猪去氧胆酸对脂肪变性肝细胞活性的影响及其机制
DOI: 10.12449/JCH240212
Effect of hyodeoxycholic acid on the activity of steatosis hepatocytes and its mechanism
-
摘要:
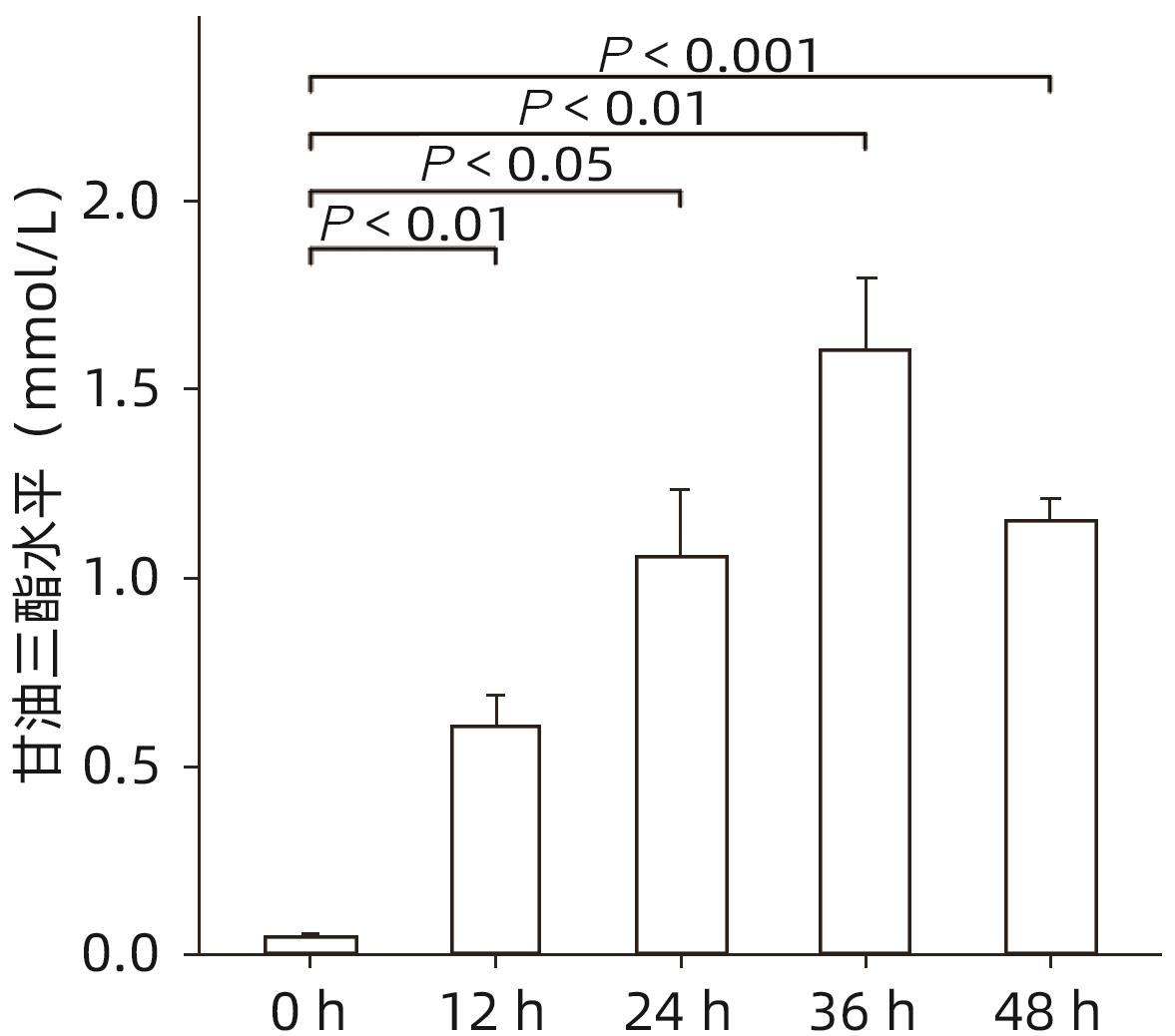
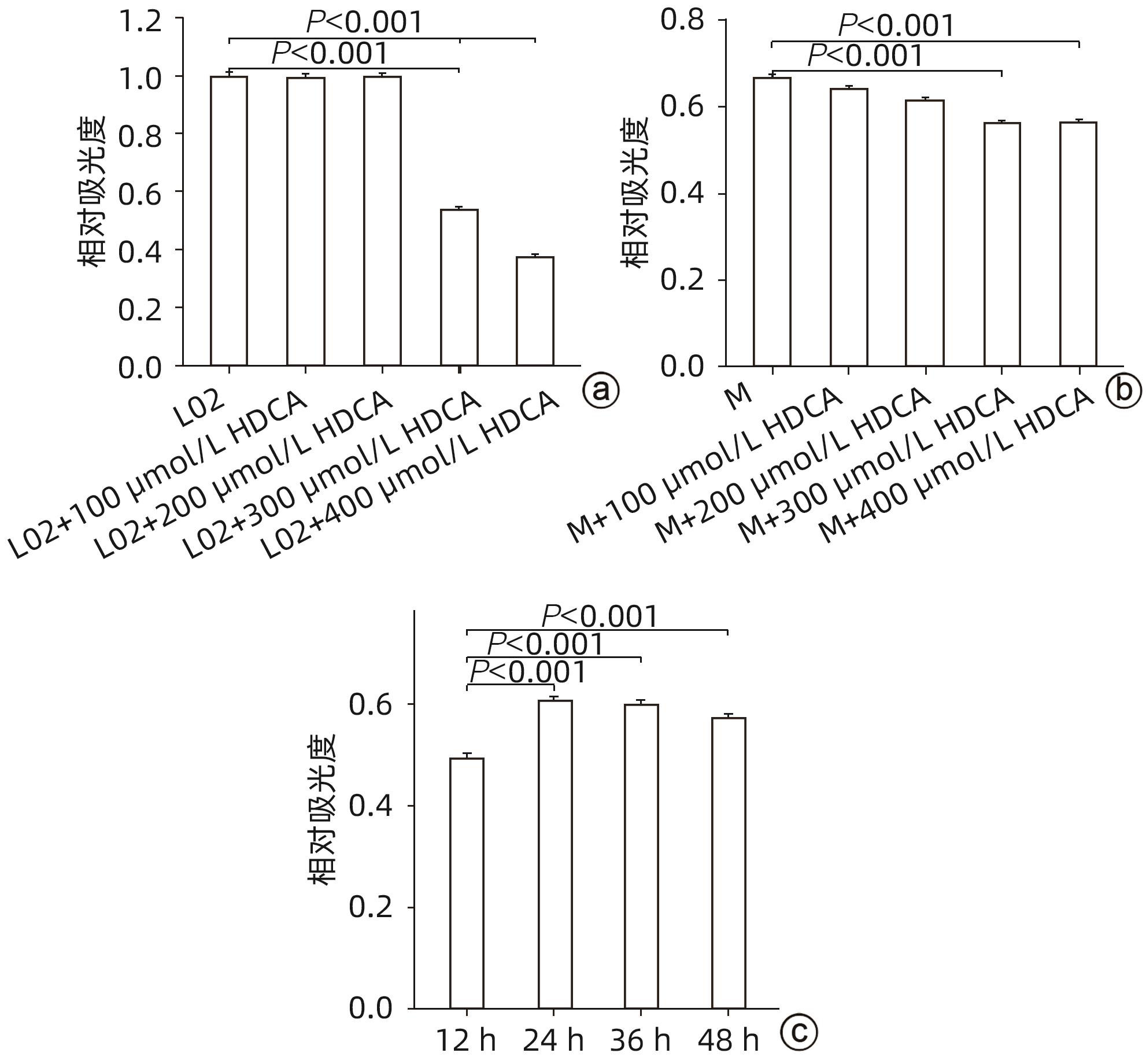
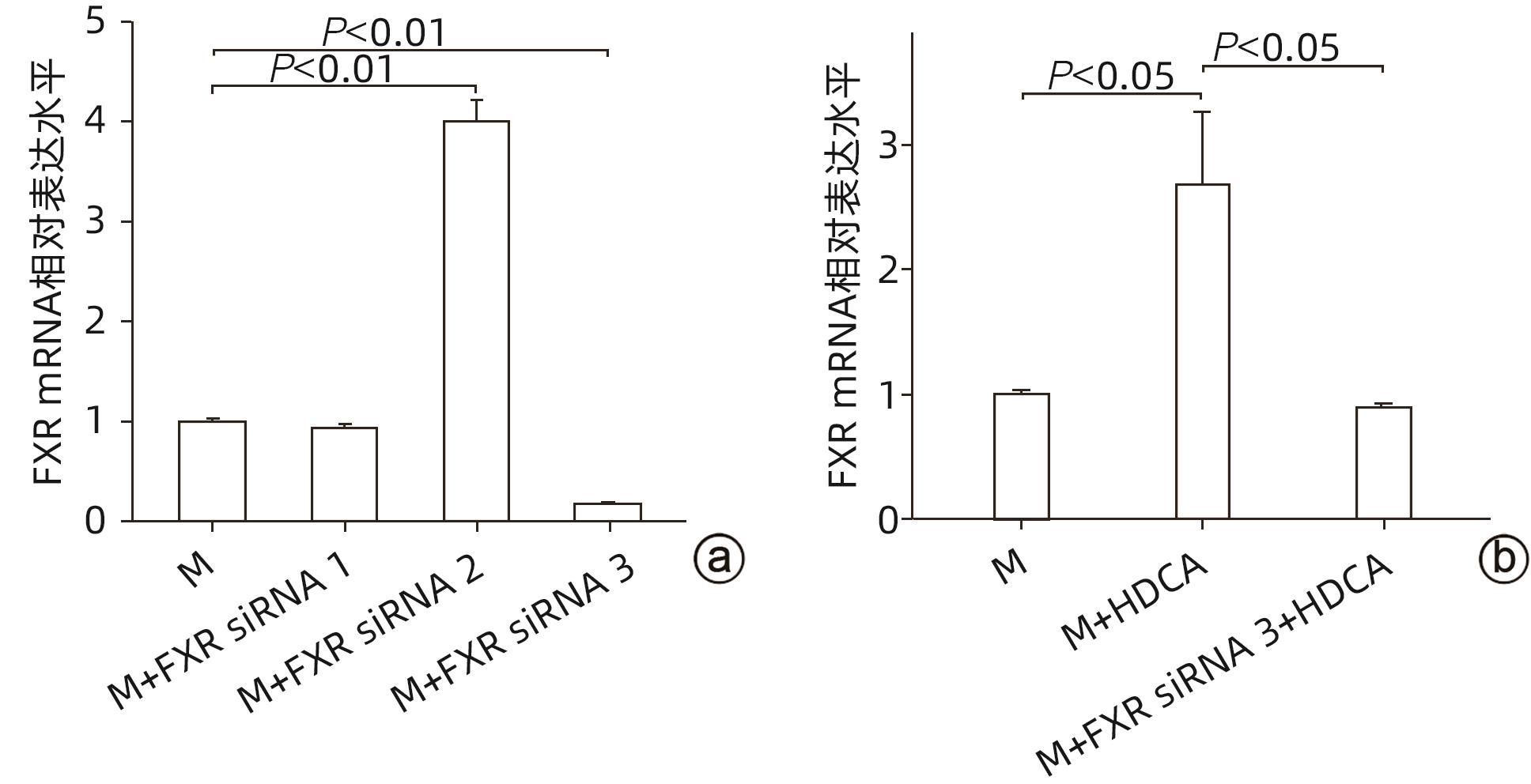
目的 探讨猪去氧胆酸(HDCA)在代谢相关脂肪性肝病(MAFLD)发展中的作用及机制,为进一步阐明MAFLD的发病机制提供新的理论依据。 方法 L02肝细胞作为实验细胞,利用棕榈酸诱导L02细胞发生脂肪变性。采用FXR siRNA干扰链技术,构建FXR低表达的肝细胞株。CCK8实验检测HDCA在不同浓度(0、100、200、300、400 μmol/L)和时间(12、24、36、48 h)对L02脂肪变性肝细胞的影响。通过qRT-PCR检测法尼醇X受体(FXR)、增殖细胞核抗原(PCNA)、周期蛋白D1(Cyclin D1)、磷脂酰肌醇-3-激酶(PI3K)和蛋白激酶B(AKT)mRNA表达;Western Blot检测FXR、Cyclin D1、PCNA、PI3K、p-PI3K、AKT和p-AKT蛋白表达。计量资料服从正态分布且方差齐时多组间比较采用单因素方差分析,进一步两两比较采用Tukey HSD检验;服从正态分布但方差不齐时采用Welch方差分析,进一步两两比较采用Games-Howell检验。两组间比较采用成组t检验。 结果 CCK8检测结果显示,300 μmol/L HDCA处理的L02细胞和脂肪变性肝细胞活性明显下降(P值均<0.05);qRT-PCR检测结果显示,FXR mRNA表达增强,PCNA、Cyclin D1、PI3K、AKT的mRNA表达下降,差异均有统计学意义(P值均<0.05)。Western Blot检测结果显示,FXR蛋白表达明显上升(P<0.05);干扰L02细胞FXR的表达后,PCNA、PI3K、p-PI3K、AKT和p-AKT的蛋白表达均明显增加(P值均<0.05)。 结论 HDCA通过上调FXR表达抑制PI3K/AKT信号通路,从而造成脂肪变性肝细胞活性下降。 Abstract:Objective To investigate the role and mechanism of hyodeoxycholic acid (HDCA) in the progression of metabolic associated fatty liver disease (MAFLD), and to provide a new theoretical basis for further clarifying the pathogenesis of MAFLD. Methods L02 hepatocytes were used as experimental cells, and palmitic acid was used to induce steatosis in L02 cells. The