磁共振成像胆道评分和肝肌比值评估肝纤维化病理分级的价值
DOI: 10.12449/JCH240413
Value of MRI biliary score and liver/muscle ratio in evaluating the pathological grade of liver fibrosis
-
摘要:
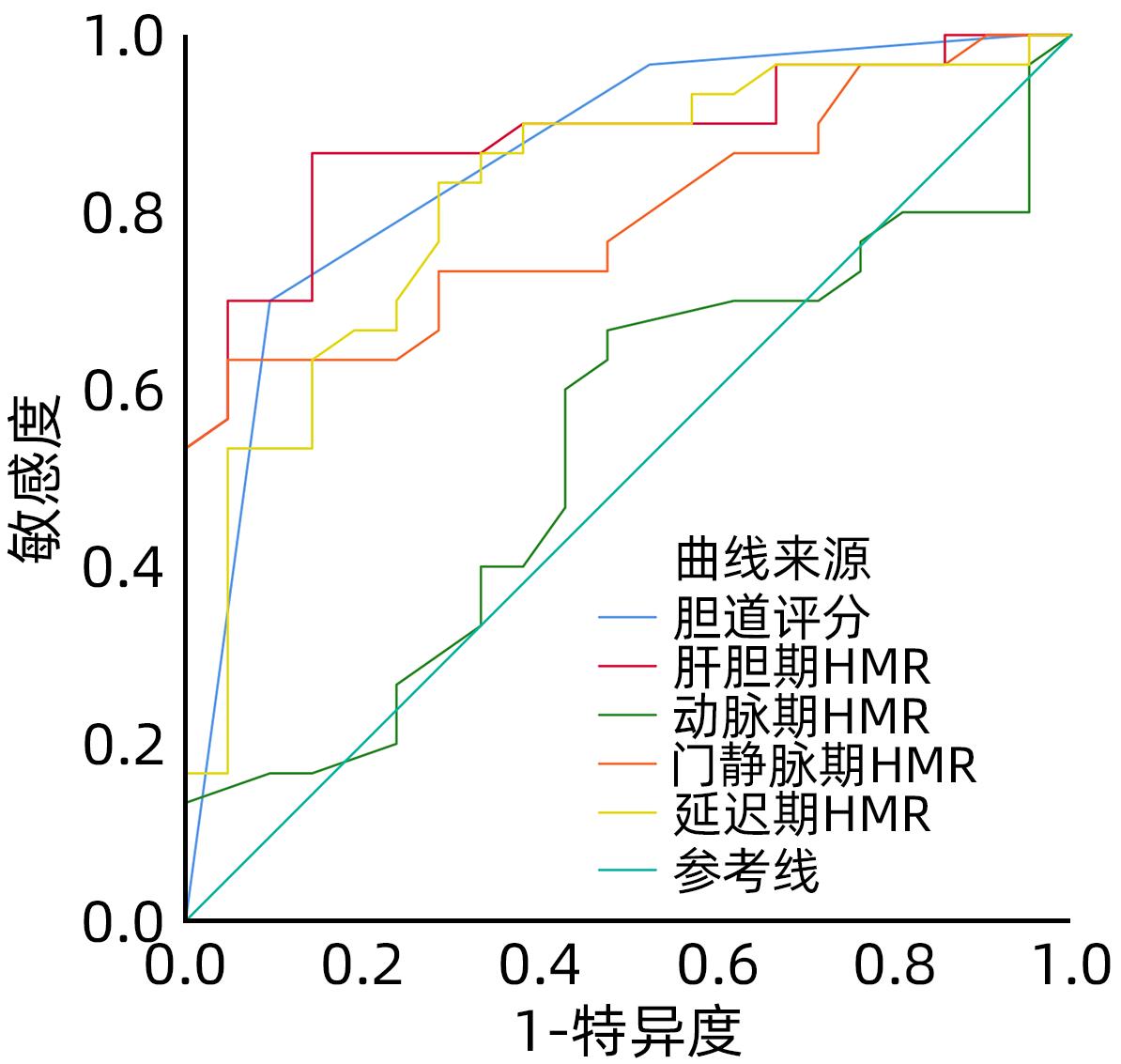
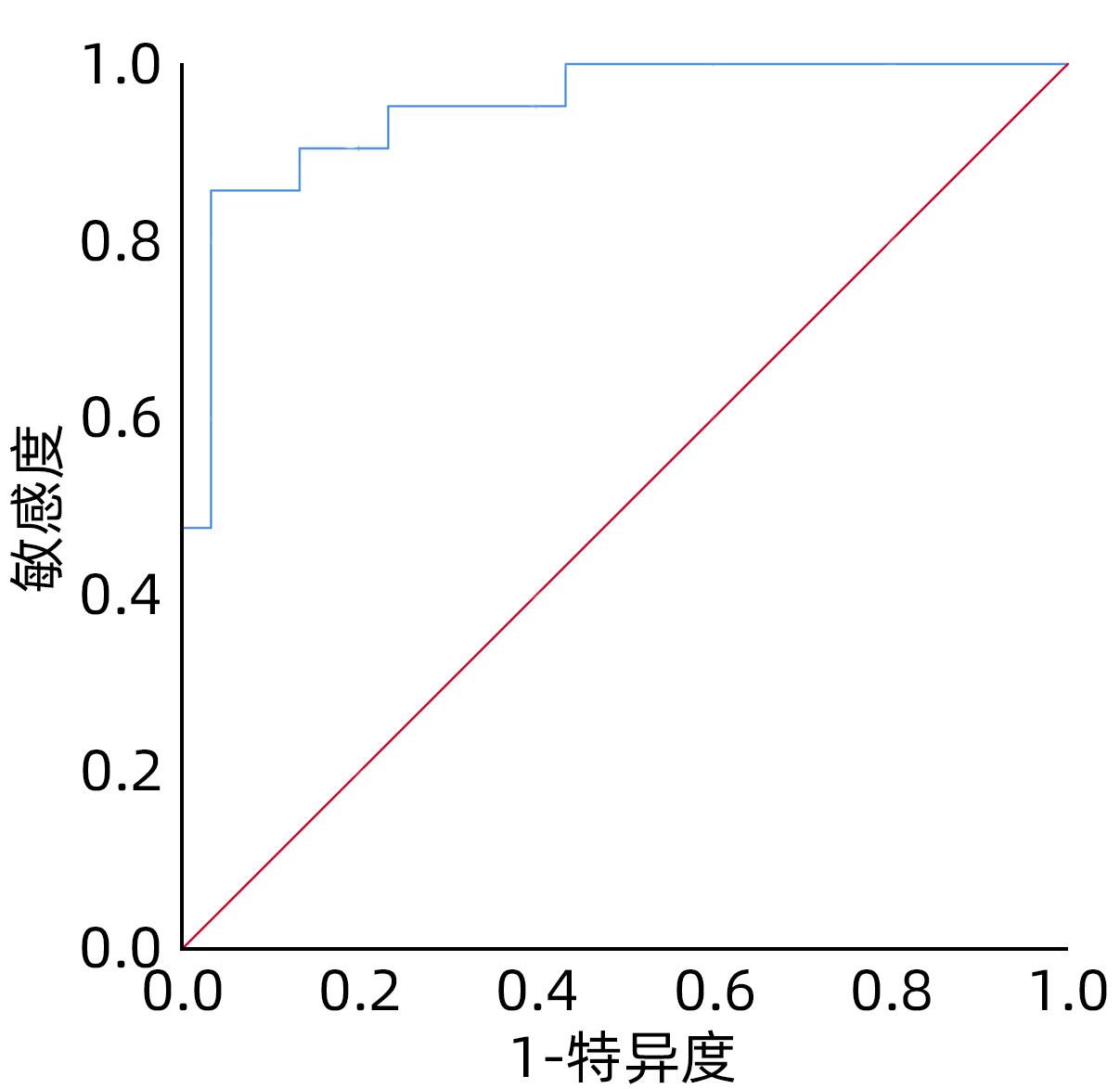
目的 探讨钆塞酸二钠多期增强MRI扫描所得胆道评分和肝肌比值在评估肝纤维化病理分级中的应用价值。 方法 回顾性分析2020年1月—2023年5月广西医科大学附属武鸣医院51例慢性乙型肝炎肝纤维化患者的MRI和临床资料。将51例肝纤维化患者分为两组,其中S1、S2期为低级别组(n=30),S3、S4期为高级别组(n=21)。扫描采用GE Architact 3.0T磁共振扫描仪,包括常规肝脏平扫,动脉期、门静脉期、延迟期、肝胆期、排泄期的增强扫描。对不同级别肝纤维化患者进行胆道评分和测量肝肌比值。计量资料组间比较采用成组t检验,计数资料组间比较采用χ2检验或Fisher确切检验法。绘制受试者工作特征曲线(ROC曲线)评价MRI指标对肝纤维化病理分级的诊断价值。 结果 低级别组胆道评分(3.67±0.55)大于高级别组(2.57±0.75)(t=6.05,P<0.001);低级别组门静脉期、延迟期、肝胆期的肝肌比值(2.38±0.76,2.48±0.70,4.10±0.63)均大于高级别组(1.97±0.18,1.99±0.27,3.16±0.47)(t值分别为2.41、3.09、5.81,P值分别为0.020、0.003、<0.001)。上述指标区分低、高级别肝纤维化的ROC曲线下面积分别为0.86、0.79、0.82、0.88,诊断高级别肝纤维化的敏感度分别为70%、63.3%、83.3%、96.7%,特异度分别为90%、95.2%、74.1%、100%。胆道评分联合肝胆期肝肌比值的ROC曲线下面积达0.95,敏感度85.7%,特异度96.7%。 结论 MRI钆塞酸二钠多期增强扫描所得胆道评分和肝胆期肝肌比值在区分低、高级别肝纤维化方面具有较高的诊断效能,对临床肝纤维化的诊治有一定的指导价值。 Abstract:Objective To investigate the value of biliary score and hepatic signal intensity-to-muscle signal intensity ratio (HMR) obtained by multiphase contrast-enhanced MRI scan using Gd-EOB-DTPA in evaluating the pathological grade of liver fibrosis. Methods A retrospective analysis was performed for the MRI and clinical data of 51 patients with chronic hepatitis B liver fibrosis in Wuming Hospital Affiliated to Guangxi Medical University from January 2020 to May 2023. The 51 patients with liver fibrosis were divided into low-grade group (S1-S2) and high-grade group (S3-S4). GE Architact 3.0T MR scanner was used to perform MRI scans, including routine plain scan and contrast-enhanced scan at arterial phase, portal venous phase, delayed phase, hepatobiliary phase, and excretory phase, and biliary score and HMR were measured for the patients with different grades of liver fibrosis. The t-test was used for comparison of continuous data between groups, and the chi-square test or the Fisher’s exact test was used for comparison of categorical data between groups. The receiver operating characteristic (ROC) curve was plotted to evaluate the value of MRI indicators in determining the pathological grade of liver fibrosis. Results Among the 51 patients with liver fibrosis, there were 30 patients in the low-grade group and 21 in the high-grade group. Compared with the high-grade group, the low-grade group had significantly higher biliary score (3.67±0.55 vs 2.57±0.75, t=6.05, P<0.001) and HMR at portal venous phase (2.38±0.76 vs 1.97±0.18, t=2.41, P=0.020), delayed phase (2.48±0.70 vs 1.99±0.27, t=3.09, P=0.003), and hepatobiliary phase (4.10±0.63 vs 3.16±0.47, t=5.81, P<0.001). The above indicators had an area under the ROC curve (AUC) of 0.86, 0.79, 0.82, and 0.88, respectively, in distinguishing low- and high-grade liver fibrosis, with a positive rate of 70%, 63.3%, 83.3%, and 96.7%, respectively, and a negative rate of 90%, 95.2%, 74.1%, and 100%, respectively, in the diagnosis of high-grade liver fibrosis. Biliary score combined HMR had an AUC of 0.95, with a positive rate of 85.7% and a negative rate of 96.7%. Conclusion Biliary score and HMR at hepatobiliary phase obtained by multiphase contrast-enhanced MRI scan using Gd-EOB-DTPA has a relatively high diagnostic efficacy in distinguishing between low- and high-grade liver fibrosis and a certain guiding value for the diagnosis and treatment of liver fibrosis in clinical practice. -
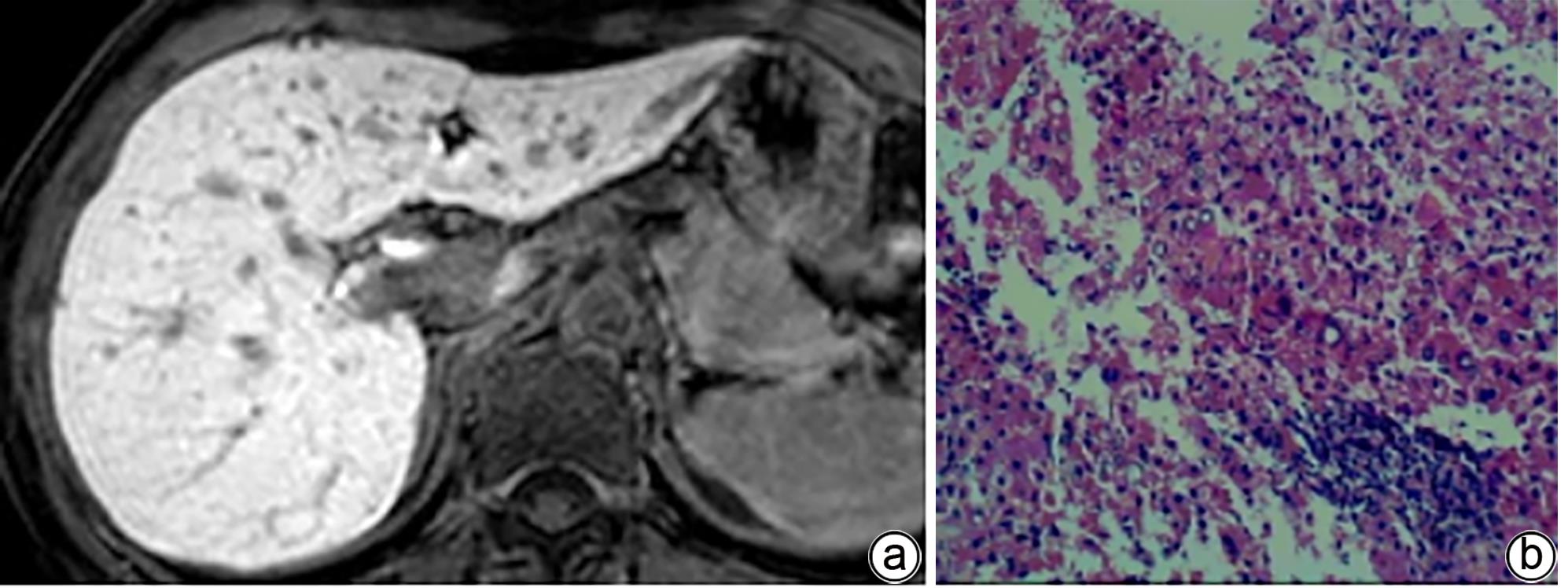
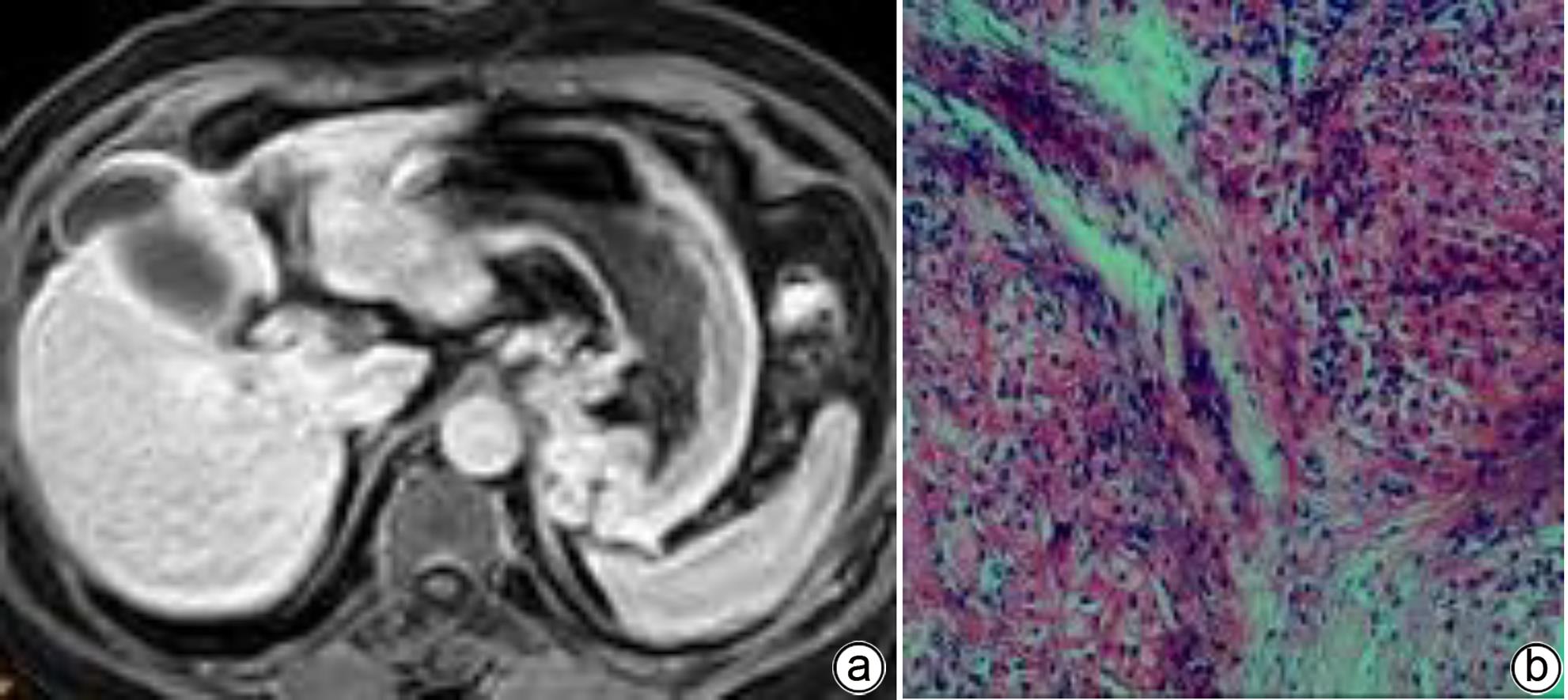
注: a,男,68岁,胆汁排泄期示,胆道各区均未见显影,胆道评分1分;b,肝穿活检病理(HE染色,×200),镜下见类圆形肝细胞团内的肝细胞排列稍紊乱,可见较多肝细胞水肿,部分细胞气球样变,小范围的点状坏死,汇管区扩大,淋巴细胞浸润,纤维组织增生并分割包绕肝细胞团。免疫组化:Hepar-1(+),HBsAg(+),CK7(+),符合慢性乙型肝炎改变(G2S4)。
图 1 肝纤维化S4期MRI增强扫描及肝活检病理结果
Figure 1. MRI enhanced scan image of the bile excretion phase of the S4 stage of liver fibrosis + Pathological image of liver biopsy
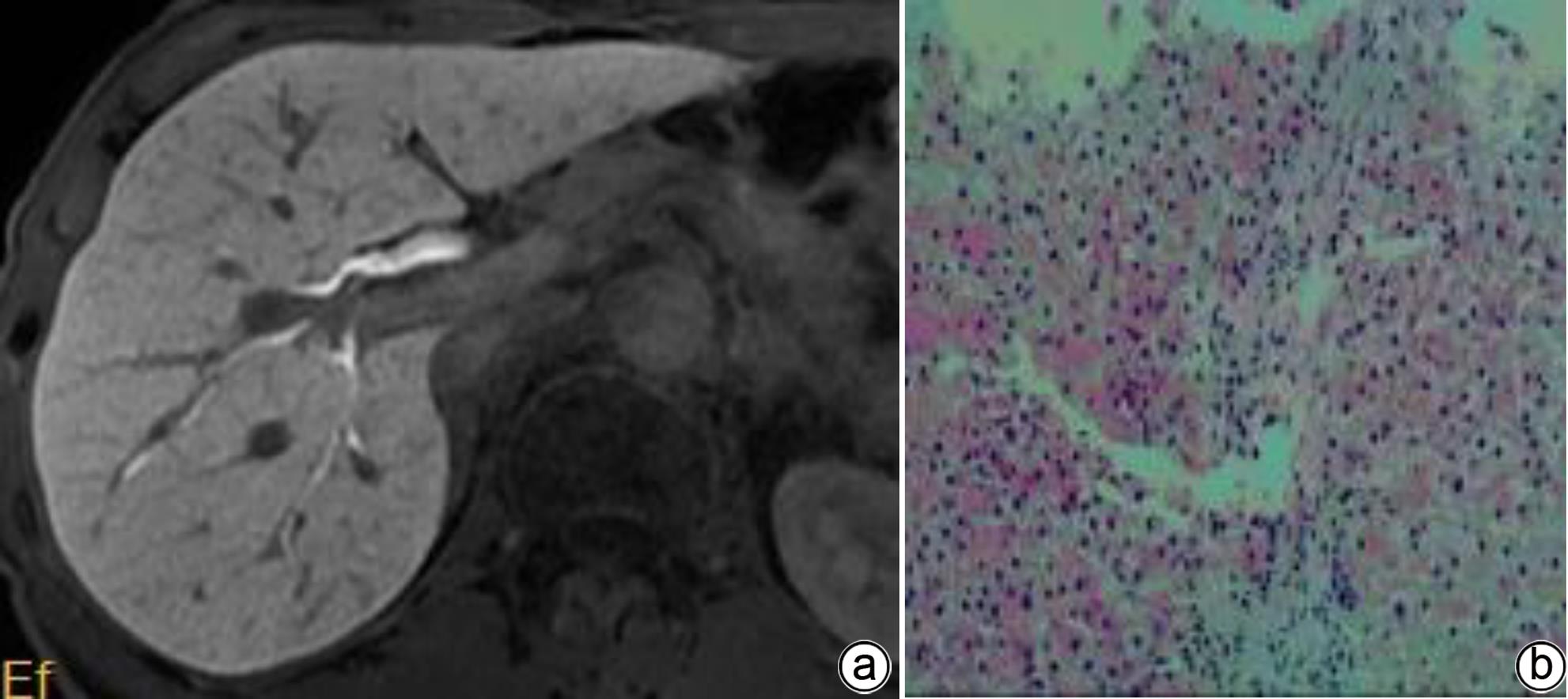
注: a,女,52岁,胆汁排泄期示,胆道各区模糊可见,胆道评分2分;b,肝活检病理(HE染色,×200),镜下见肝小叶结构存在,部分肝细胞排列稍紊乱,小灶见碎片状坏死;汇管区扩大,可见淋巴细胞浸润及纤维组织增生。免疫组化:Hepar-1(+),HBsAg(+),CK7(+),符合慢性乙型肝炎改变(G2S2)。
图 2 肝纤维化S2期MRI增强扫描及肝活检病理结果
Figure 2. MRI enhanced scan image of the bile excretion phase of the S2 stage of liver fibrosis + Pathological image of liver biopsy
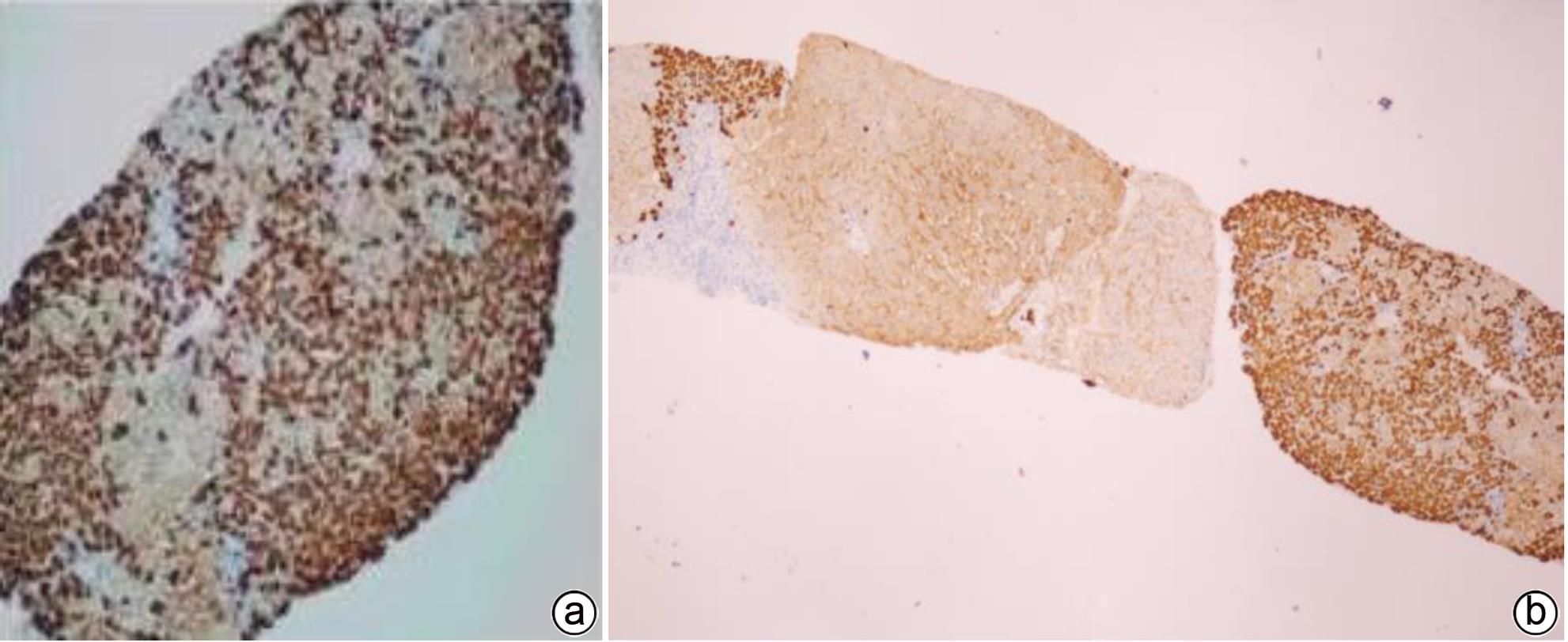
注: a,男,36岁,胆汁排泄期示,各区均可见,但部分胆管或汇合处未见显示,胆道评分3分;b,肝活检病理(HE染色,×200),镜下见肝细胞排列紊乱,可见点灶状坏死及碎片状坏死;汇管区扩大,淋巴细胞浸润及纤维组织增生。免疫组化:Hepar-1(+),HBsAg(+),CK7(+),符合慢性乙型肝炎改变(G2S3)。
图 3 肝纤维化S3期MRI增强扫描及肝活检病理结果
Figure 3. MRI enhanced scan image of the bile excretion phase of the S3 stage of liver fibrosis + Pathological image of liver biopsy
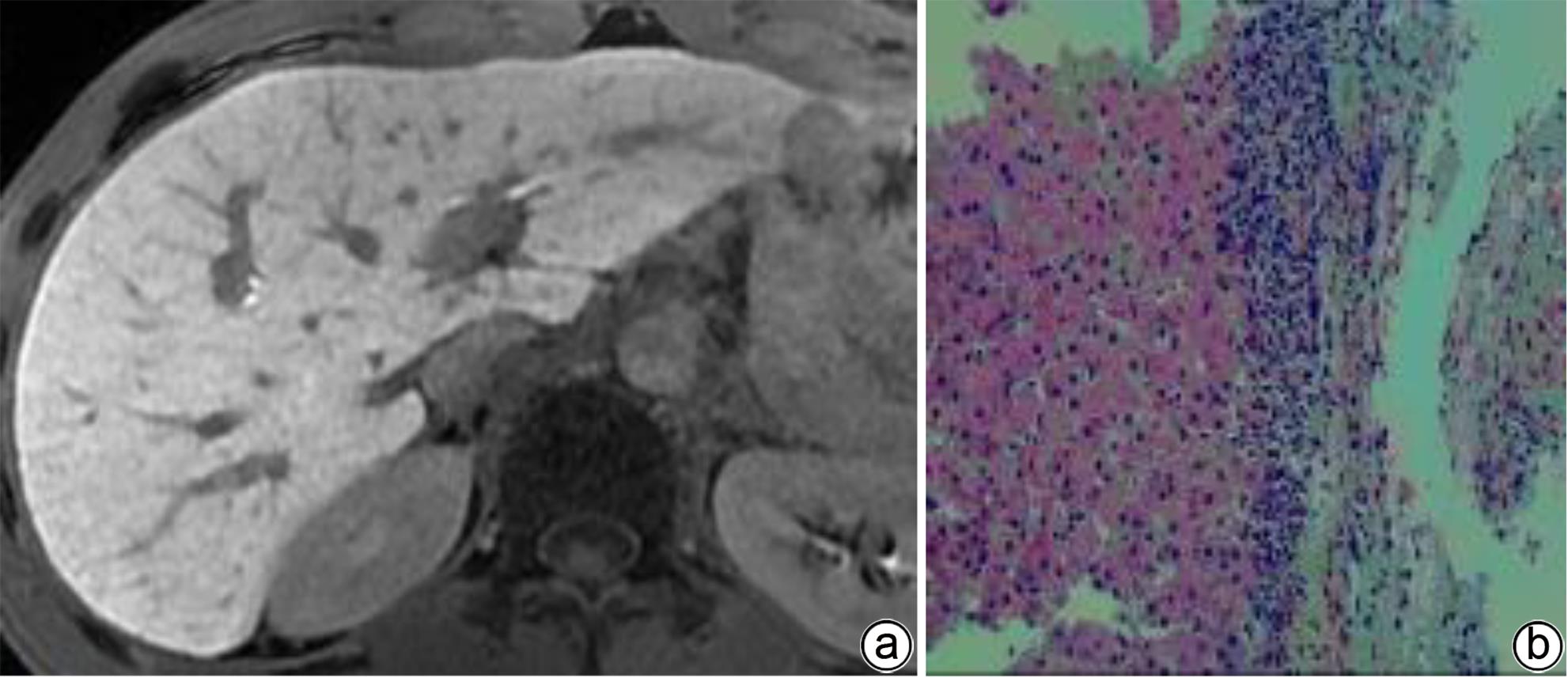
注: a,男,71岁,胆汁排泄期示,各区胆管从近端至远端均清晰可见,胆道评分4分;b,肝活检病理(HE染色,×200),镜下见肝小叶结构存在,肝细胞排列整齐,部分区域水肿变性,可见点灶状坏死;汇管区纤维组织增生,少量淋巴细胞浸润。免疫组化:Hepar-1(+),HBsAg(+),CK7(+),符合慢性乙型肝炎改变(G1S1)。
图 4 肝纤维化S1期MRI增强扫描及肝活检病理结果
Figure 4. MRI enhanced scan image of the bile excretion phase of the S1 stage of liver fibrosis + Pathological image of liver biopsy
表 1 低级别组和高级别组之间一般情况比较
Table 1. General comparison between low-grade group and high-grade group
指标 低级别组(n=30) 高级别组(n=21) 男/女(例) 27/3 17/4 年龄(岁) 51.74±9.02 44.14±7.26 体质量(kg) 51.44±7.28 50.58±10.8 身高(cm) 165.01±5.31 162.92±7.56 BMI(kg/m2) 20.55±2.72 21.31±3.17 对比剂用量(mL) 7.88±2.01 7.01±1.35 表 2 低级别与高级别组肝纤维化胆道评分和各期HMR比较
Table 2. Comparison of biliary scores between the low-grade and high-grade liver fibrosis groups, and that of hepatic muscle ratios of each stage
项目 低级别组(n=30) 高级别组(n=21) t值 P值 胆道评分 3.67±0.55 2.57±0.75 6.05 <0.001 动脉期HMR 1.64±0.28 1.58±0.13 0.85 0.400 门静脉期HMR 2.38±0.76 1.97±0.18 2.41 0.020 延迟期HMR 2.48±0.70 1.99±0.27 3.09 0.003 肝胆期HMR 4.10±0.63 3.16±0.47 5.81 <0.001 -
[1] Chinese Society of Hepatology, Chinese Medical Association; Chinese Society of Gastroenterology, Chinese Medical Association; Chinese Society of Infectious Diseases, Chinese Medical Association. Consensus on the diagnosis and therapy of hepatic fibrosis(2019)[J]. J Clin Hepatol, 2019, 35( 10): 2163- 2172. DOI: 10.3969/j.issn.1001-5256.2019.10.007.中华医学会肝病学分会, 中华医学会消化病学分会, 中华医学会感染病学分会. 肝纤维化诊断及治疗共识(2019年)[J]. 临床肝胆病杂志, 2019, 35( 10): 2163- 2172. DOI: 10.3969/j.issn.1001-5256.2019.10.007. [2] GUO JS. The critical importance of diagnosing and treating liver fibrosis[J]. J Clin Hepatol, 2018, 34( 1): 16- 19. DOI: 10.3969/j.issn.1001-5256.2018.01.002.郭津生. 重视肝纤维化的诊治[J]. 临床肝胆病杂志, 2018, 34( 1): 16- 19. DOI: 10.3969/j.issn.1001-5256.2018.01.002. [3] BEDOSSA P, DARGÈRE D, PARADIS V. Sampling variability of liver fibrosis in chronic hepatitis C[J]. Hepatology, 2003, 38( 6): 1449- 1457. DOI: 10.1053/jhep.2003.09022. [4] LI MB, LI JY, FENG DP. Research advances in the reversal of liver fibrosis[J]. J Clin Hepatol, 2023, 39( 1): 193- 198. DOI: 10.3969/j.issn.1001-5256.2023.01.030.李满彪, 李金玉, 冯对平. 肝纤维化逆转的研究进展[J]. 临床肝胆病杂志, 2023, 39( 1): 193- 198. DOI: 10.3969/j.issn.1001-5256.2023.01.030. [5] LI ZC, HUANG Y, ZHANG ZY, et al. Analysis of prediction indicators and prediction model construction for severity of liver fibrosis[J]. Chin J Gen Surg, 2020, 29( 11): 1364- 1369. DOI: 10.7659/j.issn.1005-6947.2020.11.010.李哲成, 黄云, 张泽宇, 等. 肝纤维化严重程度预测指标分析与预测模型构建[J]. 中国普通外科杂志, 2020, 29( 11): 1364- 1369. DOI: 10.7659/j.issn.1005-6947.2020.11.010. [6] DONG SQ, MA QZ, WU CN, et al. Imaging quality of biliary tract and detecting diseases with hepatobiliary phase Gd-EOB-DTPA contrast-enhanced MR cholangiography based on 3D-VIBE and 3D-FLASH sequences[J]. Chin J Med Imag Technol, 2023, 39( 6): 866- 871. DOI: 10.13929/j.issn.1003-3289.2023.06.015.董思情, 马巧稚, 吴春楠, 等. 3D-VIBE与3D-FLASH序列肝胆特异期Gd-EOB-DTPA增强MR胆道成像显示胆道系统图像质量及检出病变[J]. 中国医学影像技术, 2023, 39( 6): 866- 871. DOI: 10.13929/j.issn.1003-3289.2023.06.015. [7] DU WT, REN WL, HU DD, et al. Research progress on reversible animal model of liver fibrosis[J/OL]. Chin J Liver Dis Electron Version, 2022, 14( 3): 18- 21. DOI: 10.3969/j.issn.1674-7380.2022.03.005.杜文涛, 任万雷, 胡豆豆, 等. 肝纤维化可逆转动物模型研究进展[J/OL]. 中国肝脏病杂志(电子版), 2022, 14( 3): 18- 21. DOI: 10.3969/j.issn.1674-7380.2022.03.005. [8] DEWIDAR B, MEYER C, DOOLEY S, et al. TGF-β in hepatic stellate cell activation and liver fibrogenesis-updated 2019[J]. Cells, 2019, 8( 11): 1419. DOI: 10.3390/cells8111419. [9] LIU JH, LI XY, WANG ML, et al. Advantages and characteristics of traditional Chinese medicine in the treatment of liver fibrosis[J]. J Clin Hepatol, 2023, 39( 2): 267- 272. DOI: 10.3969/j.issn.1001-5256.2023.02.003.刘俊宏, 李欣瑜, 王淼蕾, 等. 中医药治疗肝纤维化的优势与特色[J]. 临床肝胆病杂志, 2023, 39( 2): 267- 272. DOI: 10.3969/j.issn.1001-5256.2023.02.003. [10] LU LG, YOU H, XIE WF, et al. Consensus on the diagnosis and therapy of hepatic fibrosis[J]. J Pract Hepatol, 2019, 22( 6): 793- 803.陆伦根, 尤红, 谢渭芬, 等. 肝纤维化诊断及治疗共识(2019年)[J]. 实用肝脏病杂志, 2019, 22( 6): 793- 803. [11] SUN YM, CHEN SY, YOU H. Regression of liver fibrosis: Evidence and challenges[J]. Chin Med J, 2020, 133( 14): 1696- 1702. DOI: 10.1097/CM9.0000000000000835. [12] TONG HF, WANG ZH, CHEN H, et al. Prediction of the histological grades of liver fibrosis and cirrhosis by quantitative analysis of gadoxetic acid contrast-enhanced MRI[J]. J Wenzhou Med Univ, 2020, 50( 9): 717- 722. DOI: 10.3969/j.issn.2095-9400.2020.09.006.童洪飞, 王兆洪, 陈辉, 等. 定量分析钆塞酸二钠增强MRI预测肝纤维化和肝硬化病理分级[J]. 温州医科大学学报, 2020, 50( 9): 717- 722. DOI: 10.3969/j.issn.2095-9400.2020.09.006. [13] JIANG Y, SHU J, LU X, et al. A preliminary study for the quantitative assessment of chronic hepatitis by dynamic contrast-enhanced magnetic resonance imaging[J]. J Clin Radiol, 2019, 38( 7): 1324- 1329. DOI: 10.13437/j.cnki.jcr.20190731.014.蒋颖, 舒健, 卢欣, 等. 钆塞酸二钠DCE-MRI定量评估慢性肝炎的初步研究[J]. 临床放射学杂志, 2019, 38( 7): 1324- 1329. DOI: 10.13437/j.cnki.jcr.20190731.014. [14] ZHANG JY, LU J, ZHANG XQ, et al. Feasibility and repeatability of Gd-EOB-DTPA enhanced MRI in evaluating hepatic function[J]. J Nantong Univ Med Sci, 2021, 41( 6): 534- 538. DOI: 10.16424/j.cnki.cn32-1807/r.2021.06.013.张继云, 陆健, 张学琴, 等. Gd-EOB-DTPA增强MRI评估肝段肝功能的可行性及可重复性研究[J]. 南通大学学报(医学版), 2021, 41( 6): 534- 538. DOI: 10.16424/j.cnki.cn32-1807/r.2021.06.013. [15] WANG AR, WANG Q. The Current status of gadolinium disodium enhanced MRI in quantitative assessment of liver function[J]. Chin J Oncol Prev Treat, 2021, 13( 1): 94- 99. DOI: 10.3969/j.issn.1674-5671.2021.01.18.王安荣, 王强. 钆塞酸二钠增强MRI在定量肝功能评估的应用现状[J]. 中国癌症防治杂志, 2021, 13( 1): 94- 99. DOI: 10.3969/j.issn.1674-5671.2021.01.18. [16] Foundation for Hepatitis Prevention and Control; Chinese Society of Infectious Disease and Chinese Society of Hepatology, Chinese Medical Association. Consensus on clinical application of transient elastography detecting liver fibrosis: A 2018 update[J]. Chin J Hepatol, 2019, 27( 3): 182- 191. DOI: 10.3760/cma.j.issn.1007-3418.2019.03.004.中国肝炎防治基金会, 中华医学会感染病学分会, 中华医学会肝病学分会和中国研究型医院学会肝病专业委员会, 等. 瞬时弹性成像技术诊断肝纤维化专家共识(2018年更新版)[J]. 中华肝脏病杂志, 2019, 27( 3): 182- 191. DOI: 10.3760/cma.j.issn.1007-3418.2019.03.004. [17] IMAJO K, KESSOKU T, HONDA Y, et al. Magnetic resonance imaging more accurately classifies steatosis and fibrosis in patients with nonalcoholic fatty liver disease than transient elastography[J]. Gastroenterology, 2016, 150( 3): 626- 637.e7. DOI: 10.1053/j.gastro.2015.11.048. [18] LIU YR, WANG M, ZENG G, et al. Diagnostic efficacy of significant liver fibrosis by apparent diffusion coefficient in patients with primary sclerosing cholangitis[J]. J Pract Hepatol, 2021, 24( 3): 375- 378. DOI: 10.3969/j.issn.1672-5069.2021.03.018.刘彦荣, 王苗, 曾果, 等. MRI弥散加权成像对原发性硬化性胆管炎患者肝纤维化评估价值分析[J]. 实用肝脏病杂志, 2021, 24( 3): 375- 378. DOI: 10.3969/j.issn.1672-5069.2021.03.018. -



 PDF下载 ( 1168 KB)
PDF下载 ( 1168 KB)

 下载:
下载: