Clinical effect of vitamin E in treatment of nonalcoholic fatty liver disease in children: A Meta-analysis
-
摘要:
目的评估维生素E对儿童非酒精性脂肪性肝病(NAFLD)的治疗效果。方法检索2019年12月前发表的关于维生素E治疗儿童NAFLD疗效的文章,检索范围包括Pub Med、Web of Science、The Cochran Library、Embase、OVID/NEJM、中国知网、万方数据库。分析数据包含BMI、肝酶水平(ALT、AST)、血脂水平(TG、TC、LDL、HDL)、肝脂肪变性缓解率共8个参数。使用REVMAN5. 3进行Meta分析。连续变量选择标准化均数差(SMD)和95%可信区间(CI)计算和分析干预前后参数的差异,分类变量选择风险差(RD)和95%CI进行分析。非异质性数据采用固定效应模型,否则采用随机效应模型。发表偏倚的评估通过漏斗图完成。结果共检索到599篇文章,其中9篇纳入Meta分析,受试者607例。维生素E显著改善了患儿的ALT水平(SMD=-0. 27,95%CI:-0. 48~-0. 06,P=0. 01),但是对患儿的BMI水平(SMD=-0. 09,95%CI:-0. 28~0. 10,P=0. 34)、AST水平(SMD=-0. 20,95%CI...
-
关键词:
- 非酒精性脂肪性肝病 /
- 维生素E /
- 儿童 /
- Meta分析(主题)
Abstract:Objective To investigate the clinical effect of vitamin E in the treatment of nonalcoholic fatty liver disease( NAFLD) in children. Methods PubMed,Web of Science,The Cochran Library,Embase,OVID/NEJM,CNKI,and Wanfang Data were searched for the articles on vitamin E in the treatment of NAFLD in children published up to December 2019. The data of 8 parameters were analyzed,i. e.,body mass index( BMI),liver enzymes [alanine aminotransferase( ALT) and aspartate aminotransferase( AST) ],blood lipid levels [triglyceride( TG),total cholesterol( TC),low-density lipoprotein( LDL),and high-density lipoprotein( HDL) ],and remission rate of hepatic steatosis. RevMan 5. 3 was used to perform a Meta-analysis. Continuous variables were analyzed by standardized mean difference( SMD) and 95% confidence interval( CI),and the changes after intervention were analyzed; categorical variables were analyzed by risk difference( RD) and 95% CI. A fixed effects model was used for homogeneous data,and a random effects model was used for heterogeneous data. Funnel plots were used to evaluate publication bias. Results A total of 599 articles were retrieved,among which 9 were included in the Meta-analysis,with 607 subjects in total. Vitamin E significantly improved the level of ALT( SMD =-0. 27,95% CI:-0. 48 to-0. 06,P = 0. 01),but it did not improve the levels of BMI( SMD =-0. 09,95% CI:-0. 28 to 0. 10,P = 0. 34),AST( SMD =-0. 20,95% CI:-0. 42 to 0. 02,P = 0. 07),TG( SMD =-0. 19,95% CI:-0. 51 to 0. 12,P = 0. 22),TCHO( SMD =-0. 11,95% CI:-0. 31 to 0. 08,P = 0. 24),HDL( SMD =-0. 02,95% CI:-0. 27 to 0. 23,P = 0. 88),LDL( SMD =-0. 04,95% CI:-0. 27 to0. 19,P = 0. 72),and the remission rate of hepatic steatosis( RD = 0. 06,95% CI:-0. 05 to 0. 17,P = 0. 29). Conclusion Vitamin E can significantly improve the level of ALT in children with NAFLD and can be considered as an adjuvant drug for clinical treatment.
-
Key words:
- non-alcoholic fatty liver disease /
- vitamin E /
- child /
- Meta-analysis as topic
-
自身免疫性肝炎(AIH)以T淋巴细胞介导的自身免疫反应为特征,常发生于遗传易感个体,最终可能引起肝纤维化、肝硬化、肝细胞癌,甚至死亡。肝纤维化在组织学上是可逆的,而肝硬化逆转较为困难。17.7%的AIH患者发现时已形成肝硬化[1]。AIH患者的癌变风险虽低于病毒性肝炎,但其进展为肝硬化时发生肝细胞癌的风险增加了29.18倍,有3.3%~5.1%的AIH肝硬化患者发生肝细胞癌[2-3]。肝纤维化是所有慢性肝病向肝硬化演变的关键病理过程,如何早期识别AIH患者肝纤维化、防治肝硬化发生具有重要的临床价值。
目前认为,肝活组织检查(简称肝活检)是判断肝纤维化分期的金标准,但肝活检为有创操作,疼痛、出血和穿孔等潜在并发症限制了其应用。此外,肝纤维化分布不均匀、肝活检取样误差,均可能引起错误的病理纤维化分期。2019年美国肝病学会意见[4]指出确诊AIH时必须有病理结果,但肝纤维化病理评分为半定量评分系统,无法灵敏的发现肝纤维化程度的细微变化,随访价值有限,非侵入性肝纤维化诊断在随访中更有意义。实时剪切波弹性成像(shear wave elastography,SWE)以二维形式结合传统超声成像从而无创、定量、实时显示肝脏弹性值(杨氏模量测值),其值越大表示肝脏硬度越大,已被广泛应用于评估病毒性肝炎引起的肝纤维化或肝硬化[5]。本研究旨在探究SWE对AIH患者肝纤维化分期的诊断效能,扩大SWE评估不同病因肝纤维化的适用范围,为临床医师评估患者病情提供依据。
1. 资料与方法
1.1 研究对象
选取2013年1月—2022年4月就诊于本院的AIH患者75例。纳入标准:(1)符合2008年IAIHG提出的AIH简化诊断标准[6],评分≥7分,确诊AIH;评分为6分者,采用1999年提出的AIH综合诊断积分系统进一步评估,治疗前≥16分,治疗后≥18分诊断为AIH[7]。(2)均行超声引导下肝活检,活检标本长度≥15 mm。(3)年龄>18岁。排除标准:(1)合并病毒性肝炎、脂肪肝、酒精肝、其他自身免疫性肝病和恶性肿瘤等。(2)病史资料不全者。(3)肝移植患者。根据SWE与病理肝纤维化分期结果是否相符,将AIH患者分为准确组和不准确组。
1.2 研究方法
1.2.1 一般资料
收集患者年龄、性别及在肝活检前1周测得的血常规、血生化和自身免疫性肝病抗体等实验室指标。
1.2.2 肝组织病理学检查
彩超引导下经皮肝穿刺获取肝组织,所有肝组织标本均由经验丰富且对SWE结果不知情的病理医师进行病理学诊断。肝组织纤维化分期依据Scheuer评分系统。S0~1表示无显著纤维化;显著纤维化为S2~4(≥S2);进展期肝纤维化为S3~4(≥S3),肝硬化(包含失代偿期肝硬化)为S4。
1.2.3 SWE检测
使用法国Supersonic Imagine公司生产AixPlorer全数字化彩色多普勒超声波诊断仪,设置凸阵探头,频率为1~6 MHz,模式为SWE模式。患者需禁食6 h以上,嘱患者屏息,取仰卧位,右臂伸展至头部上方。将直径约2 cm的感兴趣区(ROI)放置垂直于肝包膜下方约1~2 cm的右侧肝实质区域,避开肝脏大血管、胆管和肋骨影,获取ROI内肝脏杨氏模量测值(最大值、最小值和平均值)。重复测量5次取平均值(kPa)。本研究中的杨氏模量测值均取自肝活检前后1个月内。
1.3 统计学方法
应用SPSS 26.0及MedCalc软件进行统计学分析。正态分布的计量资料以x±s表示,两组间比较采用独立样本t检验;非正态分布的计量资料以M(P25~P75)表示,两组间比较采用Mann-Whitney U检验,多组间比较采用Kruskal-Wallis H检验,进一步两两比较采用Bonferroni法校正P值。相关性采用Spearman相关分析。绘制ROC曲线,计算其曲线下面积(AUC)。应用Logistic回归分析探究影响诊断准确性的因素。P<0.05为差异有统计学意义。
2. 结果
2.1 一般情况
共纳入AIH患者75例,男性6例,女性69例,平均年龄(51.96±14.19)岁。抗核抗体阳性患者73例,抗平滑肌抗体阳性20例,抗肝肾微粒体1型抗体阳性1例,抗可溶性肝抗原抗体阳性5例。肝组织病理分期结果:S0期6例,S1期18例,S2期22例,S3期16例,S4期13例。不同级别肝纤维化患者的年龄、PLT、ALT、AST、TBil、ALP、GGT比较,差异均无统计学意义(P值均>0.05)(表 1)。
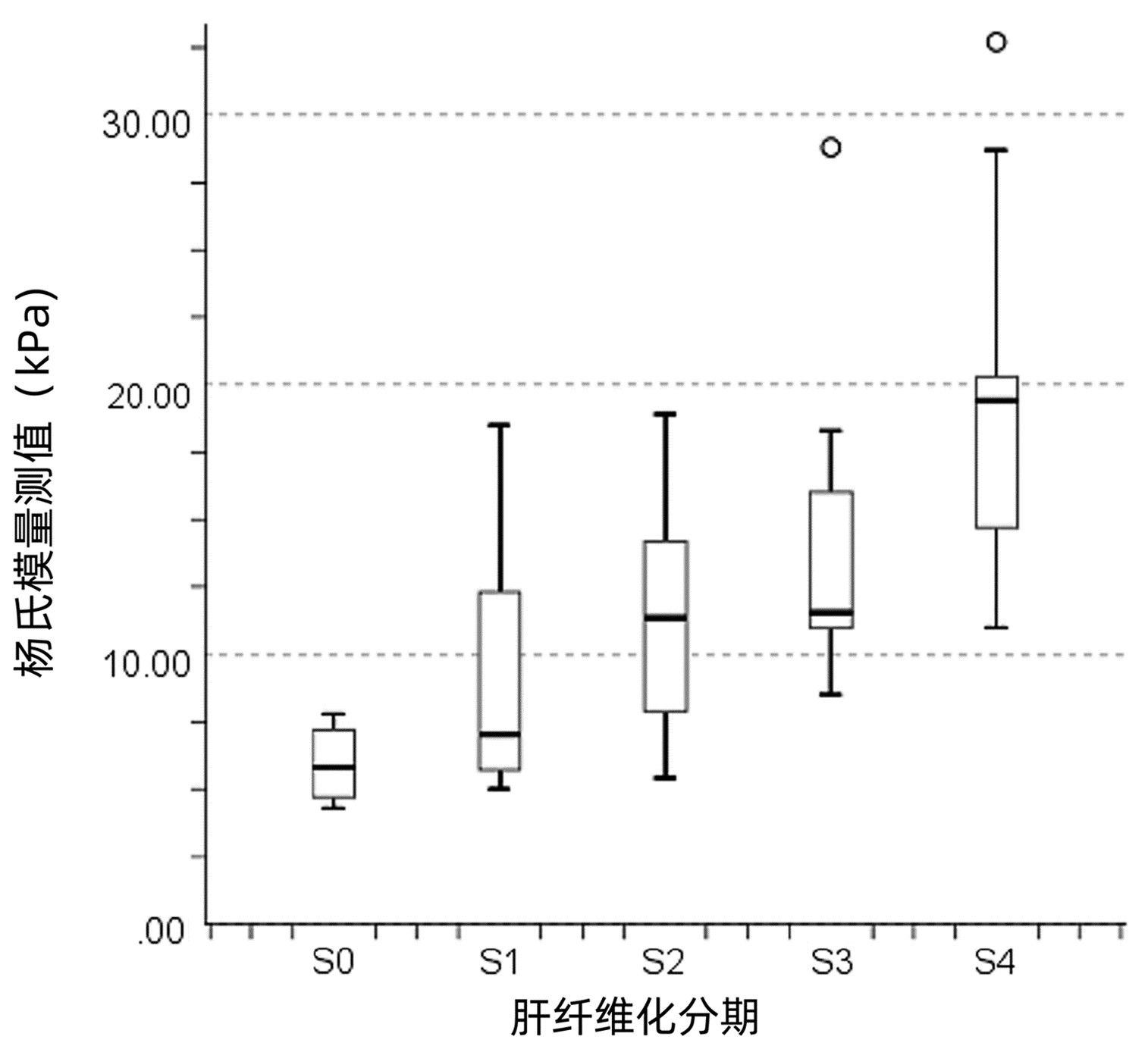
表 1 不同肝纤维化分期患者基线资料及杨氏模量测值比较Table 1. Comparison of baseline data and Young's modulus measurements between different liver fibrosis stages指标 S0期(n=6) S1期(n=18) S2期(n=22) S3期(n=16) S4期(n=13) H值 P值 年龄(岁) 48.00(26.50~61.00) 50.50(43.25~55.75) 48.50(38.25~62.50) 58.00(50.75~62.00) 56.00(51.00~71.00) 7.470 0.113 ALT(U/L) 28.00(6.50~45.25) 61.00(21.00~126.75) 89.00(33.75~236.75) 96.50(31.50~370.75) 75.00(30.50~167.00) 6.552 0.162 AST(U/L) 19.50(15.75~59.75) 94.00(23.50~178.25) 100.00(55.00~220.00) 188.50(36.50~478.50) 82.00(51.00~155.50) 8.656 0.070 ALP(U/L) 76.00(52.75~122.25) 100.50(57.25~177.00) 118.50(76.00~154.75) 129.50(85.75~199.00) 90.00(68.00~114.00) 6.875 0.143 GGT(U/L) 16.00(10.00~103.25) 75.50(20.75~262.00) 95.5(49.75~167.00) 127.00(58.00~162.00) 67.00(42.50~94.50) 7.600 0.107 TBil(μmol/L) 7.95(4.65~24.67) 16.15(7.25~38.83) 15.85(9.80~42.35) 15.78(12.08~33.68) 38.00(16.18~48.31) 7.889 0.096 PLT(×109/L) 185.50(152.00~251.75) 180.50(114.75~229.75) 119.50(90.00~189.75) 157.00(107.50~186.75) 115.00(71.50~160.00) 8.876 0.064 杨氏模量测值(kPa) 5.80(4.60~7.35)1)2) 7.01(5.68~12.33)2) 11.35(7.88~14.28)2) 11.55(11.00~16.60) 19.40(14.60~21.00) 35.186 <0.001 注: 1)与S3期比较,P<0.01;2)与S4期比较,P<0.01。 2.2 各肝纤维化分期杨氏模量测值比较
各肝纤维化分期患者杨氏模量测值比较,差异有统计学意义(P<0.001),进一步两两比较显示,S3期杨氏模量测值显著高于S0期,差异有统计学意义(P=0.009),S4期杨氏模量测值显著高于S0、S1和S2期,差异均有统计学意义(P值分别为<0.001、<0.001及0.009)(表 1)。
2.3 杨氏模量测值及血清指标学与肝纤维化分期相关性分析