farnesoid X receptor (FXR) siRNA interference chain technique was used to construct a hepatocyte cell line with low FXR expression. CCK8 assay was used to observe the effect of HDCA on L02 steatosis hepatocytes at different concentrations (0, 100, 200, 300, and 400 μmol/L) and time points (12, 24, 36, and 48 hours). The method of qRT-PCR was used to measure the mRNA expression levels of FXR, proliferating cell nuclear antigen (PCNA), Cyclin D1, phosphatidylinositol 3-kinase (PI3K), and protein kinase-B (AKT), and Western blot was used to measure the protein expression levels of FXR, Cyclin D1, PCNA, PI3K, phosphorylated PI3K (p-PI3K), AKT, and phosphorylated (p-AKT). A one-way analysis of variance was used for comparison of normally distributed continuous data with homogeneity of variance between multiple groups, and the Tukey HSD test was used for further comparison between two groups; the Welch analysis of variance was used for comparison of normally distributed continuous data with heterogeneity of variance between multiple groups, and the Games-Howell test was used for further comparison between two groups. The independent-samples t test was used for comparison between two groups. Results CCK8 assay showed a significant reduction in the viability of L02 cells and steatosis hepatocytes treated by 300 μmol/L HDCA (P<0.05), and qRT-PCR showed a significant increase in the mRNA expression level of FXR and significant reductions in the mRNA expression levels of PCNA, Cyclin D1, PI3K, and AKT (all P<0.05). Western blot showed a significant increase in the protein expression level of FRX (P<0.05), and after interference of FXR expression in L02 cells, there were significant increases in the protein expression levels of PCNA, PI3K, p-PI3K, AKT, and p-AKT (all P<0.05). Conclusion HDCA inhibits the PI3K/AKT signaling pathway by upregulating FXR expression, thereby inducing a reduction in the viability of steatosis hepatocytes. -
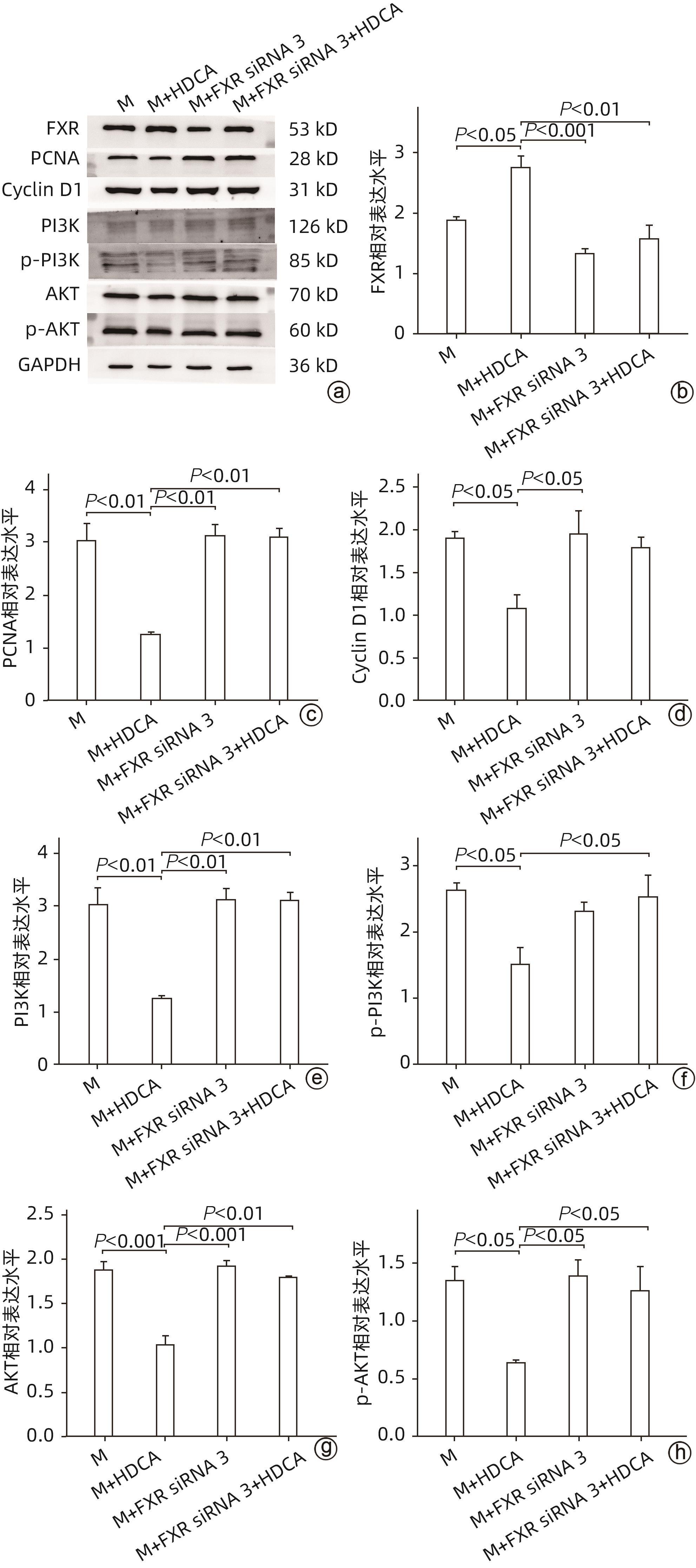
注: a,FXR、PCNA、Cyclin D1、PI3K、p-PI3K、AKT和p-AKT的蛋白印迹图;b,FXR蛋白的相对表达水平;c,PCNA蛋白的相对表达水平;d,Cyclin D1蛋白的相对表达水平;e,PI3K蛋白的相对表达水平;f,p-PI3K蛋白的相对表达水平;g,AKT蛋白的相对表达水平;h,p-AKT蛋白的相对表达水平。
图 6 抑制FXR表达后脂肪变性肝细胞FXR-PI3K/AKT通路关键分子及PCNA和Cyclin D1蛋白表达的变化
Figure 6. Changes in the expression of key molecules of FXR-PI3K/AKT pathway and PCNA and Cyclin D1 proteins in steatosis hepatocytes after inhibition of FXR expression
表 1 qRT-PCR引物序列
Table 1. qRT-PCR primer sequences
引物 序列(5'-3') FXR 上游:AACCATACTCGCAATACAGCAA 下游:ACAGCTCATCCCCTTTGATCC PCNA 上游:CCTGCTGGGATATTAGCTCCA 下游:CAGCGGTAGGTGTCGAAGC Cyclin D1 上游:GCTGCGAAGTGGAAACCATC 下游:CCTCCTTCTGCACACATTTGAA PI3K 上游:TATTTGGACTTTGCGACAAGACT 下游:TCGAACGTACTGGTCTGGATAG AKT-F 上游:AGCGACGTGGCTATTGTGAAG 下游:GCCATCATTCTTGAGGAGGAAGT 表 2 HDCA对脂肪变性肝细胞FXR、PI3K、AKT、PCNA和Cyclin D1 mRNA表达的影响
Table 2. Effect of HDCA on the mRNA expression of FXR, PI3K, AKT, PCNA, and Cyclin D1 in steatotic hepatocytes
指标 M组 M+HDCA组 t值 P值 FXR mRNA 0.485±0.162 1.010±0.013 5.576 0.005 PCNA mRNA 1.710±0.052 1.034±0.054 -15.679 <0.001 Cyclin D1 mRNA 1.435±0.124 1.041±0.053 -5.083 0.007 PI3K mRNA 1.951±0.500 1.008±0.009 -3.266 0.031 AKT mRNA 2.373±0.316 1.030±0.050 -7.266 0.002 -
[1] ESLAM M, SANYAL AJ, GEORGE J. MAFLD: A consensus-driven proposed nomenclature for metabolic associated fatty liver disease[J]. Gastroenterology, 2020, 158( 7): 1999- 2014. DOI: 10.1053/j.gastro.2019.11.312. [2] COBBINA E, AKHLAGHI F. Non-alcoholic fatty liver disease(NAFLD)-pathogenesis, classification, and effect on drug metabolizing enzymes and transporters[J]. Drug Metab Rev, 2017, 49( 2): 197- 211. DOI: 10.1080/03602532.2017.1293683. [3] DEPRINCE A, HAAS JT, STAELS B. Dysregulated lipid metabolism links NAFLD to cardiovascular disease[J]. Mol Metab, 2020, 42: 101092. DOI: 10.1016/j.molmet.2020.101092. [4] ALVES-BEZERRA M, COHEN DE. Triglyceride metabolism in the liver[J]. Compr Physiol, 2017, 8( 1): 1- 8. DOI: 10.1002/cphy.c170012. [5] CHÁVEZ-TALAVERA O, TAILLEUX A, LEFEBVRE P, et al. Bile acid control of metabolism and inflammation in obesity, type 2 diabetes, dyslipidemia, and nonalcoholic fatty liver disease[J]. Gastroenterology, 2017, 152( 7): 1679- 1694. DOI: 10.1053/j.gastro.2017.01.055. [6] WANG XW, SEED B. A PCR primer bank for quantitative gene expression analysis[J]. Nucleic Acids Res, 2003, 31( 24): e154. DOI: 10.1093/nar/gng154. [7] O'LEARY NA, WRIGHT MW, BRISTER JR, et al. Reference sequence(RefSeq) database at NCBI: Current status, taxonomic expansion, and functional annotation[J]. Nucleic Acids Res, 2016, 44( D1): D733- D745. DOI: 10.1093/nar/gkv1189. [8] YOUNOSSI ZM, RINELLA ME, SANYAL AJ, et al. From NAFLD to MAFLD: Implications of a premature change in terminology[J]. Hepatology, 2021, 73( 3): 1194- 1198. DOI: 10.1002/hep.31420. [9] Italian Association for the Study of the Liver(AISF). AISF position paper on nonalcoholic fatty liver disease(NAFLD): Updates and future directions[J]. Dig Liver Dis, 2017, 49( 5): 471- 483. DOI: 10.1016/j.dld.2017.01.147. [10] JIANG TT, SUN FF, ZENG Z, et al. Progress on metabolic associated fatty liver disease related liver cancer[J/CD]. Chin J Liver Dis(Electronic Version), 2022, 14( 3): 14- 17. DOI: 10.3969/j.issn.1674-7380.2022.03.004.蒋婷婷, 孙芳芳, 曾湛, 等. 代谢相关脂肪性肝病相关肝癌研究进展[J/CD]. 中国肝脏病杂志(电子版), 2022, 14( 3): 14- 17. DOI: 10.3969/j.issn.1674-7380.2022.03.004. [11] RIZZOLO D, BUCKLEY K, KONG B, et al. Bile acid homeostasis in a cholesterol 7α-hydroxylase and sterol 27-hydroxylase double knockout mouse model[J]. Hepatology, 2019, 70( 1): 389- 402. DOI: 10.1002/hep.30612. [12] WATANABE S, FUJITA K. Dietary hyodeoxycholic acid exerts hypolipidemic effects by reducing farnesoid X receptor antagonist bile acids in mouse enterohepatic tissues[J]. Lipids, 2014, 49( 10): 963- 973. DOI: 10.1007/s11745-014-3947-y. [13] SONG M, MA XY, ZHANG FL, et al. Effects of hyodeoxycholic acid on growth performance, energy metabolism and fat digestion and absorption of mice[J]. Chin J Anim Nutr, 2022, 34( 6): 3983- 3990. DOI: 10.3969/j.issn.1006-267x.2022.06.057.宋敏, 马现永, 张枫琳, 等. 猪去氧胆酸对小鼠生长性能、能量代谢及脂肪消化吸收的影响[J]. 动物营养学报, 2022, 34( 6): 3983- 3990. DOI: 10.3969/j.issn.1006-267x.2022.06.057. [14] SEHAYEK E, ONO JG, DUNCAN EM, et al. Hyodeoxycholic acid efficiently suppresses atherosclerosis formation and plasma cholesterol levels in mice[J]. J Lipid Res, 2001, 42( 8): 1250- 1256. [15] SHIH DM, SHAPOSHNIK Z, MENG YH, et al. Hyodeoxycholic acid improves HDL function and inhibits atherosclerotic lesion formation in LDLR-knockout mice[J]. FASEB J, 2013, 27( 9): 3805- 3817. DOI: 10.1096/fj.12-223008. [16] FORMAN BM, GOODE E, CHEN J, et al. Identification of a nuclear receptor that is activated by farnesol metabolites[J]. Cell, 1995, 81( 5): 687- 693. DOI: 10.1016/0092-8674(95)90530-8. [17] PELLICCIARI R, COSTANTINO G, FIORUCCI S. Farnesoid X receptor: From structure to potential clinical applications[J]. J Med Chem, 2005, 48( 17): 5383- 5403. DOI: 10.1021/jm0582221. [18] PARKS DJ, BLANCHARD SG, BLEDSOE RK, et al. Bile acids: Natural ligands for an orphan nuclear receptor[J]. Science, 1999, 284( 5418): 1365- 1368. DOI: 10.1126/science.284.5418.1365. [19] DOWNES M, VERDECIA MA, ROECKER AJ, et al. A chemical, genetic, and structural analysis of the nuclear bile acid receptor FXR[J]. Mol Cell, 2003, 11( 4): 1079- 1092. DOI: 10.1016/s1097-2765(03)00104-7. [20] PELLICCIARI R, FIORUCCI S, CAMAIONI E, et al. 6alpha-ethyl-chenodeoxycholic acid(6-ECDCA), a potent and selective FXR agonist endowed with anticholestatic activity[J]. J Med Chem, 2002, 45( 17): 3569- 3572. DOI: 10.1021/jm025529g. [21] SAYIN S, WAHLSTRÖM A, FELIN J, et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist[J]. Cell Metab, 2013, 17( 2): 225- 235. DOI: 10.1016/j.cmet.2013.01.003. [22] SUN LL, XIE C, WANG G, et al. Gut microbiota and intestinal FXR mediate the clinical benefits of metformin[J]. Nat Med, 2018, 24( 12): 1919- 1929. DOI: 10.1038/s41591-018-0222-4. [23] CHIANG JYL, FERRELL JM. Bile acids as metabolic regulators and nutrient sensors[J]. Annu Rev Nutr, 2019, 39: 175- 200. DOI: 10.1146/annurev-nutr-082018-124344. [24] MUELLER M, THORELL A, CLAUDEL T, et al. Ursodeoxycholic acid exerts farnesoid X receptor-antagonistic effects on bile acid and lipid metabolism in morbid obesity[J]. J Hepatol, 2015, 62( 6): 1398- 1404. DOI: 10.1016/j.jhep.2014.12.034. [25] HARRISON SA, BASHIR MR, LEE KJ, et al. A structurally optimized FXR agonist, MET409, reduced liver fat content over 12 weeks in patients with non-alcoholic steatohepatitis[J]. J Hepatol, 2021, 75( 1): 25- 33. DOI: 10.1016/j.jhep.2021.01.047. [26] HAN CY, RHO HS, KIM A, et al. FXR inhibits endoplasmic reticulum stress-induced NLRP3 inflammasome in hepatocytes and ameliorates liver injury[J]. Cell Rep, 2018, 24( 11): 2985- 2999. DOI: 10.1016/j.celrep.2018.07.068. [27] JUNG K, KIM M, SO J, et al. Farnesoid X receptor activation impairs liver progenitor cell-mediated liver regeneration via the PTEN-PI3K-AKT-mTOR axis in zebrafish[J]. Hepatology, 2021, 74( 1): 397- 410. DOI: 10.1002/hep.31679. [28] FRIEDMAN ES, LI Y, SHEN TC D, et al. FXR-dependent modulation of the human small intestinal microbiome by the bile acid derivative obeticholic acid[J]. Gastroenterology, 2018, 155( 6): 1741- 1752. DOI: 10.1053/j.gastro.2018.08.022. [29] MAKRI E, CHOLONGITAS E, TZIOMALOS K. Emerging role of obeticholic acid in the management of nonalcoholic fatty liver disease[J]. World J Gastroenterol, 2016, 22( 41): 9039- 9043. DOI: 10.3748/wjg.v22.i41.9039. [30] XU J, YAO X, LI X, et al. Farnesoid X receptor regulates PI3K/AKT/mTOR signaling pathway, lipid metabolism, and immune response in hybrid grouper[J]. Fish Physiol Biochem, 2022, 48( 6): 1521- 1538. DOI: 10.1007/s10695-022-01130-z. -



 PDF下载 ( 1921 KB)
PDF下载 ( 1921 KB)

 下载:
下载: