慢加急性肝衰竭不同预后患者血浆外泌体差异蛋白的生物信息学分析
DOI: 10.3969/j.issn.1001-5256.2021.04.022
A bioinformatics analysis of differentially expressed proteins in plasma exosome of acute-on-chronic liver failure patients with different prognoses
-
摘要:
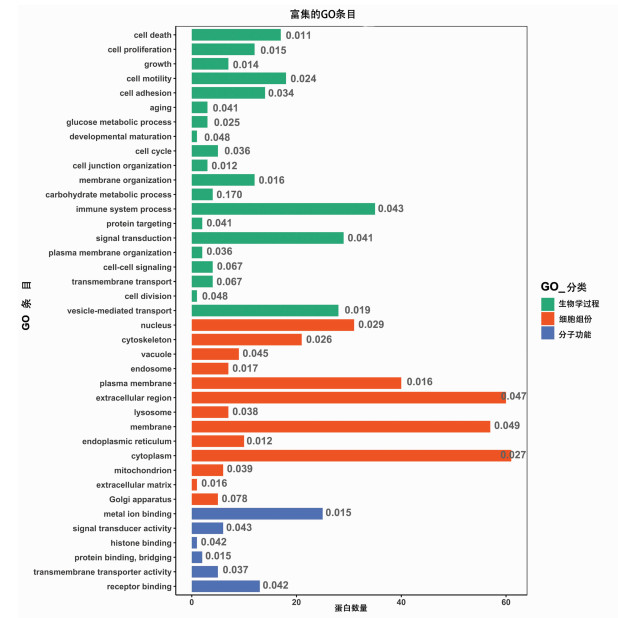
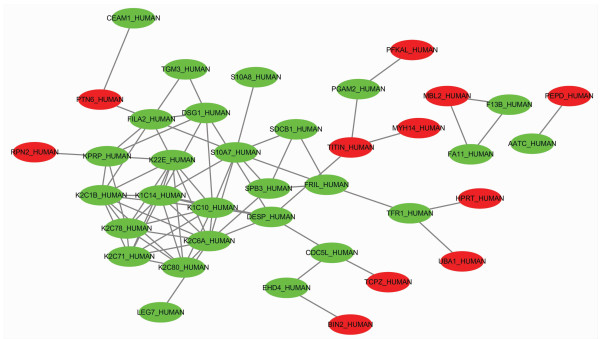
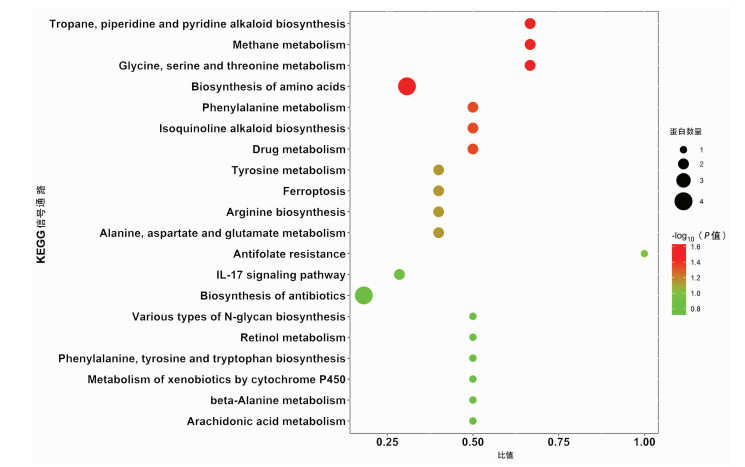
目的 筛选不同预后慢加急性肝衰竭(ACLF)患者血浆外泌体中的差异蛋白, 分析其功能及生物学过程, 为患者临床诊断提供参考依据。 方法 前瞻性选取2019年7月—10月于首都医科大学附属北京佑安医院住院确诊的ACLF患者10例, 随访90 d, 患者死亡或肝移植归入肝移植/死亡组(n=5), 患者存活归入生存组(n=5), 两组一般资料指标比较采用Mann-Whitney U秩和检验。采用非标记(Label-free) 定量蛋白质组学技术对血浆外泌体蛋白进行鉴定和定量分析, 筛选差异蛋白并进行功能富集分析, 使用R-3.5.1软件对差异蛋白进行层次聚类分析, 分析其参与的生物学过程。 结果 外泌体蛋白质组学分析共鉴定出860种蛋白, 以倍数上调>1.2倍或下调>1.2倍且P<0.05的标准筛选出差异表达蛋白116种, 与肝移植/死亡组相比, 生存组上调蛋白62种、下调蛋白54种。生物信息学分析结果显示, 这些蛋白主要参与了免疫反应、信号转导、囊泡介导的转运、细胞死亡和增殖等生物学过程, 并与炎症反应、糖类和氨基酸代谢、肝细胞损伤及再生等信号通路密切相关。 结论 非标记定量蛋白质组学技术筛选出的差异蛋白可能作为ACLF早期诊断及预后判断的血清学标志物。 Abstract:Objective To investigate the differentially expressed proteins in the plasma exosome of acute-on-chronic liver failure (ACLF) patients with different prognoses, to analyze their functions and biological processes, and to provide a basis for clinical diagnosis. Methods A prospective study was performed for 10 ACLF patients who were hospitalized and diagnosed in Beijing YouAn Hospital, Capital Medical University, from July 2019 to October 2019, and the patients were followed up for 90 days. The patients who died or received liver transplantation were enrolled as liver transplantation/death group (5 patients), and the patients who survived were enrolled as survival group (5 patients). The Mann-Whitney U test was used for comparison of general data between the two groups. The label-free quantitative proteomic method was used for identification and quantitative analysis of plasma exosome proteins to screen out differentially expressed proteins, and a functional enrichment analysis was performed. R-3.5.1 software was used to perform a hierarchical cluster analysis of differentially expressed proteins to analyze the biological processes involving these proteins. Results A total of 860 proteins were identified by the exosome proteomic analysis, and according to the criteria of upregulation > 1.2 folds or downregulation > 1.2 folds (P < 0.05), there were 116 differentially expressed proteins. Compared with the liver transplantation/death group, the survival group had 62 upregulated proteins and 54 downregulated proteins. The bioinformatics analysis showed that these differentially expressed proteins mainly participated in immune reaction, signal transduction, vesicle-mediated transport, cell death, and cell proliferation and were closely associated with the signaling pathways including inflammatory response, carbohydrate and amino acid metabolism, hepatocyte injury, and hepatocyte regeneration. Conclusion Differentially expressed proteins screened out by the label-free quantitative proteomic method can be used as serological markers for the early diagnosis and prognostic evaluation of ACLF. -
Key words:
- Acute-On-Chronic Liver Failure /
- Exosomes /
- Proteomics /
- Computational Biology
-
原发性胆汁性胆管炎(PBC)是一种慢性进行性的胆汁淤积性肝病,90%以上的患者血清抗线粒体抗体(AMA)阳性,部分患者血清IgM水平升高,随着病情的缓解而有所下降[1],因此明确IgM水平与PBC发生、发展及转归的关系,对于指导PBC治疗及预后评估具有重要的临床意义。
1. 资料与方法
1.1 研究对象
选取2010年1月—2020年1月于解放军总医院第五医学中心就诊的熊去氧胆酸(UDCA)初治PBC患者637例,所有患者均具有治疗1年以上的临床数据。纳入标准:(1)符合PBC诊断标准[2-3];(2)UDCA规范治疗(13~15 mg/kg)1年以上。排除标准:(1)合并其他的慢性肝病;(2)肝移植术后;(3)合并其他免疫性疾病、恶性肿瘤及感染性疾病;(4)资料不完整。
1.2 研究方法
采用回顾性分析的方法,采集患者性别、年龄及入院基线的血液学指标、生化指标、免疫学指标及影像学指标;并根据巴黎标准进行UDCA疗效判定[4-5],将PBC患者分为UDCA完全应答组和UDCA应答不良组(包括无应答及部分应答)。
1.3 统计学方法
应用SPSS 22.0软件进行统计学分析。计量资料进行S-K正态性检验,符合正态分布的计量资料用x±s表示,两组间比较采用独立样本t检验;非正态分布的计量资料用M(P25~P75)表示,两组间比较采用Mann-Whitney U检验。计数资料两组间比较采用χ2检验。绘制受试者工作特征曲线(ROC曲线),根据ROC曲线下面积(AUC)评估其预测效能,计算约登指数确定最佳临界值。亚组分析使用Cochran-Mantel-Haenszel(CMH)检验,并绘制风险值森林图。P<0.05为差异有统计学意义。
2. 结果
2.1 一般情况
本研究共纳入PBC患者637例,其中男126例,女511例,平均56岁(50~66岁),基线IgM升高者357例(56.0%),UDCA完全应答者436例(68.4%),其他基线资料见表 1。
表 1 PBC患者基线特征及UDCA治疗1年后的应答情况Table 1. Characteristics of patients with PBC at baseline and biochemical response to UDCA after 1 year of treatment基线特征 数值 性别[例(%)] 男 126(19.8) 女 511(80.2) 发病年龄(岁) 56(50~66) 肝硬化[例(%)] 487(76.5) PLT(×109/L) 121(77~182) Alb(g/L) 35(31~38) TBil(μmol/L) 20.7(13.8~38.2) ALT(U/L) 53(27~95) AST(U/L) 69(41~105) ALP(U/L) 247(158~432) GGT(U/L) 163(81~374) TBA(μmol/L) 28(12~65) ChE(U/L) 4405(2922~5947) TC(mmol/L) 4.4(3.3~5.8) PT(s) 11.6(10.5~12.9) INR 1.01(0.94~1.12) IgA(g/L) 2.68(1.84~3.87) IgG(g/L) 15.67(12.83~20.41) IgM(g/L) 2.76(1.61~4.43) IgM>ULN[例(%)] 357(56.0) ANA阳性[例(%)] 469(73.6) 抗Sp100阳性[例(%)] 94(14.8) 抗Gp210阳性[例(%)] 218(34.2) MRS[6] 5.84±1.45 Globe评分[7] 1.21(0.34~2.11) UK-PBC评分[8] 0.037(0.015~0.112) UDCA治疗1年[例(%)] 完全应答 436(68.4) 应答不良 201(31.6) 注:TBA,总胆汁酸;ChE,胆碱酯酶。 2.2 UDCA不同疗效的基线临床资料比较
按照UDCA治疗应答情况,637例患者中436例发生完全应答,201例为应答不良。性别分布中,完全应答组与应答不良组分布无统计学意义(P值均>0.05),基线存在肝硬化的患者UDCA应答率更高(78.9% vs 71.1%,P=0.032),生化指标中,TBil、AST、ALP、TBA和TC在两组间存在统计学差异(P值均<0.001)。免疫指标中,应答不良组IgA、IgM水平及抗Gp210阳性率均较高(P值均<0.05)。预后风险评分中,应答不良组MRS、Globe评分、UK-PBC评分均高于完全应答组(P值均<0.001)(表 2)。
表 2 UDCA治疗完全应答与应答不良患者基线指标及风险评分差异Table 2. Differences of baseline characteristics and risk scores between PBC with complete response and poor response to UDCA treatment指标 UDCA完全应答组(n=436) UDCA应答不良组(n=201) 统计值 P值 性别[例(%)] χ2=3.112 0.078 男 78(17.9) 48(23.9) 女 358(82.1) 153(76.1) 年龄(岁) 57(50~66) 54(49~65) Z=-1.447 0.148 肝硬化[例(%)] 344(78.9) 143(71.1) χ2=4.596 0.032 TBil(μmol/L) 17.5(12.1~26.1) 39.2(20.6~62.7) Z=-9.932 <0.001 AST(U/L) 55(38~87) 99(69~135) Z=-8.931 <0.001 ALP(U/L) 201(150~340) 390(237~599) Z=-8.361 <0.001 TBA(μmol/L) 22(9~43) 54(20~121) Z=-7.836 <0.001 TC(mmol/L) 4.2(3.2~5.3) 5.1(3.5~7.1) Z=-4.694 <0.001 IgA(g/L) 2.55(1.75~3.61) 3.01(2.01~4.38) Z=-3.242 0.001 IgG(g/L) 15.59(12.63~20.50) 15.73(12.97~20.16) Z=-0.227 0.821 IgM(g/L) 2.60(1.56~4.30) 3.33(1.78~5.02) Z=-2.115 0.034 ANA阳性[例(%)] 313(71.8) 156(77.6) χ2=2.402 0.121 抗Sp100阳性[例(%)] 66(15.1) 28(13.9) χ2=0.159 0.690 抗Gp210阳性[例(%)] 127(29.1) 91(45.3) χ2=15.931 <0.001 MRS 5.68±1.45 6.18±1.41 t=4.092 <0.001 Globe评分 0.83(0.14~1.66) 2.09(1.29~2.81) Z=-10.910 <0.001 UK-PBC评分 0.025(0.012~0.058) 0.128(0.050~0.324) Z=-11.646 <0.001 2.3 不同IgM水平PBC患者临床特征及UDCA疗效比较
2.3.1 IgM升高与IgM正常PBC患者基线特征及治疗应答差异
按照IgM升高与IgM正常将PBC患者分为两组,分析患者基线特征及治疗应答。性别分布、年龄、肝硬化占比在两组中比较差异均无统计学意义(P值均>0.05);生化指标中,AST、ALP、TC在IgM升高组显著高于IgM正常组(P值均<0.001);免疫指标中,IgA、IgG水平及抗Gp210阳性率在IgM升高组显著高于IgM正常组(P值均<0.05),但两组在UDCA治疗应答方面并无统计学差异(P>0.05)(表 3)。
表 3 IgM升高与IgM正常的PBC患者基线特征及治疗应答差异Table 3. Differences of baseline characteristics and biochemical response to UDCA treatment between IgM-normal and IgM-elevated PBC指标 IgM升高组(n=357) IgM正常组(n=280) 统计值 P值 性别[例(%)] χ2=0.525 0.469 男 67(18.8) 59(21.1) 女 290(81.2) 221(78.9) 年龄(岁) 55(49~65) 57(50~67) Z=-1.776 0.076 肝硬化[例(%)] 275(77.0) 212(75.7) χ2=0.151 0.698 TBil(μmol/L) 20.4(14.0~38.2) 21.1(13.6~38.6) Z=-0.066 0.947 AST(U/L) 75(45~106) 55(35~100) Z=-3.774 <0.001 ALP(U/L) 275(173~478) 201(144~347) Z=-5.063 <0.001 TBA(μmol/L) 29(13~60) 28(11~70) Z=-0.587 0.558 TC(mmol/L) 4.8(3.6~6.2) 4.2(3.1~5.3) Z=-4.344 <0.001 IgA(g/L) 2.83(1.96~3.91) 2.53(1.70~3.82) Z=-2.051 0.040 IgG(g/L) 16.88(14.00~21.42) 14.04(11.87~18.50) Z=-6.144 <0.001 ANA阳性[例(%)] 270(75.6) 199(71.1) χ2=1.680 0.195 抗Sp100阳性[例(%)] 56(15.7) 38(13.6) χ2=0.558 0.455 抗Gp210阳性[例(%)] 152(42.6) 66(23.6) χ2=25.180 <0.001 MRS 5.78±1.46 5.91±1.43 t=-1.071 0.284 Globe评分 1.18(0.29~2.12) 1.27(0.36~2.07) Z=-0.433 0.665 UK-PBC评分 0.038(0.015~0.114) 0.037(0.015~0.108) Z=-0.416 0.677 UDCA治疗1年[例(%)] χ2=2.580 0.108 完全应答 235(65.8) 201(71.8) 应答不良 122(34.2) 79(28.2) 2.3.2 应用ROC曲线确定IgM最佳临界值
将各项基线指标进行ROC曲线分析(图 1,表 4),结果显示预测UDCA治疗1年后应答不良的生化指标包括TBil、AST、ALP、TBA、TC,免疫指标包括IgA、IgM、IgG,计算IgM最佳临界值为1.5×ULN。
表 4 PBC患者基线指标预测UDCA治疗1年后应答不良的AUCTable 4. AUC values of characteristics at baseline in predicting poor biochemical response to UDCA after 1 year of treatment指标 AUC 95%CI TBil 0.745 0.709~0.778 AST 0.720 0.683~0.755 ALP 0.706 0.669~0.741 TBA 0.693 0.655~0.728 TC 0.615 0.576~0.653 IgA 0.576 0.537~0.615 IgM 0.552 0.512~0.591 IgG 0.505 0.466~0.545 2.3.3 分析不同IgM水平PBC患者基线特征及治疗应答差异
根据IgM预测UDCA应答不良的最佳临界值将患者分为IgM≥1.5×ULN及IgM<1.5×ULN两组,性别分布、年龄、肝硬化占比在两组中比较差异均无统计学意义(P值均>0.05);IgM≥1.5×ULN组AST、ALP、TC显著高于IgM<1.5×ULN组(P值均<0.05);IgM≥1.5×ULN组IgG水平及抗Gp210阳性率显著高于IgM<1.5×ULN组(P值均<0.001)。IgM≥1.5×ULN组经过UDCA治疗后发生应答不良患者显著高于IgM<1.5×ULN组(38.3% vs 27.1%,P=0.003)(表 5)。
表 5 不同IgM水平PBC患者基线临床特征及UDCA疗效比较Table 5. Comparison of baseline characteristics and the outcome of UDCA treatment between PBC with different levels of IgM指标 IgM≥1.5×ULN组(n=253) IgM<1.5×ULN组(n=384) 统计值 P值 性别[例(%)] χ2=0.038 0.846 男 51(20.2) 75(19.5) 女 202(79.8) 309(80.5) 年龄(岁) 56(49~65) 56(50~66) Z=-1.077 0.281 肝硬化[例(%)] 195(77.1) 292(76.0) χ2=0.090 0.764 TBil(μmol/L) 21.0(14.3~38.0) 20.5(13.6~39.7) Z=-0.244 0.808 AST(U/L) 78(46~112) 61(38~99) Z=-4.193 <0.001 ALP(U/L) 288(179~492) 210(150~358) Z=-5.044 <0.001 TBA(μmol/L) 28(13~57) 28(10~70) Z=-0.210 0.834 TC(mmol/L) 4.8(3.6~6.3) 4.3(3.2~5.5) Z=-3.250 0.001 IgA(g/L) 2.81(1.90~3.85) 2.63(1.76~3.87) Z=-1.021 0.307 IgG(g/L) 17.60(14.09~22.10) 14.89(12.15~19.32) Z=-5.465 <0.001 ANA阳性[例(%)] 192(75.9) 277(72.1) χ2=1.107 0.293 抗Sp100阳性[例(%)] 44(17.4) 50(13.0) χ2=2.316 0.128 抗Gp210阳性[例(%)] 116(45.8) 102(26.6) χ2=25.204 <0.001 MRS 5.71±1.43 5.92±1.46 t=-1.792 0.074 Globe评分 1.17(0.36~2.14) 1.28(0.29~2.07) Z=-0.161 0.872 UK-PBC评分 0.038(0.014~0.119) 0.037(0.015~0.109) Z=-0.481 0.631 UDCA治疗1年[例(%)] χ2=8.948 0.003 完全应答 156(61.7) 280(72.9) 应答不良 97(38.3) 104(27.1) 2.4 不同IgM水平预测应答不良的风险分析森林图
对IgM≥1.5×ULN组及IgM<1.5×ULN组两组的各项指标进行预测应答不良的风险值分析,结果显示IgM≥1.5×ULN组相对IgM<1.5×ULN组预测整体应答不良的风险值为1.416 (95%CI:1.129~1.776, P=0.003)。亚组分析中,无肝硬化患者,IgM≥1.5×ULN预测应答不良风险值为1.821(95%CI:1.224~2.711, P=0.003)。其他情况详见图 2。
3. 讨论
IgM具有强大的杀菌、激活补体、免疫调理和凝集作用,通常出现在初次体液免疫应答的最早阶段,是连接固有免疫和获得性免疫的纽带,也参与某些自身免疫疾病和超敏反应的病理过程。PBC是一种器官特异性的自身免疫性疾病,IgM升高是PBC典型的血清学特征之一,但是其升高的机制至今尚未完全阐明,外源微生物的分子模拟、免疫耐受性的破坏、免疫功能紊乱可能是导致高IgM水平的重要原因[9-10],IgM在PBC疾病进程中可能发挥免疫调节及免疫损伤的双重作用[9]。不同于PBC,自身免疫性肝炎(AIH)患者以IgG升高为主,其水平随着病情缓解而逐渐下降,IgG是AIH疾病转归的重要监测指标之一。作为PBC常规检测项目,IgM并未被国内外指南纳入诊疗和预后评估的指标。IgM在PBC发生、发展、疗效及转归中是否具有预测价值,目前尚缺乏大量的临床研究结果作为指导。
UDCA是PBC治疗的一线药物,可显著改善部分PBC患者非肝移植存活率[11-12],但仍有30%~40%的患者对UDCA治疗无应答,本研究中显示UDCA完全应答率为68.4%,对于这部分患者需要及时联合一种或两种二线药物来改善胆汁淤积以预防疾病进展[13]。UDCA治疗可能影响IgM水平,研究[14]发现UDCA能够显著降低细菌CpG诱导的总IgM和IgM-AMA的产生,但对IgG-AMA的水平却无影响。IgM与肝硬化相关症状和肝脏相关事件的发生关系密切,在UDCA联合苯扎贝特治疗过程中,不论ALP及GGT下降与否,当IgM水平持续异常时,患者的预后均较差,其生存期显著低于IgM水平正常化的患者,治疗过程中IgM水平的逐步正常化可能提示预后较好[15]。对于UDCA不完全应答的PBC患者,在给予联合利妥昔单抗治疗后,IgM水平随着肝功能指标的好转而逐步下降[16]。IgM正常化可作为长期预后的预测指标,但初始IgM正常患者IgM水平的变化情况及预测因素尚缺乏研究。在本研究中,UDCA治疗基线IgM平均水平为2.76 g/L,IgM升高的占比为56.0%,在UDCA完全应答组与应答不良组之间,基线IgM水平存在显著性差异(P=0.034),进一步分析IgM升高与IgM正常组患者的临床特征及在UDCA治疗1年后的疗效差异,发现IgM正常组UDCA应答率高于IgM升高组(71.8% vs 65.8%),但两组差异无统计学意义(P=0.108),通过ROC曲线分析获得IgM预测UDCA治疗1年后应答不良风险的最佳临界值(1.5×ULN),按最佳临界值进行分组后,结果显示IgM<1.5×ULN组的PBC患者发生UDCA应答率显著高于IgM≥1.5×ULN组(72.9% vs 61.7%, P=0.003),IgM≥1.5×ULN组发生UDCA应答不良风险是前者的1.416倍(95%CI: 1.129~1.776),因此基线IgM水平可能有助于预测PBC治疗应答。
IgM是进展期PBC的危险因素,与PBC胆管损伤及纤维化密切相关。进展期PBC的肝组织中IgM表达水平显著升高[17],在胆道闭锁的患儿中,靶向胆管上皮细胞的IgM自身抗体能够激活补体并参与肝纤维化的发生,从而使疾病不断进展[18-19]。在本研究中血清IgM升高组的PBC患者,其肝硬化占比也偏高,因是回顾性研究,患者缺乏UDCA治疗基线及治疗后的组织学证据,无法评估IgM与疾病进展、转归之间的相关性,今后尚需要进一步完善。
UDCA生化应答的PBC患者具有较好的预后,即使进展为肝硬化的患者,如仍处于代偿期,也可达到延长生存期、降低肝病相关病死率及肝移植需求的目标[20]。MRS是一种常用的预测生存概率模型,最初用于筛选肝移植对象和确定肝移植手术时机[6],MRS对于失代偿期肝硬化的PBC患者也具有较强的预测性。UK-PBC评分[8]及GLOBE评分[7]是近年来被采用的PBC预后模型。在接受UDCA治疗的PBC患者中,MRS、UK-PBC评分和GLOBE评分对患者肝移植或死亡的风险预测均具有较好的准确性[21]。本研究结果显示MRS、Globe评分、UK-PBC评分在UDCA完全应答组及应答不良组之间均具有显著性差异,提示UDCA完全应答组的非肝移植存活率均显著高于应答不良组,但在IgM升高组和正常组之间以及IgM≥1.5×ULN组和IgM<1.5×ULN组之间,三种预测模型评分均无明显差异,这说明基线IgM水平还不能作为独立预测非肝移植存活率的指标。
总之,本研究结果提示基线IgM水平对于预测UDCA应答具有重要价值,基线IgM水平较高的PBC患者,治疗中应密切监测IgM水平,如持续异常,应及时联合二线药物治疗。
-
表 1 液相色谱洗脱条件
时间(min) 流动相B比例(%) 0 6 8 15 60 32 79 40 80 95 85 95 86 6 90 3 表 2 两组患者一般资料比较
项目 生存组(n=5) 肝移植/死亡组(n=5) Z值 P值 男性[例(%)] 3(60) 3(60) 年龄(岁) 43.80(42.00~53.00) 44.60(33.00~51.00) 0.17 0.22 ALT(U/L) 115.46(9.70~421.20) 242.58(31.30~781.90) 1.36 0.22 AST(U/L) 131.70(49.90~168.90) 228.06(50.90~496.50) 0.52 0.69 凝血酶原活动度 23.20(10.00~34.00) 31.06(15.30~48.00) 0.94 0.42 TBil(μmol/L) 360.30(213.40~498.60) 450.46(340.00~690.80) 0.52 0.69 DBil(μmol/L) 226.90(142.40~311.10) 295.70(102.30~418.70) 0.73 0.55 AFP(ng/ml) 91.20(6.25~290.10) 184.21(7.42~591.4) 0.52 0.69 WBC(×109/L) 4.23(1.48~5.71) 8.50(7.40~10.43) 0.94 0.42 RBC(×1012/L) 3.22(2.44~3.74) 3.45(2.99~4.26) 0.31 0.84 PLT(×109/L) 88.00(35.00~163.00) 85.40(42.00~121.00) 0.31 0.84 注: HLA, 人类淋巴细胞抗原。 表 3 (肝移植/死亡组)/生存组上调差异蛋白
基因 蛋白编号 差异蛋白 差异倍数 P值 IGHV4-4 A0A075B6R2 Ig重变4-4 747.718 0.049 58 IGHV3-21 A0A0B4J1V1 Ig重变3-21 458.288 0.043 69 IGHV3-73 A0A0B4J1V6 Ig重变3-73 450.090 0.010 41 IGKV1-8 A0A0C4DH67 Igκ变量1-8 222.641 0.001 08 IGKV1D-12 P01611 Igκ变量1D-12 181.438 0.046 98 DNM1L G8JLD5 动力相关蛋白1(Drp1) 178.461 0.010 70 DIAPH1 H9KV28 透明基因相关成蛋白1(Diaph1) 153.400 0.041 52 NCOR1 J3KRE4 核受体共抑制因子1(NCoR1) 128.299 0.006 20 HPRT1 P00492 次黄嘌呤磷酸核糖基转移酶 113.501 0.008 19 IGLV3-19 P01714 Igλ变量3-19 108.399 0.032 01 IGHV4-39 P01824 Ig重变4-39 101.752 0.004 34 ASL P04424 精氨琥珀酸裂解酶(ASL) 97.605 0.001 68 RPN2 P04844 核糖体结合蛋白Ⅱ 93.996 0.034 03 HLA-A P05534 HLAⅠ类组织相容性抗原, A-24链 88.826 0.000 19 GSTP1 P09211 谷胱甘肽S-转移酶P 83.988 0.028 45 表 4 (肝移植/死亡组)/生存组下调差异蛋白
基因 蛋白编号 差异蛋白 差异倍数 P值 SDCBP SDCBP 同线蛋白1 0.104 87 0.048 88 H3F3B K7EMV3 组蛋白H3 0.092 72 0.042 38 WBP2 K7EIJ0 WW结构域结合蛋白2 0.092 49 0.042 25 DSP P15924 桥粒斑蛋白 0.090 24 0.041 04 SLC3A2 F5GZS6 4F2细胞表面抗原重链 0.089 90 0.040 86 FTL P02792 铁蛋白轻链 0.086 97 0.039 28 KRT6A P02538 角蛋白, Ⅱ型细胞骨架6A 0.085 11 0.038 28 KRT2 P35908 角蛋白Ⅱ型表皮细胞骨架2 0.081 73 0.036 46 EHD4 Q9H223 EH含域蛋白4 0.081 67 0.036 42 SDCBP G5EA09 同线蛋白, 同型CRA_a 0.081 40 0.036 29 KRT14 P02533 角蛋白, Ⅱ型细胞骨架14 0.080 83 0.035 97 F11 P03951 凝血因子Ⅺ 0.080 10 0.035 57 ANXA6 P08133 膜联蛋白A6 0.075 99 0.033 36 TFRC P02786 转铁蛋白受体1 0.075 67 0.033 19 KPRP Q5T749 角化细胞富含脯氨酸蛋白 0.073 74 0.032 15 -
[1] TANG CM, YAU TO, YU J. Management of chronic hepatitis B infection: Current treatment guidelines, challenges, and new developments[J]. World J Gastroenterol, 2014, 20(20): 6262-6278. DOI: 10.3748/wjg.v20.i20.6262. [2] SARIN SK, KEDARISETTY CK, ABBAS Z, et al. Acute-on-chronic liver failure: Consensus recommendations of the Asian Pacific Association for the Study of the Liver (APASL) 2014[J]. Hepatol Int, 2014, 8(4): 453-471. DOI: 10.1007/s12072-014-9580-2. [3] SARIN SK, CHOUDHURY A. Acute-on-chronic liver failure: Terminology, mechanisms and management[J]. Nat Rev Gastroenterol Hepatol, 2016, 13(3): 131-149. DOI: 10.1038/nrgastro.2015.219. [4] HIRSOVA P, IBRAHIM SH, VERMA VK, et al. Extracellular vesicles in liver pathobiology: Small particles with big impact[J]. Hepatology, 2016, 64(6): 2219-2233. DOI: 10.1002/hep.28814. [5] XU S, ZHANG LP. Research advances in the role of exosomes in non-neoplastic liver diseases[J]. J Clin Hepatol, 2020, 36(4): 940-943. DOI: 10.3969/j.issn.1001-5256.2020.04.052.徐帅, 张立平. 外泌体在非肿瘤性肝脏疾病中的研究进展[J]. 临床肝胆病杂志, 2020, 36(4): 940-943. DOI: 10.3969/j.issn.1001-5256.2020.04.052. [6] VERMA VK, LI H, WANG R, et al. Alcohol stimulates macrophage activation through caspase-dependent hepatocyte derived release of CD40L containing extracellular vesicles[J]. J Hepatol, 2016, 64(3): 651-660. DOI: 10.1016/j.jhep.2015.11.020. [7] NOJIMA H, FREEMAN CM, SCHUSTER RM, et al. Hepatocyte exosomes mediate liver repair and regeneration via sphingosine-1-phosphate[J]. J Hepatol, 2016, 64(1): 60-68. DOI: 10.1016/j.jhep.2015.07.030. [8] Liver Failure and Artificial Liver Group, Chinese Society of Infectious Diseases, Chinese Medical Association; Severe Liver Disease and Artificial Liver Group, Chinese Society of Hepatology, Chinese Medicial Association. Guideline for diagnosis and treatment of liver failure(2018)[J]. J Clin Hepatol, 2019, 35(1): 38-44. DOI: 10.3969/j.issn.1001-5256.2019.01.007.中华医学会感染病学分会肝衰竭与人工肝学组, 中华医学会肝病学分会重型肝病与人工肝学组. 肝衰竭诊治指南(2018年版)[J]. 临床肝胆病杂志, 2019, 35(1): 38-44. DOI: 10.3969/j.issn.1001-5256.2019.01.007. [9] JING HM, ZHANG T, YANG F, et al. Manganese-related differential proteomic studies of cerebrospinal fluid from rats by label free method[J]. J Toxicol, 2012, 26(4): 247-253. https://www.cnki.com.cn/Article/CJFDTOTAL-WSDL201204001.htm敬海明, 张拓, 杨帆, 等. Label free方法研究大鼠脑脊液中锰毒性相关的差异蛋白质组[J]. 毒理学杂志, 2012, 26(4): 247-253. https://www.cnki.com.cn/Article/CJFDTOTAL-WSDL201204001.htm [10] DEMORY BECKLER M, HIGGINBOTHAM JN, FRANKLIN JL, et al. Proteomic analysis of exosomes from mutant KRAS colon cancer cells identifies intercellular transfer of mutant KRAS[J]. Mol Cell Proteomics, 2013, 12(2): 343-355. DOI: 10.1074/mcp.M112.022806. [11] JI H, GREENING DW, BARNES TW, et al. Proteome profiling of exosomes derived from human primary and metastatic colorectal cancer cells reveal differential expression of key metastatic factors and signal transduction components[J]. Proteomics, 2013, 13(10-11): 1672-1686. DOI: 10.1002/pmic.201200562. [12] ALBILLOS A, LARIO M, ÁLVAREZ-MON M. Cirrhosis-associated immune dysfunction: Distinctive features and clinical relevance[J]. J Hepatol, 2014, 61(6): 1385-1396. DOI: 10.1016/j.jhep.2014.08.010. [13] GUO YY, YUAN CB, YU HY, et al. Difference of T lymphocyte subsets in peripheral blood of children with chronic hepatitis B and liver failure[J]. Chin Hepatol, 2014, 19(7): 515-517. https://www.cnki.com.cn/Article/CJFDTOTAL-ZUAN201407011.htm郭银燕, 袁春蓓, 俞海英, 等. 慢性乙型肝炎与肝衰竭患儿外周血T淋巴细胞亚群的差异[J]. 肝脏, 2014, 19(7): 515-517. https://www.cnki.com.cn/Article/CJFDTOTAL-ZUAN201407011.htm [14] VEIGA-PARGA T, SEHRAWAT S, ROUSE BT. Role of regulatory T cells during virus infection[J]. Immunol Rev, 2013, 255(1): 182-196. DOI: 10.1111/imr.12085. [15] SMIRNOVA E, GRIPARIC L, SHURLAND DL, et al. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells[J]. Mol Biol Cell, 2001, 12(8): 2245-2256. DOI: 10.1091/mbc.12.8.2245. [16] YANG X, WANG H, NI HM, et al. Inhibition of Drp1 protects against senecionine-induced mitochondria-mediated apoptosis in primary hepatocytes and in mice[J]. Redox Biol, 2017, 12: 264-273. DOI: 10.1016/j.redox.2017.02.020. [17] XU S, PI H, CHEN Y, et al. Cadmium induced Drp1-dependent mitochondrial fragmentation by disturbing calcium homeostasis in its hepatotoxicity[J]. Cell Death Dis, 2013, 4: e540. DOI: 10.1038/cddis.2013.7. [18] PALMA E, MA X, RIVA A, et al. Dynamin-1-like protein inhibition drives megamitochondria formation as an adaptive response in alcohol-induced hepatotoxicity[J]. Am J Pathol, 2019, 189(3): 580-589. DOI: 10.1016/j.ajpath.2018.11.008. [19] GALLOWAY CA, LEE H, BROOKES PS, et al. Decreasing mitochondrial fission alleviates hepatic steatosis in a murine model of nonalcoholic fatty liver disease[J]. Am J Physiol Gastrointest Liver Physiol, 2014, 307(6): G632-641. DOI: 10.1152/ajpgi.00182.2014. [20] YU T, WANG L, LEE H, et al. Decreasing mitochondrial fission prevents cholestatic liver injury[J]. J Biol Chem, 2014, 289(49): 34074-34088. DOI: 10.1074/jbc.M114.588616. [21] STRITT S, NURDEN P, TURRO E, et al. A gain-of-function variant in DIAPH1 causes dominant macrothrombocytopenia and hearing loss[J]. Blood, 2016, 127(23): 2903-2914. DOI: 10.1182/blood-2015-10-675629. [22] LYNCH ED, LEE MK, MORROW JE, et al. Nonsyndromic deafness DFNA1 associated with mutation of a human homolog of the Drosophila gene diaphanous[J]. Science, 1997, 278(5341): 1315-1318. DOI: 10.1126/science.278.5341.1315 [23] YANG J, ZHOU L, ZHANG Y, et al. DIAPH1 Is Upregulated and inhibits cell apoptosis through ATR/p53/Caspase-3 signaling pathway in laryngeal squamous cell carcinoma[J]. Dis Markers, 2019, 2019: 6716472. DOI: 10.1155/2019/6716472. [24] LIU D, FU X, WANG Y, et al. Protein diaphanous homolog 1 (Diaph1) promotes myofibroblastic activation of hepatic stellate cells by regulating Rab5a activity and TGFβ receptor endocytosis[J]. FASEB J, 2020, 34(6): 7345-7359. DOI: 10.1096/fj.201903033R. [25] SUN Z, FENG D, FANG B, et al. Deacetylase-independent function of HDAC3 in transcription and metabolism requires nuclear receptor corepressor[J]. Mol Cell, 2013, 52(6): 769-782. DOI: 10.1016/j.molcel.2013.10.022. [26] BURRAGE LC, MADAN S, LI X, et al. Chronic liver disease and impaired hepatic glycogen metabolism in argininosuccinate lyase deficiency[J]. JCI Insight, 2020, 5(4). DOI: 10.1172/jci.insight.132342. [27] UCHIHARA T, MIYAKE K, YONEMURA A, et al. Extracellular vesicles from cancer-associated fibroblasts containing annexin A6 induces FAK-YAP activation by stabilizing β1 integrin, enhancing drug resistance[J]. Cancer Res, 2020, 80(16): 3222-3235. DOI: 10.1158/0008-5472.CAN-19-3803. [28] ALVAREZ-GUAITA A, BLANCO-MUÑOZ P, MENESES-SALAS E, et al. Annexin A6 is critical to maintain glucose homeostasis and survival during liver regeneration in mice[J]. Hepatology, 2020, 72(6): 2149-2164. DOI: 10.1002/hep.31232. [29] TORTI SV, TORTI FM. Iron and cancer: More ore to be mined[J]. Nat Rev Cancer, 2013, 13(5): 342-355. DOI: 10.1038/nrc3495. [30] ALKHATEEB AA, CONNOR JR. The significance of ferritin in cancer: Anti-oxidation, inflammation and tumorigenesis[J]. Biochim Biophys Acta, 2013, 1836(2): 245-254. DOI: 10.1016/j.bbcan.2013.07.002. [31] LUNOVA M, GOEHRING C, KUSCUOGLU D, et al. Hepcidin knockout mice fed with iron-rich diet develop chronic liver injury and liver fibrosis due to lysosomal iron overload[J]. J Hepatol, 2014, 61(3): 633-641. DOI: 10.1016/j.jhep.2014.04.034. [32] WANG AR, LI XL, MEN QS, et al. Serum ferritin and liver disease[J/CD]. Chin J Liver Dis(Electronic Edition), 2020, 12(4): 34-37. DOI: 10.3969/j.issn.1674-7380.2020.04.006.王傲然, 李晓玲, 门秋爽, 等. 血清铁蛋白与肝脏疾病研究进展[J/CD]. 中国肝脏病杂志(电子版), 2020, 12(4): 34-37. DOI: 10.3969/j.issn.1674-7380.2020.04.006. [33] DENG JH, CHEN ZP, HUANG MM, et al. Clinical significance of Ferritin Light Chain and its expression in serum of patients with chronic hepatopathy[J]. Modern Prevent Med, 2016, 43(18): 3444-3447. https://www.cnki.com.cn/Article/CJFDTOTAL-XDYF201618046.htm邓敬桓, 陈智平, 黄美梦, 等. FTL在慢性肝病患者血清中的表达及临床意义[J]. 现代预防医学, 2016, 43(18): 3444-3447. https://www.cnki.com.cn/Article/CJFDTOTAL-XDYF201618046.htm 期刊类型引用(7)
1. 张玉婷,李嘉泰,宋晓静,丁方回,岳平,闫少林,李俊峰,张立婷,李汛. 不同IgM水平的原发性胆汁性胆管炎患者治疗应答及预后特点分析. 胃肠病学和肝病学杂志. 2025(02): 250-253 .  百度学术
百度学术2. 张玲玲,张会品,李桂英,孔燕,马兰. 抗线粒体抗体、红细胞体积分布宽度与原发性胆汁性胆管炎熊去氧胆酸治疗应答效果的关系. 中国医药导报. 2024(04): 100-103 .  百度学术
百度学术3. 张晓芳,徐海峰,章颖. LSM、PNI、Mayo评分对PBC相关肝硬化的预测价值. 南通大学学报(医学版). 2024(02): 131-135 .  百度学术
百度学术4. 何学元,马建勋,杨屹立,张敏,潘新民. 布地奈德治疗熊去氧胆酸治疗不应答的原发性胆汁性胆管炎患者疗效及血清氧化应激指标的变化. 实用肝脏病杂志. 2023(03): 376-379 .  百度学术
百度学术5. 邢雪梅,杨建睿,李治君,陈雅娟,姚凤霞,刘征. 免疫球蛋白用于原发性胆汁性胆管炎、戊型肝炎、肝硬化的鉴别诊断. 基础医学与临床. 2023(08): 1271-1274 .  百度学术
百度学术6. 李兆明,章颖,邹美银. TBA、IgM、FIB-4评分对原发性胆汁性胆管炎患者肝纤维化的诊断价值. 胃肠病学和肝病学杂志. 2023(11): 1254-1257 .  百度学术
百度学术7. 赵永春,刘锦云,刘晓瑞. 醋酸泼尼松龙联合熊去氧胆酸对PBC患者预后的影响. 河南医学高等专科学校学报. 2023(06): 637-640 .  百度学术
百度学术其他类型引用(1)
-




 PDF下载 ( 4411 KB)
PDF下载 ( 4411 KB)

 下载:
下载:


 下载:
下载:
 百度学术
百度学术



