干细胞衍生的外泌体在肝脏疾病治疗中的作用
DOI: 10.3969/j.issn.1001-5256.2023.03.034
-
摘要: 肝脏容易受多种病因影响发生肝损伤,严重情况下可引起其合成、解毒、代谢和生物转化等功能发生障碍,当前针对肝衰竭、失代偿性肝硬化等终末期肝病仍缺乏高效的临床治疗手段。近年来间充质干细胞的临床疗效已被证实,基于干细胞外泌体的相关治疗成为研究热点。本文介绍了干细胞外泌体治疗的优势、机制研究进展、临床前研究现状等。从目前研究结果来看,干细胞衍生的外泌体治疗肝脏疾病具有良好应用前景,但临床前研究仍需进一步深入,临床研究有待开展。Abstract: The liver is easily affected by a variety of factors to induce liver damage, which can cause disorders in the synthesis, detoxification, metabolism, and biotransformation functions of the liver in severe cases, and at present, there is still a lack of efficient clinical treatment methods for end-stage liver diseases such as liver failure and decompensated liver cirrhosis. Recent studies have confirmed the clinical efficacy of stem cells, and treatment methods based on stem cell-derived exosomes have become a research hotspot. This article introduces the advantages of treatment based on stem cell-derived exosomes, the research advances in related mechanisms, and the current status of preclinical research. Current research findings suggest that treatment based on stem cell-derived exosomes has a good application prospect in the treatment of liver diseases, but it is still needed to conduct in-depth preclinical and clinical studies.
-
Key words:
- Liver Diseases /
- Stem Cells /
- Mesenchymal Stem Cells /
- Exosomes
-
表 1 干细胞外泌体治疗肝脏疾病的实验研究
Table 1. Experimental studies of stem cells-derived exosomes in the treatment of liver diseases
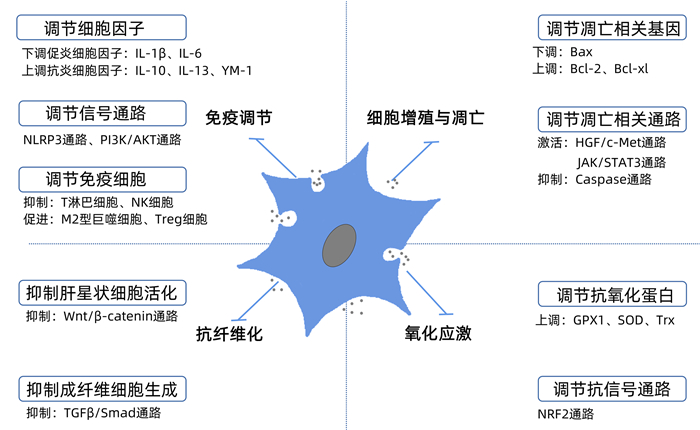
外泌体来源 重要活性因子 疾病模型 动物/细胞 结果 BMSC-Exo - LO2细胞损伤 LO2细胞 促进自噬,减少肝细胞凋亡[32] BMSC-Exo MIP2、IL-6、Y-RNA-1 暴发性肝衰竭 C57BL/6小鼠 减轻炎症反应,提高存活率[33] BMSC-Exo PPARγ、β-catenin等 肝纤维化 Sprague-Dawley大鼠 抑制Wnt/β-catenin信号通路,减轻纤维化程度,抑制炎症,促进肝细胞再生[34] BMSC-Exo miR223-3p 自身免疫性肝炎 C57BL/6小鼠 调节STAT3相关信号通路,抑制炎性细胞因子释放,减轻炎症反应[35] AMSC-Exo miR-17 ALF C57BL/6小鼠 靶向TXNIP,抑制炎症反应,改善肝损伤[36] AMSC-EV - NASH Mc4r-KO小鼠 减轻炎症反应并抑制纤维化[15] AMSC-EV lncRNA H19 ALF Sprague-Dawley大鼠 减轻炎症反应,促进肝细胞的增殖、抑制凋亡[37] ucMSC-Exo - ALF C57BL/6小鼠、LO2细胞 激活IGF-1R/PI3K/AKT和ERK1/2信号通路,减少氧化应激、抑制细胞凋亡[38] ucMSC-Exo GPX1 急性肝损伤 BALB/c-nu/nu小鼠 抑制IKKB/NF-κB/caspase3/9通路,减少氧化应激、抑制细胞凋亡[24] MenSC-Exo ICAM-1、血管生成素-2、Axl 爆发性肝衰竭 C57BL/6小鼠 抑制caspase-3的表达,减少细胞凋亡,降低病死率[39] 注:MIP2,巨噬细胞炎性蛋白2;PPARγ,过氧化物酶体增殖物激活受体γ;ALF,急性肝衰竭;GPX1,谷胱甘肽过氧化物酶1;ICAM,细胞间黏附分子;MenSC-Exo,经血来源间充质干细胞外泌体。 表 2 培养环境变化对干细胞外泌体治疗肝脏疾病影响的实验研究
Table 2. Studies on the influence of different culture environments on stem cell-derived exosomes for liver diseases
外泌体来源 预处理方式 模型 动物/细胞 结果 ucMSC-Exo TNFα ALF C57BL/6小鼠 抑制NLRP3信号通路,抑制炎症反应[40] ucMSC-Exo IL-6 急性肝损伤 C57BL/6小鼠 减轻巨噬细胞浸润和局部肝损伤,改善肝脏组织形态和全身疾病[19] AMCS-EV IFNγ 肝硬化 C57BL/6小鼠 减轻炎症反应,抑制肝纤维化[16] AMSC-CM 低氧 部分肝切除 BALB/c小鼠 促进肝再生、抑制促炎细胞因子和降低异常升高的肝酶[22] AMSC-CM IFNγ 缺血再灌注 M1型巨噬细胞和THLE-2细胞 阻断caspase3/7的激活,抑制炎症反应和细胞凋亡[17] BMSC-CM 低氧 ALF BaLb小鼠 减轻ALF的损伤程度,抗炎、抗氧化[25] BMSC-CM 肝细胞共培养 ALF Sprague-Dawley大鼠、L02细胞 增强细胞活力,促进肝脏结构恢复[41] 注:NLRP3,NOD样受体热蛋白结构域相关蛋白3。 -
[1] BARTUNEK J, BEHFAR A, DOLATABADI D, et al. Cardiopoietic stem cell therapy in heart failure: the C-CURE (Cardiopoietic stem Cell therapy in heart failURE) multicenter randomized trial with lineage-specified biologics[J]. J Am Coll Cardiol, 2013, 61(23): 2329-2338. DOI: 10.1016/j.jacc.2013.02.071. [2] SILVA JD, LOPES-PACHECO M, PAZ A, et al. Mesenchymal stem cells from bone marrow, adipose tissue, and lung tissue differentially mitigate lung and distal organ damage in experimental acute respiratory distress syndrome[J]. Crit Care Med, 2018, 46(2): e132-e140. DOI: 10.1097/CCM.0000000000002833. [3] SUK KT, YOON JH, KIM MY, et al. Transplantation with autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: Phase 2 trial[J]. Hepatology, 2016, 64(6): 2185-2197. DOI: 10.1002/hep.28693. [4] JAIBAJI M, JAIBAJI R, VOLPIN A. Mesenchymal stem cells in the treatment of cartilage defects of the knee: A systematic review of the clinical outcomes[J]. Am J Sports Med, 2021, 49(13): 3716-3727. DOI: 10.1177/0363546520986812. [5] CHRIST B, BRVCKNER S, WINKLER S. The Therapeutic promise of mesenchymal stem cells for liver restoration[J]. Trends Mol Med, 2015, 21(11): 673-686. DOI: 10.1016/j.molmed.2015.09.004. [6] KALLURI R, LEBLEU VS. The biology, function, and biomedical applications of exosomes[J]. Science, 2020, 367(6478): eaau6977. DOI: 10.1126/science.aau6977. [7] PAN BT, JOHNSTONE RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor[J]. Cell, 1983, 33(3): 967-978. DOI: 10.1016/0092-8674(83)90040-5. [8] PEGTEL DM, GOULD SJ. Exosomes[J]. Annu Rev Biochem, 2019, 88: 487-514. DOI: 10.1146/annurev-biochem-013118-111902. [9] RANI S, RYAN AE, GRIFFIN MD, et al. Mesenchymal stem cell-derived extracellular vesicles: Toward cell-free therapeutic applications[J]. Mol Ther, 2015, 23(5): 812-823. DOI: 10.1038/mt.2015.44. [10] THÉRY C, WITWER KW, AIKAWA E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines[J]. J Extracell Vesicles, 2018, 7(1): 1535750. DOI: 10.1080/20013078.2018.1535750. [11] MELDOLESI J. Exosomes and ectosomes in intercellular communication[J]. Curr Biol, 2018, 28(8): R435-R444. DOI: 10.1016/j.cub.2018.01.059. [12] TSUCHIYA A, KOJIMA Y, IKARASHI S, et al. Clinical trials using mesenchymal stem cells in liver diseases and inflammatory bowel diseases[J]. Inflamm Regen, 2017, 37: 16. DOI: 10.1186/s41232-017-0045-6. [13] BORRELLI DA, YANKSON K, SHUKLA N, et al. Extracellular vesicle therapeutics for liver disease[J]. J Control Release, 2018, 273: 86-98. DOI: 10.1016/j.jconrel.2018.01.022. [14] RONG X, LIU J, YAO X, et al. Human bone marrow mesenchymal stem cells-derived exosomes alleviate liver fibrosis through the Wnt/β-catenin pathway[J]. Stem Cell Res Ther, 2019, 10(1): 98. DOI: 10.1186/s13287-019-1204-2. [15] WATANABE T, TSUCHIYA A, TAKEUCHI S, et al. Development of a non-alcoholic steatohepatitis model with rapid accumulation of fibrosis, and its treatment using mesenchymal stem cells and their small extracellular vesicles[J]. Regen Ther, 2020, 14: 252-261. DOI: 10.1016/j.reth.2020.03.012. [16] TAKEUCHI S, TSUCHIYA A, IWASAWA T, et al. Small extracellular vesicles derived from interferon-γ pre-conditioned mesenchymal stromal cells effectively treat liver fibrosis[J]. NPJ Regen Med, 2021, 6(1): 19. DOI: 10.1038/s41536-021-00132-4. [17] ZITO G, MICELI V, CARCIONE C, et al. Human amnion-derived mesenchymal stromal/stem cells pre-conditioning inhibits inflammation and apoptosis of immune and parenchymal cells in an in vitro model of liver ischemia/reperfusion[J]. Cells, 2022, 11(4): 709. DOI: 10.3390/cells11040709. [18] LIU Y, LOU G, LI A, et al. AMSC-derived exosomes alleviate lipopolysaccharide/d-galactosamine-induced acute liver failure by miR-17-mediated reduction of TXNIP/NLRP3 inflammasome activation in macrophages[J]. EBioMedicine, 2018, 36: 140-150. DOI: 10.1016/j.ebiom.2018.08.054. [19] SHAO M, XU Q, WU Z, et al. Exosomes derived from human umbilical cord mesenchymal stem cells ameliorate IL-6-induced acute liver injury through miR-455-3p[J]. Stem Cell Res Ther, 2020, 11(1): 37. DOI: 10.1186/s13287-020-1550-0. [20] FAN Y, HERR F, VERNOCHET A, et al. Human fetal liver mesenchymal stem cell-derived exosomes impair natural killer cell function[J]. Stem Cells Dev, 2019, 28(1): 44-55. DOI: 10.1089/scd.2018.0015. [21] GUO L, LAI P, WANG Y, et al. Extracellular vesicles derived from mesenchymal stem cells prevent skin fibrosis in the cGVHD mouse model by suppressing the activation of macrophages and B cells immune response[J]. Int Immunopharmacol, 2020, 84: 106541. DOI: 10.1016/j.intimp.2020.106541. [22] LEE SC, JEONG HJ, LEE SK, et al. Hypoxic conditioned medium from human adipose-derived stem cells promotes mouse liver regeneration through JAK/STAT3 signaling[J]. Stem Cells Transl Med, 2016, 5(6): 816-825. DOI: 10.5966/sctm.2015-0191. [23] TAN CY, LAI RC, WONG W, et al. Mesenchymal stem cell-derived exosomes promote hepatic regeneration in drug-induced liver injury models[J]. Stem Cell Res Ther, 2014, 5(3): 76. DOI: 10.1186/scrt465. [24] YAN Y, JIANG W, TAN Y, et al. hucMSC exosome-derived GPX1 is required for the recovery of hepatic oxidant injury[J]. Mol Ther, 2017, 25(2): 465-479. DOI: 10.1016/j.ymthe.2016.11.019. [25] TEMNOV A, ROGOV K, ZHALIMOV V, et al. The effect of a mesenchymal stem cell conditioned medium fraction on morphological characteristics of hepatocytes in acetaminophen-induced acute liver failure: a preliminary study[J]. Hepat Med, 2019, 11: 89-96. DOI: 10.2147/HMER.S196354. [26] HONG HE, KIM OH, KWAK BJ, et al. Antioxidant action of hypoxic conditioned media from adipose-derived stem cells in the hepatic injury of expressing higher reactive oxygen species[J]. Ann Surg Treat Res, 2019, 97(4): 159-167. DOI: 10.4174/astr.2019.97.4.159. [27] SUN C, SHI C, DUAN X, et al. Exosomal microRNA-618 derived from mesenchymal stem cells attenuate the progression of hepatic fibrosis by targeting Smad4[J]. Bioengineered, 2022, 13(3): 5915-5927. DOI: 10.1080/21655979.2021.2023799. [28] ÁLVAREZ-VIEJO M. Mesenchymal stem cells from different sources and their derived exosomes: A pre-clinical perspective[J]. World J Stem Cells, 2020, 12(2): 100-109. DOI: 10.4252/wjsc.v12.i2.100. [29] LOU G, CHEN Z, ZHENG M, et al. Mesenchymal stem cell-derived exosomes as a new therapeutic strategy for liver diseases[J]. Exp Mol Med, 2017, 49(6): e346. DOI: 10.1038/emm.2017.63. [30] PIRES AO, MENDES-PINHEIRO B, TEIXEIRA FG, et al. Unveiling the differences of secretome of human bone marrow mesenchymal stem cells, adipose tissue-derived stem cells, and human umbilical cord perivascular cells: a proteomic analysis[J]. Stem Cells Dev, 2016, 25(14): 1073-1083. DOI: 10.1089/scd.2016.0048. [31] WANG ZG, HE ZY, LIANG S, et al. Comprehensive proteomic analysis of exosomes derived from human bone marrow, adipose tissue, and umbilical cord mesenchymal stem cells[J]. Stem Cell Res Ther, 2020, 11(1): 511. DOI: 10.1186/s13287-020-02032-8. [32] ZHAO S, LIU Y, PU Z. Bone marrow mesenchymal stem cell-derived exosomes attenuate D-GaIN/LPS-induced hepatocyte apoptosis by activating autophagy in vitro[J]. Drug Des Devel Ther, 2019, 13: 2887-2897. DOI: 10.2147/DDDT.S220190. [33] HAGA H, YAN IK, TAKAHASHI K, et al. Extracellular vesicles from bone marrow-derived mesenchymal stem cells improve survival from lethal hepatic failure in mice[J]. Stem Cells Transl Med, 2017, 6(4): 1262-1272. DOI: 10.1002/sctm.16-0226. [34] RONG X, LIU J, YAO X, et al. Human bone marrow mesenchymal stem cells-derived exosomes alleviate liver fibrosis through the Wnt/β-catenin pathway[J]. Stem Cell Res Ther, 2019, 10(1): 98. DOI: 10.1186/s13287-019-1204-2. [35] LU FB, CHEN DZ, CHEN L, et al. Attenuation of experimental autoimmune hepatitis in mice with bone mesenchymal stem cell-derived exosomes carrying MicroRNA-223-3p[J]. Mol Cells, 2019, 42(12): 906-918. DOI: 10.14348/molcells.2019.2283. [36] LIU Y, LOU G, LI A, et al. AMSC-derived exosomes alleviate lipopolysaccharide/d-galactosamine-induced acute liver failure by miR-17-mediated reduction of TXNIP/NLRP3 inflammasome activation in macrophages[J]. EBioMedicine, 2018, 36: 140-150. DOI: 10.1016/j.ebiom.2018.08.054. [37] JIN Y, WANG J, LI H, et al. Extracellular vesicles secreted by human adipose-derived stem cells (hASCs) improve survival rate of rats with acute liver failure by releasing lncRNA H19[J]. EBioMedicine, 2018, 34: 231-242. DOI: 10.1016/j.ebiom.2018.07.015. [38] WU HY, ZHANG XC, JIA BB, et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate acetaminophen-induced acute liver failure through activating ERK and IGF-1R/PI3K/AKT signaling pathway[J]. J Pharmacol Sci, 2021, 147(1): 143-155. DOI: 10.1016/j.jphs.2021.06.008. [39] CHEN L, XIANG B, WANG X, et al. Exosomes derived from human menstrual blood-derived stem cells alleviate fulminant hepatic failure[J]. Stem Cell Res Ther, 2017, 8(1): 9. DOI: 10.1186/s13287-016-0453-6. [40] ZHANG S, JIANG L, HU H, et al. Pretreatment of exosomes derived from hUCMSCs with TNF-α ameliorates acute liver failure by inhibiting the activation of NLRP3 in macrophage[J]. Life Sci, 2020, 246: 117401. DOI: 10.1016/j.lfs.2020.117401. [41] CHEN L, ZHANG J, YANG L, et al. The effects of conditioned medium derived from mesenchymal stem cells cocultured with hepatocytes on damaged hepatocytes and acute liver failure in rats[J]. Stem Cells Int, 2018, 2018: 9156560. DOI: 10.1155/2018/9156560. [42] JIAO Y, LU W, XU P, et al. Hepatocyte-derived exosome may be as a biomarker of liver regeneration and prognostic valuation in patients with acute-on-chronic liver failure[J]. Hepatol Int, 2021, 15(4): 957-969. DOI: 10.1007/s12072-021-10217-3. [43] MA L, WEI J, ZENG Y, et al. Mesenchymal stem cell-originated exosomal circDIDO1 suppresses hepatic stellate cell activation by miR-141-3p/PTEN/AKT pathway in human liver fibrosis[J]. Drug Deliv, 2022, 29(1): 440-453. DOI: 10.1080/10717544.2022.2030428. [44] BANG OY, KIM EH. Mesenchymal stem cell-derived extracellular vesicle therapy for stroke: challenges and progress[J]. Front Neurol, 2019, 10: 211. DOI: 10.3389/fneur.2019.00211. [45] EGGENHOFER E, BENSELER V, KROEMER A, et al. Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion[J]. Front Immunol, 2012, 3: 297. DOI: 10.3389/fimmu.2012.00297. [46] VOLAREVIC V, MARKOVIC BS, GAZDIC M, et al. Ethical and safety issues of stem cell-based therapy[J]. Int J Med Sci, 2018, 15(1): 36-45. DOI: 10.7150/ijms.21666. [47] LÖRINCZ ÁM, TIMÁR CI, MAROSVÁRI KA, et al. Effect of storage on physical and functional properties of extracellular vesicles derived from neutrophilic granulocytes[J]. J Extracell Vesicles, 2014, 3: 25465. DOI: 10.3402/jev.v3.25465. [48] SHOKRAVI S, BORISOV V, ZAMAN BA, et al. Mesenchymal stromal cells (MSCs) and their exosome in acute liver failure (ALF): a comprehensive review[J]. Stem Cell Res Ther, 2022, 13(1): 192. DOI: 10.1186/s13287-022-02825-z. [49] CHENG Y, ZENG Q, HAN Q, et al. Effect of pH, temperature and freezing-thawing on quantity changes and cellular uptake of exosomes[J]. Protein Cell, 2019, 10(4): 295-299. DOI: 10.1007/s13238-018-0529-4. [50] WIKLANDER OP, NORDIN JZ, O'LOUGHLIN A, et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting[J]. J Extracell Vesicles, 2015, 4: 26316. DOI: 10.3402/jev.v4.26316. -



 PDF下载 ( 2300 KB)
PDF下载 ( 2300 KB)

 下载:
下载: