蒙药蓝盆花抑制肝星状细胞增殖的作用及机制初探
DOI: 10.3969/j.issn.1001-5256.2023.06.015
Role and mechanism of action of the Mongolian medicine Scabiosa atropurea in inhibiting the proliferation of hepatic stellate cells
-
摘要:
目的 利用细胞实验探究蓝盆花对肝星状细胞增殖的作用及机制。 方法 取20只Wistar大鼠随机分为对照组和给药组,每组10只,对照组以生理盐水灌胃,给药组予蓝盆花灌胃制备含药血清。分别加入对照组血清(10%)、蓝盆花含药血清低剂量(10%)、蓝盆花含药血清中剂量(15%)及蓝盆花含药血清高剂量(20%),用以孵育HSC-T6细胞。MTT法检测不同药物浓度在不同时间段对细胞的影响;流式细胞术检测细胞凋亡情况;qRT-PCR及Western blot检测HSC细胞中纤维化标志物(α-SMA、Collagen Ⅰ)及PI3K/Akt信号通路相关因子mRNA、蛋白表达情况。多组间比较采用单因素方差分析,进一步两两比较采用LSD-t检验。 结果 与对照组比较,蓝盆花含药血清低、中、高剂量组细胞OD值均显著降低(P值均<0.05),细胞总凋亡率均显著增高(P值均<0.05)。qRT-PCR结果显示,与对照组比较,蓝盆花含药血清低、中、高剂量组α-SMA、Collagen Ⅰ、PI3K、Akt mRNA表达显著下调,PTEN mRNA表达显著增高(P值均<0.05);Western blot结果显示,与对照组比较,蓝盆花含药血清低、中、高剂量组α-SMA、Collagen Ⅰ、PI3K、Akt、p-Akt蛋白表达显著下调,PTEN蛋白表达显著增高(P值均<0.05)。 结论 蒙药蓝盆花可抑制HSC-T6细胞增殖且促进其凋亡,其机制可能是通过调控纤维化标志物和PI3K/Akt信号通路来发挥抗肝纤维化作用。 Abstract:Objective To investigate the role and mechanism of action of Scabiosa atropurea in inhibiting the proliferation of hepatic stellate cells using cell experiment. Methods A total of 20 Wistar rats were randomly divided into control group and administration group, with 10 rats in each group. The rats in the control group were given normal saline by gavage, and those in the administration group were given Scabiosa atropurea by gavage to prepare drug-containing serum. HSC-T6 cells were incubated with the serum from the control group (10%) or the low-, middle-, and high-dose serum containing Scabiosa atropurea (10%, 15%, and 20%, respectively). MTT assay was used to observe the effect of different drug concentrations on cells in different periods of time; flow cytometry was used to measure cell apoptosis; qRT-PCR and Western blot were used to measure the mRNA and protein expression levels of fibrosis markers (α-SMA, collagen Ⅰ) and PI3K/Akt signaling pathway-related factors in hepatic stellate cells (HSCs). A one-way analysis of variance was used for comparison between multiple groups, and the least significant difference t- test was used for further comparison between two groups. Results Compared with the control group, the low-, middle-, and high-dose serum containing Scabiosa atropurea groups had a significant reduction in the OD value of cells (all P < 0.05) and a significant increase in the overall apoptosis rate of cells (all P < 0.05). The results of qRT-PCR showed that compared with the control group, the low-, middle-, and high-dose serum containing Scabiosa atropurea groups had significant reductions in the mRNA expression levels of α-SMA, collagen Ⅰ, PI3K, and Akt and a significant increase in the mRNA expression level of PTEN (all P < 0.05); Western blot showed that compared with the control group, the low-, middle-, and high-dose serum containing Scabiosa atropurea groups had significant reductions in the protein expression levels of α-SMA, collagen Ⅰ, PI3K, Akt, and p-Akt and a significant increase in the protein expression level of PTEN (all P < 0.05). Conclusion The Mongolian medicine Scabiosa atropurea can inhibit the proliferation of HSC-T6 cells and promote their apoptosis, possibly by regulating fibrosis markers and the PI3K/Akt signaling pathway to exert an anti-liver fibrosis effect. -
Key words:
- Hepatic Fibrosis /
- Hepatic Stellate Cells /
- Scabiosa Atropurea /
- Signal Transduction
-
肝细胞癌(简称肝癌)术后复发是导致患者死亡的主要原因[1]。对于复发性肝癌,临床上常用的治疗方法包括再次肝切除术、射频消融(radiofrequency ablation,RFA)、肝移植、介入、靶向药物治疗等[2]。然而,目前国内外肝癌诊疗指南均未对复发性肝癌提供具体的诊疗推荐。再次肝切除术和RFA属于根治性治疗方法,临床应用广泛,文献报道较多。但单个研究样本量小,且研究间的结论不统一,临床上对复发性肝癌的根治性治疗方案尚存争议。本文使用Meta分析的方法,客观分析两种治疗措施对复发性肝癌的疗效与安全性。
1. 资料与方法
1.1 文献检索
系统检索PubMed、中国知网和万方数据库,检索日期自建库至2020年6月15日。英文检索词包括:recurrent、recurrence、hepatocellular carcinoma、liver cancer、hepatic resection、hepatectomy、resection、radiofrequency ablation等,中文检索词包括:复发、肝癌、肝细胞癌、手术、再次肝切除术、射频消融等。
1.2 纳入标准
(1) 研究:比较再次肝切除术与RFA治疗复发性肝癌的随机对照试验或队列研究,英文或中文发表。(2)患者:罹患原发性肝癌,接受首次肝切除术治疗后,肝癌复发,复发肿瘤符合米兰标准,且无大血管侵犯及肝外转移。(3)干预措施:肝癌复发后,接受再次肝切除术或RFA治疗。(4)结局指标:报道了总生存期(overall survival,OS)、无瘤生存期(recurrence-free survival,RFS)、围手术期并发症和围手术期病死率中的任何一项。
1.3 排除标准
(1) 肝切除和RFA治疗原发性肝癌、胆管细胞癌或转移性肝癌的研究;(2)单组样本量过小(<10例)或单臂研究;(3)未提供病例具体纳入时间段的研究;(4)肝癌复发后,接受再次肝切除术或RFA治疗前,接受其他的治疗方案(如介入、靶向药物等)。再次肝切除术或RFA治疗后,序贯介入或其他治疗措施,不在排除标准范围之内。对于重复性发表的研究,仅纳入样本量最大的一项。
1.4 数据提取
两位评价者独立检索并提取纳入文献的数据,数据提取过程中若有分歧则通过双方讨论或询问第3位评价者解决。提取的数据包括人口统计学资料及临床基线数据、OS、RFS、围手术期并发症发生率及病死率。若原始文献未详细描述生存数据,则从其Kaplan-Meier生存曲线图估算。
1.5 统计学方法
统计分析采用RevMan 5.4.1软件(Cochrane协作组,牛津,英国)。采用从纳入文献提取的风险比(HR)进行OS和RFS的Meta分析。统计学异质性的评估采用I2检验。I2≥50%时采用随机效应模型,I2<50%时采用固定效应模型。P<0.05为差异有统计学意义。采用漏斗图估计发表偏倚。由于纳入文献提供的数据存在一定的局限性,无法根据患者性别、肿瘤大小、肿瘤个数、肿瘤位置等进行亚组分析。
2. 结果
2.1 基线特点
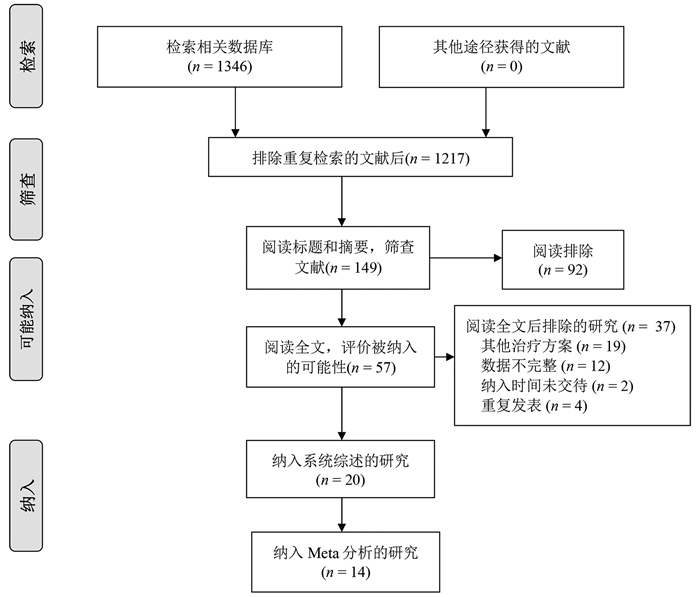
根据纳入和排除标准,18篇回顾性队列研究[3-20]和2篇随机对照试验[21-22]比较了再次肝切除术和RFA治疗复发性肝癌的临床疗效与安全性,共2903例患者(图 1)。全部纳入患者均来自亚洲国家,基线数据详见表 1。
表 1 纳入研究的基线特点第一作者和年份 国家/地区 纳入时间(年) 组别 样本量 男/女(例) 年龄(岁) 单个/多个肿瘤(例) 复发肿瘤直径(cm) 肝硬化[例(%)] CTP分级(A/B/C,例) 随访(月) Wang 2015[3] 中国 2004—2010 肝切除 128 113/15 50.2±10.1 89/39 2.4±0.9 66(51.6) - - RFA 162 148/14 52.7±10.9 107/55 2.3±0.7 - - - Sun 2017[4] 中国台湾 2002—2014 肝切除 43 34/9 60(35~76) - 1.9(0.8~3.0) 36(83.7) 42/1 53 RFA 57 38/19 63(27~81) - 1.8(1.0~3.0) 50(87.7) 57/0 54 Umeda 2011[5] 日本 1998—2007 肝切除 29 - - - 3.20±0.57 - 29/0 48 RFA 58 - - - 2.1±0.3 - 51/7 48 Song 2015[6] 韩国 1994—2012 肝切除 39 31/8 52.5±9.8 32/7 2.2±1.1 23(59) 39/0 36.3(0.8~126.6) RFA 178 145/33 55.4±10.6 156/22 1.7±0.6 130(73.0) 172/6 44.7(5.6~139.8) Liang 2008[7] 中国 1999—2007 肝切除 44 39/5 48.8±12.0 34/10 ≤3 (26) - 44/0 33.5±24.1 RFA 66 54/12 54.6±10.8 48/18 ≤3 (44) - 64/2 21.1±19.1 Ho 2012[8] 中国台湾 2001—2007 肝切除 54 40/14 56.3±12.3 - 2.9±1.8 26(48.1) 51/2/1 32(0~79) RFA 50 39/11 61.0±11.1 - 2.3±1.9 28(56.0) 50/0 27(0~96) Chan 2012[9] 中国香港 2001—2008 肝切除 29 - 52(38~79) 21/8 2.1(0.8~5.5) 25(86.2) 29/0 44.9 RFA 45 - 59(36~80) 29/16 2.2(0.8~6.0) 40(88.9) 40/5 44.9 Chen 2018[10] 中国 2009—2015 肝切除 48 41/7 73.5±3.5 28/20 2.6±1.1 41(85.4) 39/9 36.9(2~78) RFA 57 51/6 73.7±2.9 30/27 2.5±1.2 49(86.0) 45/12 37.3(2~78) 岑峰2016[11] 中国 2011—2015 肝切除 28 22/6 53.6 12/16 - 23(82.1) 18/10 - RFA 24 19/5 55.7 10/14 - 19(79.2) - - 陈康2019[12] 中国 2005—2014 肝切除 77 65/12 - - - 57(74) 76/1 57(2~168) RFA 82 72/10 - - - 50(61) 77/5 51(4~111) 黄新辉2013[13] 中国 2007—2011 肝切除 66 51/15 50.5±10.1 66/0 2.9±1.1 57(86.3) 66/0 - RFA 46 36/10 54.1±12.1 46/0 2.6±0.9 39(84.8) 46/0 - 梁惠宏2011[14] 中国 1999—2009 肝切除 72 65/7 49±12 72/0 2.1±0.1 - 70/2 36±25 RFA 79 69/10 55±11 79/0 2.5±0.1 - 73/6 32±21 任正刚2008[15] 中国 2000—2005 肝切除 145 127/18 51 127/18 2.0 - 145/0 23(3~88) RFA 68 64/4 52 52/16 2.0 - 68/0 23(3~88) 田正灵2016[16] 中国 2012—2015 肝切除 30 28/2 48.8±9.6 24/6 - 24(80) - 17(6.0~42.5) RFA 27 26/1 50.9±10.1 19/8 - 23(85.2) - 17(6.0~42.5) 张辉2013[17] 中国 2003—2011 肝切除 69 - - - 3.5 61(88.4) 54/15 - RFA 99 - - 2.1 76(76.8) 71/28 - 张婷婷2014[18] 中国 1998—2010 肝切除 27 25/2 47±13 25/2 3.2±1.1 - 27/0 32(9~118) RFA 39 37/2 52±13 37/2 2.7±1.1 - 37/2 28(2~79) Peng 2018[19] 中国 2006—2015 肝切除 79 67/2 55 59/20 ≤3(48) - - 53.2(4~96) RFA 107 95/12 57 75/32 ≤3(73) - - 52.3(3~96) Lu 2020[20] 中国 2004—2015 肝切除 138 124/16 50.1±10.9 112/26 2.8±1.9 96(70) 138/0 37.6 RFA 194 172/22 52.9±11.8 162/32 1.9±0.9 134(69) 194/0 41.6 Xia 2019[21] 中国 2010—2013 肝切除 120 107/13 52.4(25.7~60.5) 96/24 2.9(1.0~5.0) 50(41.7) 120/0 44.3(4.3~90.6) RFA 120 109/11 53.5(28.0~59.9) 94/26 2.7(1.0~4.8) 55(45.8) 120/0 44.3(4.3~90.6) 刘嘉龙2019[22] 中国 2016—2017 肝切除 39 38/1 50.0±10.0 37/2 2.1±0.7 37(94.9) 38/1 24 RFA 41 37/4 48.9±11.3 39/2 1.8±0.8 39(95.1) 39/2 24 注:-,未报道。 2.2 围术期并发症及病死率
15篇文献[4, 6-7, 9-17, 19, 21-22]报道了再次肝切除术围手术期并发症发生率为5.5%~68.2%,中位值为22.4%,常见并发症包括肝功能不全、胸腔积液、腹水、胆瘘等。14篇文献[4, 6-7, 9-10, 12-17, 19, 21-22]报道了RFA的围手术期并发症发生率为0~13%,中位值为3.3%,常见并发症包括胆漏、腹腔出血等。16篇文献[4, 6-7, 9-19, 21-22]报道了再次肝切除术和RFA围手术期病死率,其中6篇文献[4, 6, 10, 12, 17, 19]报道再次肝切除术组围手术期病死率为1.3%~2.6%,中位值为2%,其余文献报道发生率为0;仅1篇文献[9]报道RFA围手术期病死率为2.2%,其余文献报道发生率为0(表 2)。
表 2 纳入患者的治疗结局第一作者和年份 组别 并发症发生率(%) 围手术期病死率(%) 复发[例(%)] RFS OS 1年 3年 5年 P值1) 1年 3年 5年 P值1) Wang 2015[3] 肝切除 - - - - - - - 97.7 84.1 64.5 <0.001 RFA - - - - - - 96.9 73.4 37.0 Sun 2017[4] 肝切除 16 2 30(69.8) 57.0 32.1 28.6 0.89 97.6 82.7 56.4 0.69 RFA 7 0 41(71.9) 60.8 26.6 16.6 98.2 77.2 52.6 Umeda 2011[5] 肝切除 - - - - - - - 93.1 66.8 58.1 0.899 RFA - - - - - - 94.7 75.1 48.3 Song 2015[6] 肝切除 64 2.6 18(46.2) 66.1 48.5 43.1 0.834 88.8 88.8 83.9 0.686 RFA 2.2 0 117(65.7) 70.1 40.8 30.0 98.9 82.5 71.0 Liang 2008[7] 肝切除 68.2 0 86.4 - - - - 78.6 44.5 27.6 0.79 RFA 3.0 0 78.8 - - - 76.6 48.6 39.9 Ho 2012[8] 肝切除 - - - - - - - - - 72.0 - RFA - - - - - - - - 83.0 Chan 2012[9] 肝切除 24.1 0 21(72.4) 41.1 24.2 24.2 0.14 89.7 56.5 35.2 0.51 RFA 2.2 2.2 38(84.4) 32.2 12.4 9.3 83.7 43.1 29.1 Chen 2018[10] 肝切除 25 2.1 26(54.2) 73.1 49.7 40.7 0.465 76.3 52.5 42.6 0.413 RFA 0 0 27(47.4) 69.5 37.8 33.1 78.2 40.8 36.7 岑峰2016[11] 肝切除 50 0 - 46.4 0 0 - - - - - RFA - 0 - 20.8 0 0 - - - 陈康2019[12] 肝切除 17.1 1.3 - - - - - 88.8 68.8 51.1 0.258 RFA 9.8 0 - - - - 91.4 73.4 61.1 黄新辉2013[13] 肝切除 18.1 0 - 43.9 14.4 8.2 0.548 89.5 54.3 28.8 0.780 RFA 0 0 - 56.9 12.4 5.0 82.6 50.8 20.5 梁惠宏2011[14] 肝切除 36 0 ≤1年: 39>1年: 33 - - - - 95.3 65.7 54.5 >0.05 RFA 13 0 ≤1年: 37>1年: 42 - - - - 100 79.4 62.1 任正刚2008[15] 肝切除 5.5 0 ≤2年: 71>2年: 74 79.4 48.1 34.4 0.001 88.1 62.6 41.0 0.693 RFA 1.5 0 ≤2年: 37>2年: 31 58.0 27.8 12.4 94.7 65.1 37.3 田正灵2016[16] 肝切除 6.7 0 10(33.3) 73.3 64.3 - 0.002 96.7 93.3 - 0.54 RFA 3.7 0 24(88.9) 46.7 26.7 - 96.3 84.0 - 张辉2013[17] 肝切除 15.9 1.45 - - - - - 68.2 45.4 - >0.05 RFA 0 0 - - - - 73.7 53.6 - 张婷婷2014[18] 肝切除 - 0 22(56.4) 66.7 50.7 43.4 0.323 96.2 76.9 61.2 0.471 RFA - 0 14(52.9) 65.8 28.0 14.0 86.2 73.3 62.2 Peng 2018[19] 肝切除 17.7 1.3 - 64.8 41.6 38.3 0.258 84.8 60.2 51.9 0.871 RFA 4.7 0 - 58.2 35.2 29.6 84.6 66.9 49.1 Lu 2020[20] 肝切除 - - - 91.8 82.0 72.9 0.38 - - - - RFA - - - 94.4 75.4 61.7 - - - Xia 2019[21] 肝切除 22.4 0 60.8 85.0 52.4 36.2 0.09 92.5 65.8 43.6 0.17 RFA 7.3 0 64.2 74.2 41.7 30.2 87.5 52.5 38.5 刘嘉龙2019[22] 肝切除 35.9 0 19(48.7) 69.2 - - <0.001 92.3 - - 0.292 RFA 4.9 0 32(78.0) 26.8 - - 85.4 - - 注:-,未报道;1) P值来源于原文报道。 2.3 RFA与肝切除术OS的比较
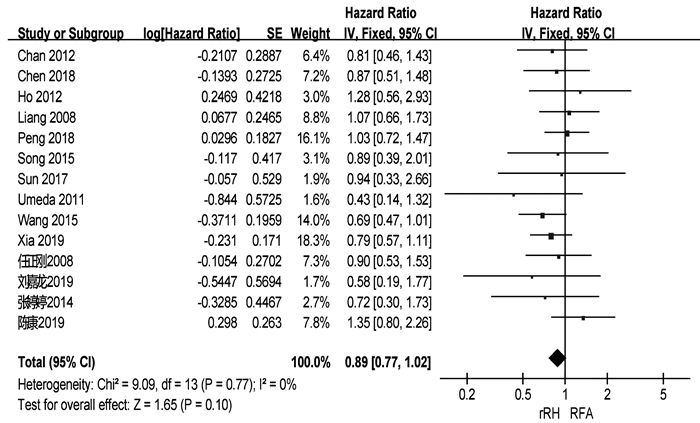
共有18篇研究[3-10, 12-18, 21-22]报道了OS(表 2)。两组患者术后1、3、5年中位OS分别为92.3%、66.3%、51.1%与91.4%、69.2%、39.9%。共有14篇研究[3-10, 12, 15, 18-19, 21-22]可提取数据统计HR,各组间不存在异质性(I2=0,P=0.77),采用固定效应模型进行分析。结果显示,两组患者的OS差异无统计学意义(HR= 0.89,95%CI:0.77~1.02,P=0.10)(图 2)。
2.4 RFA与肝切除术RFS的比较
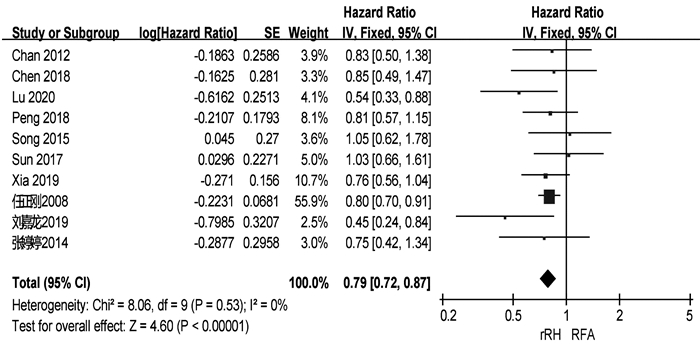
共有13篇研究[4, 6, 9-11, 13, 15-16, 18-19, 20-22]报道了RFS(表 2)。两组患者术后1、3、5年中位RFS分别为67.9%、48.3%、34.4%和57.5%、27.9%、14.0%。共有10篇研究[4, 6, 9-10, 15, 18-22]可提取数据统计HR,各组间不存在异质性(I2=0,P=0.53),采用固定效应模型进行分析。结果显示,再次肝切除术组患者的RFS显著高于RFA组(HR=0.79,95%CI:0.72~0.87,P<0.001)(图 3)。
2.5 发表偏倚
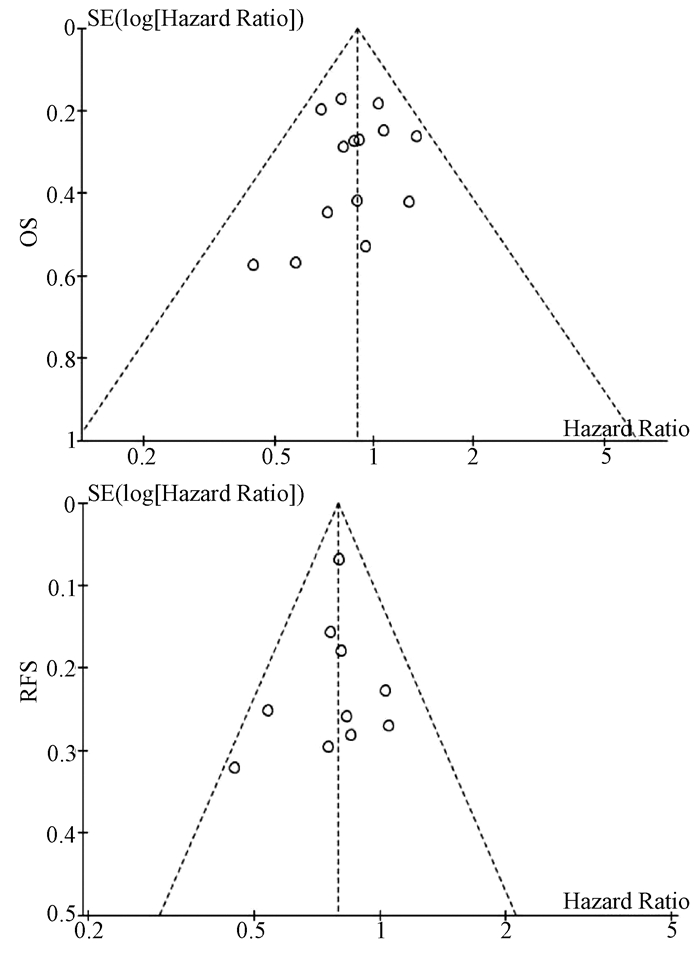
基于OS和RFS HR的漏斗图提示,发表偏倚不明显(图 4)。
3. 讨论
肝癌术后复发是影响患者术后长期生存的主要危险因素。对于复发性肝癌,目前临床上以多学科综合治疗为主。肝移植能显著提高复发性肝癌患者的RFS和远期生存率[23],但肝移植适应证严格、肝源匮乏和费用昂贵等问题仍限制其广泛的临床应用。此外,既往研究[24-25]表明再次肝切除术和RFA对复发性肝癌的疗效均优于介入。因此,针对肝内复发性肝癌患者,再次肝切除术和RFA是目前较为理想的治疗方案。有研究[3]认为再次肝切除术是治疗复发性肝癌的首选治疗方案,即使患者的原发癌更具侵袭性,肝功能水平更差,肝癌复发后行再次肝切除术仍可以获得与RFA相似的疗效。梁惠宏等[14]的研究则发现,RFA治疗复发性小肝癌的长期疗效优于再次肝切除术。因此,关于再次肝切除术和RFA治疗复发性肝癌的选择仍存在较大争议。本研究通过Meta分析的方法,综合评估再次肝切除术和RFA治疗复发性肝癌的疗效和安全性。
本研究结果显示,两组患者的OS无显著性差异,但再次肝切除术组患者的RFS显著高于RFA组。这一结论与肝切除术和RFA治疗原发性肝癌的结果一致[26-27]。RFA组患者RFS偏低的原因可能是:(1)再次肝切除术能将复发肿瘤以及潜在的瘤周子灶及血管癌栓同时切除,减少肿瘤再次局部复发和转移的可能性;(2)RFA后部分患者的肿瘤消融不完全而需进行二次消融或其他治疗;(3)术前影像学检查未能发现主瘤旁的微小卫星灶,导致消融不完全而引起肝癌再次复发。虽然RFA可用于治疗直径≤5 cm的肝癌,但目前认为其最佳适应证为直径≤3 cm的小肝癌[28]。有研究[29]发现,肝癌的直径和数目是导致RFA消融不完全的主要因素,随着肝癌直径的增加,RFA的疗效逐步下降。Livraghi等[30]的研究也表明RFA治疗直径3~5 cm的肝癌时完全消融率显著降低,导致RFA治疗复发性肝癌后局部复发和早期复发的比例明显升高。无论是原发性还是复发性肝癌,肿瘤直径在3~5 cm时经RFA治疗后其RFS和远期生存率均低于再次肝切除术[21, 31]。因此,在选择复发性肝癌治疗方案时,肿瘤直径应作为重要的参考指标。
本研究中,在围手术期并发症方面,再次肝切除术组和RFA组患者的中位发生率分别为22.4%和3.3%,与原发性肝癌接受肝切除术或RFA治疗后的并发症发生率相似[32-33]。此外,本研究还发现RFA治疗复发性肝癌的安全性明显优于再次肝切除术。RFA组患者术后并发症发生率及严重程度更低。虽然再次肝切除术能改善患者的远期生存率及RFS,但再次肝切除术的实施仍受诸多因素的限制,例如复发肿瘤的位置、数量以及肝硬化严重程度等。手术造成较大的创伤导致患者术后更容易发生严重并发症。此外,肝癌患者常伴有不同程度的肝硬化,无疑增加了手术的风险,部分患者最终死于肝衰竭而非肝癌本身带来的肿瘤负担[32]。相比于再次肝切除术,RFA在超声等影像学技术引导下精准地对复发肿瘤进行消融,从而最大限度地避免正常肝组织的破坏。对于手术无法切除、无法耐受手术的患者亦可选择RFA治疗。此外,RFA还可联合介入或无水酒精注射提高患者的OS和RFS[10, 19]。RFA本身具有微创和可重复的优点,肝癌再次复发后相当一部分患者仍可及时接受RFA或其他治疗[12],从而获得与再次肝切除相似的短期OS。
值得注意的是,本研究纳入文献中多数为回顾性研究,仅有2篇随机对照试验,因此本研究结论仍需更多的随机对照试验进行验证。其次,再次肝切除术和RFA治疗复发性肝癌的适应证存在一定的区别。然而,本Meta分析纳入的多数文献均仅纳入符合米兰标准的复发性肝癌,这些患者同时符合再次肝切除术和RFA的适应证。最后,肝癌术后复发(甚至多次)比较常见,对于复发肿瘤的治疗,多采取综合治疗方案(如介入、靶向、免疫治疗等),而非再次肝切除术或RFA等“单一”治疗模式。因此,联合治疗方案可能影响本荟萃分析对患者OS的判断。
总而言之,对于复发性肝癌的治疗,再次肝切除术能提高患者RFS,RFA则具有安全性高的优点。因此,在复发性肝癌患者的治疗中应遵循个体化和多学科治疗原则,合理选择治疗方案。
-
表 1 各组细胞OD值的检测结果
Table 1. Results of OD value of cells in each group
组别 给药24 h 给药48 h 给药72 h 对照组 0.98±0.01 0.96±0.01 0.98±0.01 低剂量组 0.63±0.042) 0.71±0.042) 0.88±0.021) 中剂量组 0.71±0.042) 0.76±0.012) 0.85±0.031) 高剂量组 0.38±0.052) 0.67±0.072) 0.81±0.032) F值 37.82 20.64 45.07 P值 <0.001 <0.001 <0.001 注:与对照组比较,1)P<0.05, 2)P<0.01。 表 2 各组细胞凋亡率的检测结果
Table 2. Detection results of apoptosis rate in each group
组别 晚期凋亡率(%) 早期凋亡率(%) 总凋亡率(%) 对照组 7.45±0.40 4.48±0.83 11.92±1.08 低剂量组 8.19±1.092) 16.51±0.852) 24.70±1.841) 中剂量组 6.58±0.442) 10.87±0.922) 17.46±0.951) 高剂量组 6.92±0.622) 12.36±0.882) 19.28±1.442) F值 24.36 16.03 20.58 P值 <0.001 <0.001 <0.001 注:与对照组比较,1)P<0.05, 2)P<0.01。 表 3 HSC-T6细胞中α-SMA、Collagen Ⅰ、PI3K、Akt及PTEN的mRNA水平
Table 3. The mRNA expression levels of α-SMA, Collagen Ⅰ, PI3K, Akt and PTEN in HSC-T6 cells
组别 Collagen Ⅰ α-SMA PI3K Akt PTEN 对照组 1.000±0.000 1.000±0.000 1.000±0.000 1.000±0.000 1.000±0.000 低剂量组 0.740±0.0741) 0.697±0.0792) 0.119±0.0142) 0.417±0.1402) 4.820±0.8572) 中剂量组 0.334±0.1282) 0.422±0.0082) 0.274±0.0532) 0.399±0.0992) 2.856±0.0801) 高剂量组 0.284±0.1092) 0.301±0.0202) 0.170±0.0562) 0.341±0.0802) 5.848±0.7742) F值 41.74 62.62 269.90 32.15 41.29 P值 <0.001 <0.001 <0.001 <0.001 <0.001 注:与对照组比较,1)P<0.05, 2)P<0.01。 表 4 HSC-T6细胞中α-SMA、Collagen Ⅰ、PI3K、Akt、p-Akt及PTEN的蛋白表达水平
Table 4. The protein expression levels of α-SMA, Collagen Ⅰ, PI3K, Akt, p-Akt and PTEN in HSC-T6 cells
组别 Collagen Ⅰ α-SMA PI3K Akt p-Akt PTEN 对照组 0.081±0.011 0.346±0.087 0.095±0.013 0.840±0.053 0.632±0.043 1.005±0.005 低剂量组 0.044±0.0052) 0.176±0.0322) 0.038±0.0032) 0.613±0.0202) 0.291±0.0692) 2.779±0.3022) 中剂量组 0.052±0.0052) 0.196±0.0191) 0.049±0.0012) 0.492±0.0512) 0.356±0.0572) 2.889±0.0142) 高剂量组 0.031±0.0052) 0.151±0.0132) 0.045±0.0022) 0.511±0.0562) 0.229±0.0212) 3.413±0.0542) F值 27.86 10.28 44.10 34.36 36.74 139.80 P值 <0.001 <0.001 <0.001 <0.001 <0.001 <0.001 注:与对照组比较,1)P<0.05, 2)P<0.01。 -
[1] ZHANG CY, YUAN WG, HE P, et al. Liver fibrosis and hepatic stellate cells: Etiology, pathological hallmarks and therapeutic targets[J]. World J Gastroenterol, 2016, 22(48): 10512-10522. DOI: 10.3748/wjg.v22.i48.10512. [2] TRAUTWEIN C, FRIEDMAN SL, SCHUPPAN D, et al. Hepatic fibrosis: Concept to treatment[J]. J Hepatol, 2015, 62(1 Suppl): S15-S24. DOI: 10.1016/j.jhep.2015.02.039. [3] HIGASHI T, FRIEDMAN SL, HOSHIDA Y. Hepatic stellate cells as key target in liver fibrosis[J]. Adv Drug Deliv Rev, 2017, 121: 27-42. DOI: 10.1016/j.addr.2017.05.007. [4] ZHANG M, SERNA-SALAS S, DAMBA T, et al. Hepatic stellate cell senescence in liver fibrosis: Characteristics, mechanisms and perspectives[J]. Mech Ageing Dev, 2021, 199: 111572. DOI: 10.1016/j.mad.2021.111572. [5] EZHILARASAN D, SOKAL E, NAJIMI M. Hepatic fibrosis: It is time to go with hepatic stellate cell-specific therapeutic targets[J]. Hepatobiliary Pancreat Dis Int, 2018, 17(3): 192-197. DOI: 10.1016/j.hbpd.2018.04.003. [6] AYD1N MM, AKÇAL1 KC. Liver fibrosis[J]. Turk J Gastroenterol, 2018, 29(1): 14-21. DOI: 10.5152/tjg.2018.17330. [7] SCHUPPAN D, ASHFAQ-KHAN M, YANG AT, et al. Liver fibrosis: Direct antifibrotic agents and targeted therapies[J]. Matrix Biol, 2018, 68-69: 435-451. DOI: 10.1016/j.matbio.2018.04.006. [8] YANG HX, BAI YF, CHANG L, et al. Research progress on the resources and utilization of the Mongolian medicinal plant Cyperus genus[J]. Northwest Pharm J, 2020, 35(5): 779-784. DOI: 10.3969/j.issn.1004-2407.2020.05.031.杨宏昕, 白音夫, 常亮, 等. 蒙药材蓝盆花属植物资源及利用的研究进展[J]. 西北药学杂志, 2020, 35(5): 779-784. DOI: 10.3969/j.issn.1004-2407.2020.05.031. [9] LU C, LI Y, CUI Y, et al. Isolation and functional analysis of genes involved in polyacylated anthocyanin biosynthesis in Blue Senecio cruentus[J]. Front Plant Sci, 2021, 12: 640746. DOI: 10.3389/fpls.2021.640746. [10] JAMES ANTONY JJ, ZAKARIA S, ZAKARIA R, et al. Biochemical analyses of Dendrobium Sabin Blue PLBs during cryopreservation by vitrification[J]. Physiol Mol Biol Plants, 2019, 25(6): 1457-1467. DOI: 10.1007/s12298-019-00703-2. [11] ZHANG CY, YAN YX, GAO XY, et al. Mechanism of anti- hepatic fibrosis action of the monk's medicine seven flavors liver clearing powder: based on UHPLC-TOF-MS and network pharmacology methods[J]. J South Med Univ, 2021, 41(8): 1131-1141. DOI: 10.12122/j.issn.1673-4254.2021.08.02.张春艳, 颜羽昕, 高晓阳, 等. 蒙药七味清肝散抗肝纤维化的作用机制: 基于UHPLC-TOF-MS和网络药理学方法[J]. 南方医科大学学报, 2021, 41(8): 1131-1141. DOI: 10.12122/j.issn.1673-4254.2021.08.02. [12] LIANG J, MENG GSLM, YAN YX, et al. Study on the anti-hepatic fibrosis effect and mechanism of qiwei qinggan powder based on proteomics[J]. China Pharm, 2020, 31(11): 1294-1302. DOI: 10.6039/j.issn.1001-0408.2020.11.03.梁洁, 孟根斯立木, 颜羽昕, 等. 基于蛋白质组学研究七味清肝散的抗肝纤维化作用及机制研究[J]. 中国药房, 2020, 31(11): 1294-1302. DOI: 10.6039/j.issn.1001-0408.2020.11.03. [13] ZHANG CY, JIN R, YAN YX, et al. Exploring the mechanism of action of Bluebonnets against liver fibrosis based on pharmacodynamic and network pharmacological approaches[J]. China J Chin Mater Med, 2022, 47(13): 3609-3618. DOI: 10.19540/j.cnki.cjcmm.20220207.702.张春艳, 金蓉, 颜羽昕, 等. 基于药效学和网络药理方法探讨蓝盆花抗肝纤维化的作用机制[J]. 中国中药杂志, 2022, 47(13): 3609-3618. DOI: 10.19540/j.cnki.cjcmm.20220207.702. [14] LUBECKA K, FLOWER K, BEETCH M, et al. Loci-specific differences in blood DNA methylation in HBV-negative populations at risk for hepatocellular carcinoma development[J]. Epigenetics, 2018, 13(6): 605-626. DOI: 10.1080/15592294.2018.1481706. [15] TAO Y, WANG N, QIU T, et al. The role of autophagy and NLRP3 inflammasome in liver fibrosis[J]. Biomed Res Int, 2020, 2020: 7269150. DOI: 10.1155/2020/7269150. [16] ZHANG M, ZANG S. T cells in fibrosis and fibrotic diseases[J]. Front Immunol, 2020, 11: 1142. DOI: 10.3389/fimmu.2020.01142. [17] KOSTALLARI E, HIRSOVA P, PRASNICKA A, et al. Hepatic stellate cell-derived platelet-derived growth factor receptor-alpha-enriched extracellular vesicles promote liver fibrosis in mice through SHP2[J]. Hepatology, 2018, 68(1): 333-348. DOI: 10.1002/hep.29803. [18] MATSUDA M, SEKI E. Hepatic stellate cell-macrophage crosstalk in liver fibrosis and carcinogenesis[J]. Semin Liver Dis, 2020, 40(3): 307-320. DOI: 10.1055/s-0040-1708876. [19] KHOMICH O, IVANOV AV, BARTOSCH B. Metabolic hallmarks of hepatic stellate cells in liver fibrosis[J]. Cells, 2019, 9(1): 24. DOI: 10.3390/cells9010024. [20] SHI Z, ZHANG K, CHEN T, et al. Transcriptional factor ATF3 promotes liver fibrosis via activating hepatic stellate cells[J]. Cell Death Dis, 2020, 11(12): 1066. DOI: 10.1038/s41419-020-03271-6. [21] KAMM DR, MCCOMMIS KS. Hepatic stellate cells in physiology and pathology[J]. J Physiol, 2022, 600(8): 1825-1837. DOI: 10.1113/JP281061. [22] WANG R, SONG F, LI S, et al. Salvianolic acid A attenuates CCl4-induced liver fibrosis by regulating the PI3K/AKT/mTOR, Bcl-2/Bax and caspase-3/cleaved caspase-3 signaling pathways[J]. Drug Des Devel Ther, 2019, 13: 1889-1900. DOI: 10.2147/DDDT.S194787. [23] WU H, CHEN G, WANG J, et al. TIM-4 interference in Kupffer cells against CCL4-induced liver fibrosis by mediating Akt1/Mitophagy signalling pathway[J]. Cell Prolif, 2020, 53(1): e12731. DOI: 10.1111/cpr.12731. [24] XIE Y, SHI X, SHENG K, et al. PI3K/Akt signaling transduction pathway, erythropoiesis and glycolysis in hypoxia (Review)[J]. Mol Med Rep, 2019, 19(2): 783-791. DOI: 10.3892/mmr.2018.9713. [25] PAPA A, PANDOLFI PP. The PTEN-PI3K axis in cancer[J]. Biomolecules, 2019, 9(4): 153. DOI: 10.3390/biom9040153. [26] XIU AY, DING Q, LI Z, et al. Doxazosin attenuates liver fibrosis by inhibiting autophagy in hepatic stellate cells via activation of the PI3K/Akt/mTOR signaling pathway[J]. Drug Des Devel Ther, 2021, 15: 3643-3659. DOI: 10.2147/DDDT.S317701. [27] LIU X, LIU W, DING C, et al. Taxifolin, extracted from waste larix olgensis roots, attenuates CCl4-induced liver fibrosis by regulating the PI3K/AKT/mTOR and TGF-β1/Smads signaling pathways[J]. Drug Des Devel Ther, 2021, 15: 871-887. DOI: 10.2147/DDDT.S281369. 期刊类型引用(13)
1. 刘新,金子铮,刘宁,陈淑湘,刘辉,于艳华,娄金丽. 乙肝核心相关抗原联合AFP、PIVKA-Ⅱ对乙肝相关肝癌的诊断价值研究. 北京医学. 2023(10): 852-857 .  百度学术
百度学术2. 周莉,陈辰,翟璐,程晓静,韩旭,姚升娟,高敏,李嘉. 血清AFP、PIVKA-Ⅱ、miR-21检测对肝细胞癌的诊断意义. 实用器官移植电子杂志. 2022(02): 135-139 .  百度学术
百度学术3. 董磊. 血清FER、AFP-L3联合检测诊断肝细胞癌的价值分析. 检验医学与临床. 2020(11): 1561-1563+1567 .  百度学术
百度学术4. 李惠军,李彩东,田鹏飞,段正军,雷志萍,张旭强. 乙肝病毒相关肝癌行AFP、PIVKA-Ⅱ和AFU联合检测的价值研究. 中国卫生标准管理. 2020(13): 108-110 .  百度学术
百度学术5. 张莉. 血清铁蛋白、甲胎蛋白异质体3联合腓骨蛋白-1检测诊断肝细胞癌的价值分析. 医学理论与实践. 2020(14): 2247-2249+2240 .  百度学术
百度学术6. 王傲然,李晓玲,门秋爽,孙凤霞. 血清铁蛋白与肝脏疾病研究进展. 中国肝脏病杂志(电子版). 2020(04): 34-37 .  百度学术
百度学术7. 许羚雁. GALAD模型在原发性肝细胞癌诊断和预后评估中的价值研究. 循证医学. 2020(05): 277-281 .  百度学术
百度学术8. 高光剑,潘耀振. PIVKA-Ⅱ及AFP对原发性肝癌的诊断价值. 贵州医科大学学报. 2019(01): 100-104 .  百度学术
百度学术9. 董秋月. 慢性乙型肝炎患者32例的肿瘤标志物检测的临床意义. 世界最新医学信息文摘. 2019(53): 13-14 .  百度学术
百度学术10. 孔令希,覃山子,秦雪,陈志坚. PIVKA-Ⅱ、AFP、RDW联合检测在肝细胞癌中的诊断价值. 广西医科大学学报. 2018(06): 839-841 .  百度学术
百度学术11. 张莹,颜学兵. 血清异常凝血酶原Ⅱ对HBV相关肝细胞癌的诊断价值. 临床肝胆病杂志. 2018(07): 1470-1474 .  本站查看
本站查看12. 王欢,陆惠慧,尚琴,徐令清. PIVKA-Ⅱ在乙型肝炎病毒感染者中筛查HCC的应用价值分析. 中国医学创新. 2018(12): 62-65 .  百度学术
百度学术13. 赵芬,郭庆波,曲凯,梁欢. 联合检测PIVKAⅡ、AFP、Ferrtin对肝细胞癌的诊断价值. 国际检验医学杂志. 2018(21): 2615-2617+2621 .  百度学术
百度学术其他类型引用(2)
-




 PDF下载 ( 2342 KB)
PDF下载 ( 2342 KB)


 下载:
下载:




 下载:
下载:

 百度学术
百度学术