杨氏模量测值、TBil和年龄与肝纤维化分期呈正相关(r值分别为0.675、0.285和0.290,P值均<0.05)。随着疾病进展,杨氏模量测值增大(图 1)。PLT与肝纤维化分期呈负相关(r=-0.289,P=0.012)。ALT、AST、ALP和GGT与肝纤维化分期均无相关性(r值分别为0.169、0.191、0.050和0.097,P值均>0.05)。
2.4 SWE判断肝纤维化分期的ROC曲线
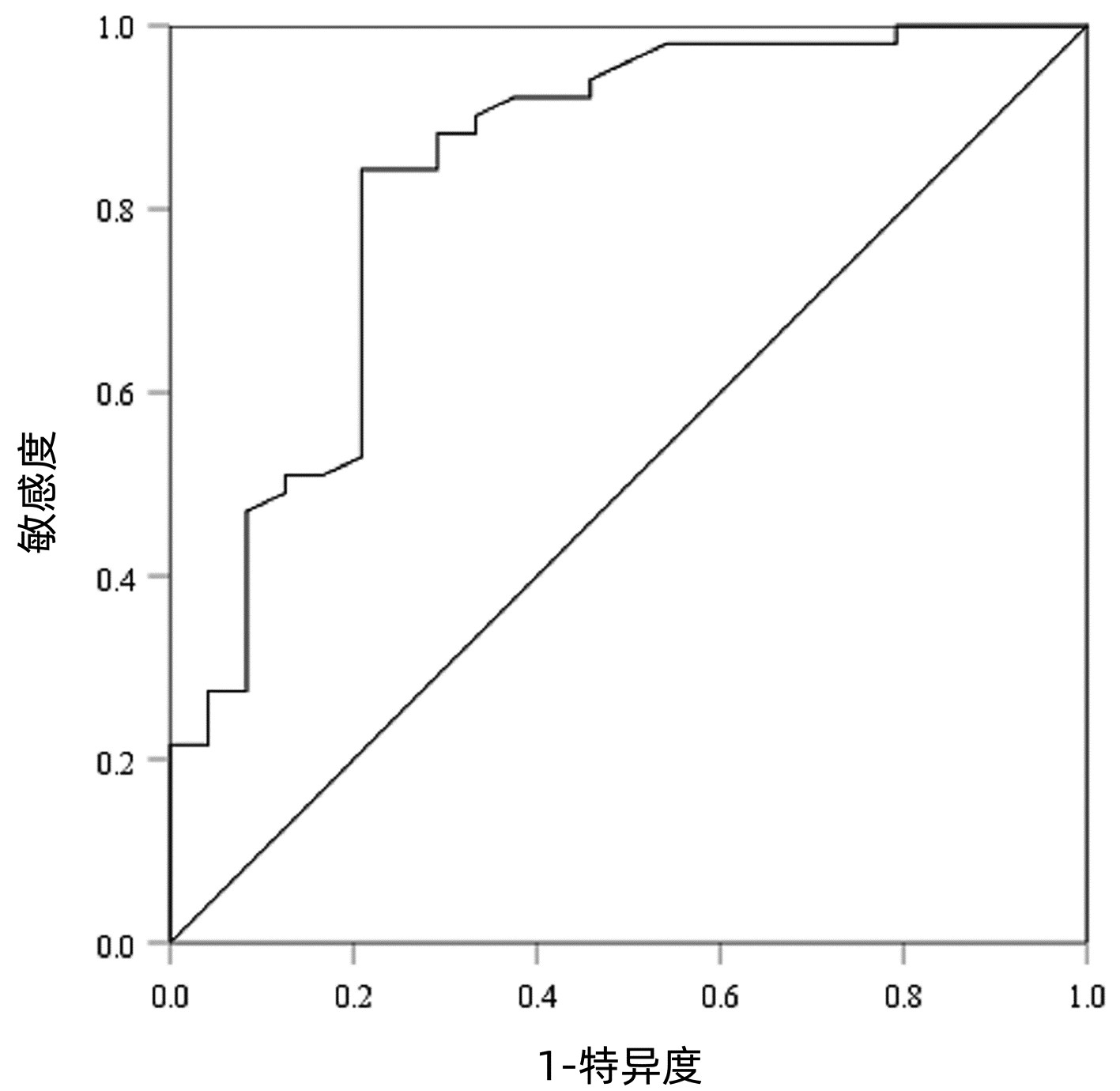
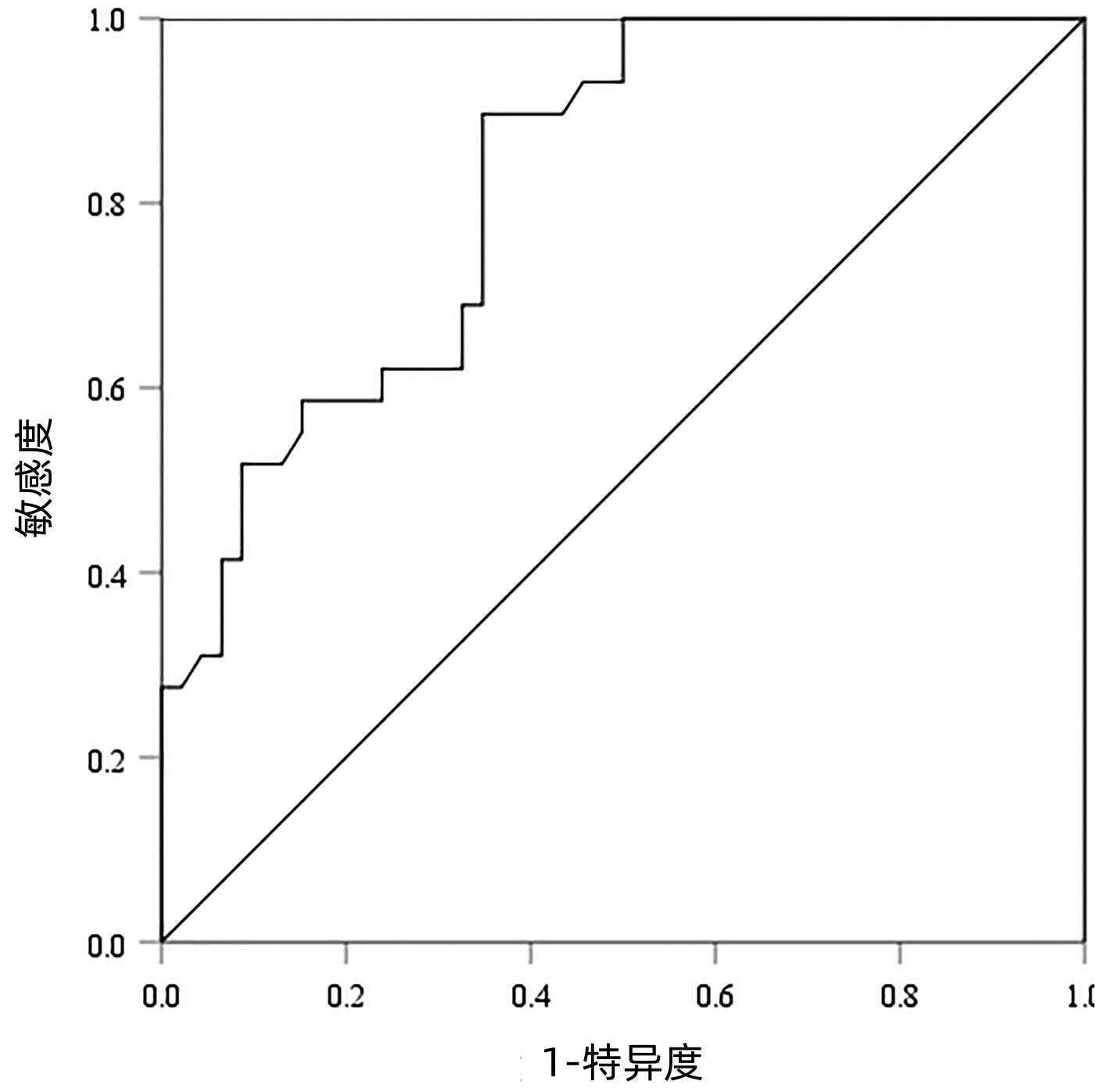
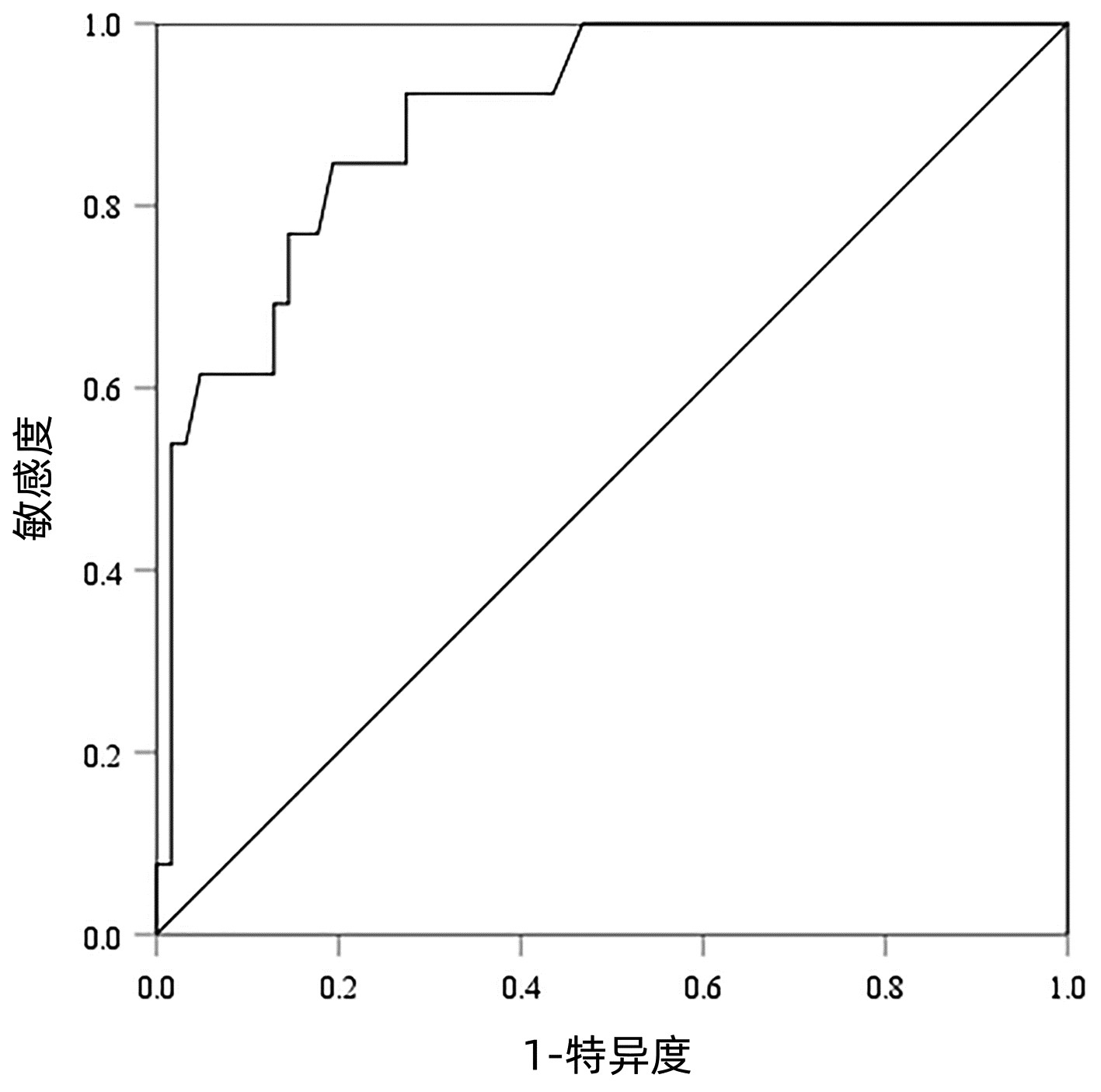
以病理检查结果为标准,分别绘制SWE诊断不同肝纤维化分期AIH患者的ROC曲线并计算其AUC、最佳截断值、灵敏度、特异度、约登指数、阳性似然比、阴性似然比等(表 2,图 2~4)。结果显示,SWE诊断≥S2、≥S3和S4的AUC分别为0.839、0.820和0.898,最佳截断值分别为9.2、10.9和14.4 kPa,灵敏度均较高,分别为84.31%、89.66%和84.62%,但诊断≥S3的特异度较低(65.22%)。
表 2 SWE判断肝纤维化分期的ROC曲线结果Table 2. The ROC curve results in determining liver fibrosis stage分期 最佳截断值(kPa) AUC 95%CI 灵敏度(%) 特异度(%) 约登指数 阳性似然比 阴性似然比 P值 ≥S2 9.2 0.839 0.736~0.913 84.31 79.17 0.634 8 4.05 0.20 <0.001 ≥S3 10.9 0.820 0.715~0.900 89.66 65.22 0.548 7 2.58 0.16 <0.001 S4 14.4 0.898 0.807~0.956 84.62 80.65 0.652 6 4.37 0.19 <0.001 2.5 SWE测得的杨氏模量测值与Scheuer评分的符合率
SWE正确分类了75例患者中的43例(57.33%),其中S0~1和S2的符合率最高,分别为70.37%和83.33%,S3和S4的符合率均<50%(表 3)。
表 3 肝脏杨氏模量测值与Scheuer评分系统对肝纤维化分期的符合率Table 3. The concordance rate of Young's modulus measurements vs Scheuer scoring system分期(杨氏模量测值) Scheuer评分(例) 合计(例) 符合率(%) S0~1期 S2期 S3期 S4期 S0~1期(≤9.2 kPa) 19 6 2 0 27 70.37 S2期(>9.2且≤10.9 kPa) 0 5 1 0 6 83.33 S3期(>10.9且≤14.4 kPa) 3 6 8 2 19 42.11 S4期(>14.4 kPa) 2 5 5 11 23 47.82 注:总体符合率为57.33%。 2.6 SWE诊断肝纤维化分期准确率的影响因素
SWE诊断肝纤维化分期准确组与不准确组之间AST、TBil、ALP和GGT差异均有统计学意义(P值均<0.05),两组间ALT和PLT差异均无统计学意义(P值均>0.05)(表 4)。多因素二元Logistic回归分析结果显示,ALP为SWE诊断肝纤维化分期准确率的独立影响因素(OR=1.009,95%CI:1.001~1.018,P=0.029)。进一步分析发现,ALP仅在两组S0~1期患者间差异有统计学意义(Z=-2.452,P=0.014)。
表 4 影响SWE诊断肝纤维化分期准确性的单因素分析Table 4. Univariate analysis of factors associated with the disagreement between SWE and liver fibrosis stage指标 准确组(n=43) 不准确组(n=32) 统计值 P值 ALT(U/L) 45.00(23.00~186.00) 104.50(36.50~205.50) Z=-1.736 0.083 AST(U/L) 62.00(22.00~144.00) 118.50(55.00~359.75) Z=-2.464 0.014 ALP(U/L) 92.00(67.00~138.00) 124.50(79.50~162.50) Z=-2.180 0.029 GGT(U/L) 65.00(22.00~129.00) 114.00(50.75~197.25) Z=-2.389 0.017 TBil(μmol/L) 15.50(7.80~32.70) 26.15(13.85~69.88) Z=-2.630 0.009 PLT(×109/L) 159.91±63.11 142.34±79.64 t=1.065 0.290 3. 讨论
AIH的诊断复杂,其诊断常借助评分系统。2021年亚太肝病学会[8]指出,在亚太地区,AIH简化诊断积分系统与综合诊断积分系统相比具有更高的敏感性和特异性,但容易漏诊部分不典型患者。我国《自身免疫性肝炎诊断和治疗指南(2021)》[9]推荐对于疑似AIH且采用简化诊断积分系统不能确诊的患者,再以综合诊断积分系统进行评估以免漏诊。肝硬度在AIH患者启动治疗时机选择和评价预后方面均有重要意义。肝活检是判断肝硬度的金标准,但慢性肝病患者常合并血小板低下、凝血功能异常和腹水等并发症,无法满足肝活检要求,此时肝纤维化无创诊断显得尤为重要。
目前广泛应用于临床的无创诊断手段包括超声弹性成像技术、血清学指标和磁共振弹性成像(MRE)等[10-11]。瞬时弹性成像(TE)、声脉冲辐射力成像(ARFI)和SWE为常用的3种超声弹性成像技术。TE对诊断中重度肝纤维化具有较高的准确性,但对腹腔积液及肥胖病人测量效果不佳。ARFI主要是指点剪切波弹性成像(pSWE),既往研究[12]显示pSWE对慢性肝病显著及进展期肝纤维化的诊断能力低于SWE。SWE将传统超声成像与实时可视化剪切波超声结合,可以精确定位ROI,具有较好的重复性及稳定性[13-14]。此外,SWE不受腹腔积液、肋间隙限制,常规超声仪器就能完成操作,可在基层及医疗资源有限地区应用。一项纳入1134例慢性肝病患者的Meta分析[15]显示,在判定显著肝纤维化及肝硬化方面,SWE比TE的诊断价值更高。与血清学指标和MRE相比,SWE也表现出优秀的诊断效能。对非酒精性脂肪肝病患者肝纤维化的Meta分析[16]结果显示,SWE诊断≥S2、≥S3和S4的灵敏度和特异度远高于APRI、FIB-4等血清学指标,其灵敏度略高于MRE。
本研究发现,杨氏模量测值在AIH患者不同纤维化分期中存在显著性差异,可用于区分不同程度肝纤维化。其数值随纤维化分期的升高而升高,与纤维化分期呈较强的正相关性(r=0.675),这与多数临床研究一致(r值为0.45~0.80)[15]。一方面,SWE对不同肝纤维化分期的诊断价值不同,本研究中SWE诊断≥S2、≥S3和S4的AUC均>0.80,但对S4诊断价值最高,与Xing等[17]的研究结果基本相同。另一方面,SWE评估不同病因肝纤维化的最佳截断值不同,可能需要不同的标准化截断值。本研究中,AIH患者≥S2、≥S3和S4对应的最佳截断值分别为9.2、10.9和14.4 kPa。既往研究[18-19]中,CHB和原发性胆汁性胆管炎患者对应的最佳截断值分别为7.0、8.1、10.0 kPa和10.7、12.2和14.1 kPa。
本研究中,SWE诊断肝纤维化分期与病理分期的总体符合率(57.33%)与Zeng等[20]的研究(53.5%)相近,但均较低,且S2和S3的杨氏模量测值中位数无显著差异,这可能与本研究样本量小有关,尚需扩大样本量进一步探究其诊断价值。本研究发现,ALP是影响S0~1期AIH患者SWE测量结果准确性的独立影响因素,对于ALP异常的患者可适当提高肝纤维化的诊断阈值,或待ALP恢复正常后再行SWE检查。由于临床普遍关注患者是否存在显著纤维化(≥S2),且本研究中纳入的S0期患者较少,为减少统计学误差,本研究将S0~1期合并为无显著纤维化组,仅分析SWE对≥S2、≥S3和S4的AIH患者的诊断价值。
国内鲜有SWE评价AIH患者肝纤维化的报道,本研究排除其他自身免疫性肝病,单独评估≥S2、≥S3和S4的AIH患者,结果更加准确和全面。但本研究存在一定的局限性:(1)本研究中S4期患者数量偏少,未进一步区分代偿期及失代偿期肝硬化,后续的研究中应扩大样本量对其进行细分;(2)肝活检标本由经验丰富的不同病理医师行病理学诊断,可能存在观察者间及观察者内差异。
本研究发现SWE能够有效判断AIH患者显著纤维化、进展期肝纤维化及肝硬化,可用于动态监测肝纤维化程度变化,拓宽了AIH患者肝纤维化无创诊断的思路,有望减少肝活检次数。但SWE测定的肝脏杨氏模量值与病理纤维化分期的总体符合率较低,尚需扩大样本量进一步探究其对AIH的应用价值。
-
[1]COBBINA E,AKHLAGHI F.Non-alcoholic fatty liver disease(NAFLD)-pathogenesis,classification,and effect on drug metabolizing enzymes and transporters[J].Drug Metab Rev,2017,49(2):197-211. [2]MENCIN AA,LAVINE JE.Nonalcoholic fatty liver disease in children[J].Curr Opin Clin Nutr Metab Care,2011,14(2):151-157. [3]BYRNE CD,TARGHER G.NAFLD:A multisystem disease[J].JHepatol,2015,62(1 Suppl):s47-s64. [4]RINELLA ME,SANYAL AJ.Management of NAFLD:A stage-based approach[J].Nat Rev Gastroenterol Hepatol,2016,13(4):196-205. [5]HARDWICK RN,FISHER CD,CANET MJ,et al.Diversity in antioxidant response enzymes in progressive stages of human nonalcoholic fatty liver disease[J].Drug Metab Dispos,2010,38(12):2293-2301. [6]PIRGON,BILGIN H,EKMEZ F,et al.Association between insulin resistance and oxidative stress parameters in obese adolescents with non-alcoholic fatty liver disease[J].J Clin Res Pediatr Endocrinol,2013,5(1):33-39. [7]DU J,MA YY,YU CH,et al.Effects of pentoxifylline on nonalcoholic fatty liver disease:A Meta-analysis[J].World J Gastroenterol,2014,20(2):569-577. [8]HIGGINS JP,THOMPSON SG,DEEKS JJ,et al.Measuring inconsistency in meta-analyses[J].BMJ,2003,327(7414):557-560. [9]VAJRO P,MANDATO C,FRANZESE A,et al.Vitamin E treatment in pediatric obesity-related liver disease:A randomized study[J].J Pediatr Gastroenterol Nutr,2004,38(1):48-55. [10]NOBILI V,MANCO M,DEVITO R,et al.Effect of vitamin E on aminotransferase levels and insulin resistance in children with nonalcoholic fatty liver disease[J].Aliment Pharmacol Ther,2006,24(11-12):1553-1561. [11]NOBILI V,MANCO M,DEVITO R,et al.Lifestyle intervention and antioxidant therapy in children with nonalcoholic fatty liver disease:Arandomized,controlled trial[J].Hepatology,2008,48(1):119-128. [12]WANG CL,LIANG L,FU JF,et al.Effect of lifestyle intervention on non-alcoholic fatty liver disease in Chinese obese children[J].World J Gastroenterol,2008,14(10):1598-1602. [13]LAVINE JE,SCHWIMMER JB,van NATTA ML,et al.Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents:The TONIC randomized controlled trial[J].JAMA,2011,305(16):1659-1668. [14]D'ADAMO E,MARCOVECCHIO ML,GIANNINI C,et al.Improved oxidative stress and cardio-metabolic status in obese prepubertal children with liver steatosis treated with lifestyle combined with Vitamin E[J].Free Radic Res,2013,47(3):146-153. [15]GHERGHEREHCHI R,HAZHIR N,MOSTAFA GHAREBAGHI M.Lifestyle intervention and vitamin E therapy in obese children with nonalcoholic fatty liver disease[J].J Compr Ped,2013,4(1):62-65. [16]MURER SB,AEBERLI I,BRAEGGER CP,et al.Antioxidant supplements reduced oxidative stress and stabilized liver function tests but did not reduce inflammation in a randomized controlled trial in obese children and adolescents[J].J Nutr,2014,144(2):193-201. [17]NOBILI V,ALISI A,MOSCA A,et al.The antioxidant effects of hydroxytyrosol and vitamin E on pediatric nonalcoholic fatty liver disease,in a clinical trial:A new treatment[J].Antioxid Redox Signal,2019,31(2):127-133. [18]HASHIMOTO E,TOKUSHIGE K,LUDWIG J.Diagnosis and classification of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis:Current concepts and remaining challenges[J].Hepatol Res,2015,45(1):20-28. [19]TILG H,MOSCHEN AR.Evolution of inflammation in nonalcoholic fatty liver disease:The multiple parallel hits hypothesis[J].Hepatology,2010,52(5):1836-1846. [20]DAY CP.Pathogenesis of steatohepatitis[J].Best Pract Res Clin Gastroenterol,2002,16(5):663-678. [21]LEVENE AP,GOLDIN RD.The epidemiology,pathogenesis and histopathology of fatty liver disease[J].Histopathology,2012,61(2):141-152. [22]SPAHIS S,DELVIN E,BORYS JM,et al.Oxidative stress as a critical factor in nonalcoholic fatty liver disease pathogenesis[J].Antioxid Redox Signal,2017,26(10):519-541. [23]HICKMAN I,MACDONALD G.Is vitamin E beneficial in chronic liver disease?[J].Hepatology,2007,46(2):288-290. [24]MENG XY,LIU J,WANG L,et al.Effects of ascorbic acid-polyethyleneimine carbon dots on proliferation,apoptosis and oxidative stress of MG63 cells by Golgi stress[J].J Jilin Univ(Med Edit),2019,45(6):1218-1223,1480.(in Chinese)孟许亚,刘杰,王璐,等.抗坏血酸-聚乙烯亚胺复合碳点通过高尔基体应激对MG63细胞增殖、凋亡和氧化应激的影响[J].吉林大学学报(医学版),2019,45(6):1218-1223,1480. [25]CHALASANI N,DEEG MA,CRABB DW.Systemic levels of lipid peroxidation and its metabolic and dietary correlates in patients with nonalcoholic steatohepatitis[J].Am J Gastroenterol,2004,99(8):1497-1502. [26]SEKI S,KITADA T,YAMADA T,et al.In situ detection of lipid peroxidation and oxidative DNA damage in non-alcoholic fatty liver diseases[J].J Hepatol,2002,37(1):56-62. [27]SARKHY AA,AL-HUSSAINI AA,NOBILI V.Does vitamin E improve the outcomes of pediatric nonalcoholic fatty liver disease?A systematic review and meta-analysis[J].Saudi J Gastroenterol,2014,20(3):143-153. [28]SATO K,GOSHO M,YAMAMOTO T,et al.Vitamin E has a beneficial effect on nonalcoholic fatty liver disease:A meta-analysis of randomized controlled trials[J].Nutrition,2015,31(7-8):923-930. [29]PHUNG N,PERA N,FARRELL G,et al.Pro-oxidant-mediated hepatic fibrosis and effects of antioxidant intervention in murine dietary steatohepatitis[J].Int J Mol Med,2009,24(2):171-180. [30]MANCO M,ALISI A,NOBILI V.Risk of severe liver disease in NAFLD with normal ALT levels:A pediatric report[J].Hepatology,2008,48(6):2087-2088. [31]WIECKOWSKA A,MCCULLOUGH AJ,FELDSTEIN AE.Noninvasive diagnosis and monitoring of nonalcoholic steatohepatitis:Present and future[J].Hepatology,2007,46(2):582-589. [32]SAKR HF,ABBAS AM,HAIDARA MA.Swimming,but not vitamin E,ameliorates prothrombotic state and hypofibrinolysis in a rat model of nonalcoholic fatty liver disease[J].J Basic Clin Physiol Pharmacol,2018,29(1):61-71. [33]KIM GH,CHUNG JW,LEE JH,et al.Effect of vitamin E in nonalcoholic fatty liver disease with metabolic syndrome:A propensity score-matched cohort study[J].Clin Mol Hepatol,2015,21(4):379-386. [34]SANYAL AJ,CHALASANI N,KOWDLEY KV,et al.Pioglitazone,vitamin E,or placebo for nonalcoholic steatohepatitis[J].N Engl JMed,2010,362(18):1675-1685. [35]WITTLIN L,LOGOMARSINO JV.Therapeutic effects of vitamin Esupplementation in liver diseases and transplantation[J].J Gastroenterol Hepatol Res,2014,3:1095-1102. 期刊类型引用(0)
其他类型引用(1)
-




 PDF下载 ( 2527 KB)
PDF下载 ( 2527 KB)

 下载:
下载:





 下载:
下载: