. Albumin-to-alkaline phosphatase ratio as an independent prognostic factor for overall survival of advanced hepatocellular carcinoma patients without receiving standard anti-cancer therapies[J]. J Cancer, 2018, 9(1): 189-197. DOI: 10.7150/jca.21799. [18] SUN P, CHEN S, LI Y. The association between pretreatment serum alkaline phosphatase and prognosis in hepatocellular carcinoma: A meta-analysis[J]. Medicine (Baltimore), 2020, 99(11): e19438. DOI: 10.1097/MD.0000000000019438. [19] WU SJ, LIN YX, YE H, et al. Prognostic value of alkaline phosphatase, gamma-glutamyl transpeptidase and lactate dehydrogenase in hepatocellular carcinoma patients treated with liver resection[J]. Int J Surg, 2016, 36(Pt A): 143-151. DOI: 10.1016/j.ijsu.2016.10.033. [20] PIRAS-STRAUB K, KHAIRZADA K, GERKEN G, et al. Glutamate dehydrogenase and alkaline phosphatase as very early predictors of hepatocellular carcinoma recurrence after liver transplantation[J]. Digestion, 2015, 91(2): 117-127. DOI: 10.1159/000370212. [21] XIA F, NDHLOVU E, LIU Z, et al. Alpha-fetoprotein+alkaline phosphatase (A-A) score can predict the prognosis of patients with ruptured hepatocellular carcinoma underwent hepatectomy[J]. Dis Markers, 2022, 2022: 9934189. DOI: 10.1155/2022/9934189. [22] HU X, CHEN R, WEI Q, et al. The landscape of alpha fetoprotein in hepatocellular carcinoma: where are we?[J]. Int J Biol Sci, 2022, 18(2): 536-551. DOI: 10.7150/ijbs.64537. [23] YANG SL, LIU LP, YANG S, et al. Preoperative serum α-fetoprotein and prognosis after hepatectomy for hepatocellular carcinoma[J]. Br J Surg, 2016, 103(6): 716-724. DOI: 10.1002/bjs.10093. [24] TSILIMIGRAS DI, HYER JM, DIAZ A, et al. Synergistic impact of alpha-fetoprotein and tumor burden on long-term outcomes following curative-intent resection of hepatocellular carcinoma[J]. Cancers (Basel), 2021, 13(4): 747. DOI: 10.3390/cancers13040747. [25] PAN YX, SUN XQ, HU ZL, et al. Prognostic values of alpha-fetoprotein and des-gamma-carboxyprothrombin in hepatocellular carcinoma in china: an analysis of 4792 patients[J]. J Hepatocell Carcinoma, 2021, 8: 657-670. DOI: 10.2147/JHC.S316223. [26] SHARMA U, PAL D, PRASAD R. Alkaline phosphatase: an overview[J]. Indian J Clin Biochem, 2014, 29(3): 269-278. DOI: 10.1007/s12291-013-0408-y. [27] YAMAMOTO K, AWOGI T, OKUYAMA K, et al. Nuclear localization of alkaline phosphatase in cultured human cancer cells[J]. Med Electron Microsc, 2003, 36(1): 47-51. DOI: 10.1007/s007950300006. [28] YU MC, CHAN KM, LEE CF, et al. Alkaline phosphatase: does it have a role in predicting hepatocellular carcinoma recurrence?[J]. J Gastrointest Surg, 2011, 15(8): 1440-1449. DOI: 10.1007/s11605-011-1537-3. [29] HUANG CW, WU TH, HSU HY, et al. Reappraisal of the role of alkaline phosphatase in hepatocellular carcinoma[J]. J Pers Med, 2022, 12(4): 518. DOI: 10.3390/jpm12040518. [30] CHICCO D, ONETO L. Computational intelligence identifies alkaline phosphatase (ALP), alpha-fetoprotein (AFP), and hemoglobin levels as most predictive survival factors for hepatocellular carcinoma[J]. Health Informatics J, 2021, 27(1): 1460458220984205. DOI: 10.1177/1460458220984205. 期刊类型引用(4)
1. 周洁,高国生,金青. 血小板与淋巴细胞比值和甲胎蛋白对肝移植患者预后的预测价值. 医药前沿. 2025(01): 23-26+31 .  百度学术
百度学术2. 林孔英,陈清静,郭洛彬,杨云,陈宇峰,张建溪,魏复群,张辉,程智清,黎蕴通,王聪仁,江亚彬,林科灿,周伟平,曾永毅. 甲胎蛋白反应评估中晚期肝癌靶免联合治疗效果和预后的多中心临床研究. 中华消化外科杂志. 2024(02): 248-256 .  百度学术
百度学术3. 柴森. 甲胎蛋白联合甲胎蛋白-L3、碱性磷酸酶和酸性水解酶检测在肝细胞癌诊断中的应用. 临床研究. 2024(07): 122-124 .  百度学术
百度学术4. 蔡日,游焜,彭亚南. MRI-DWI联合血清学检查在小肝癌诊断中的应用价值. 中国CT和MRI杂志. 2024(09): 97-99 .  百度学术
百度学术其他类型引用(0)
-




 PDF下载 ( 2441 KB)
PDF下载 ( 2441 KB)


 下载:
下载:


 下载:
下载:
 百度学术
百度学术