Value of FibroScan and platelet count-to-spleen thickness ratio in predicting the degree of esophageal and gastric varices in liver cirrhosis
-
摘要:
目的 探讨瞬时弹性成像技术(FibroScan)和血小板计数与脾脏厚径比率(PC/ST)对肝硬化食管胃底静脉曲张(EGV)程度的预测价值。 方法 选取2017年1月—2020年9月于郑州大学第五附属医院入院3 d内接受FibroScan、彩色多普勒超声、电子胃镜及血生化检查的肝硬化患者210例,以电子胃镜检查结果为“金标准”分为无、轻度、中度、重度EGV组。计量资料多组间比较采用One-Way ANOVA检验或Kruskal-Wallis H检验,并绘制肝硬度值(LSM)、PC/ST及LSM+PC/ST受试者工作特征曲线(ROC曲线),通过DeLong法比较各指标ROC曲线的诊断性能。 结果 无、轻、中、重度EGV组的LSM(F=32.00,P<0.01)和PC/ST值(H=49.58,P<0.01)组间比较差异均有统计学意义。轻度EGV组LSM、PC/ST、LSM+PC/ST的ROC曲线下面积(AUC)分别为0.762、0.656、0.770,阳性预测值及阴性预测值分别为75.4%、75.0%,60.2%、75.8%,82.5%、65.4%;中度EGV组LSM、PC/ST、LSM+PC/ST的AUC分别为0.841、0.796、0.896,阳性预测值及阴性预测值分别为86.1%、79.7%,68.0%、80.5%,74.1%、90.2%;重度EGV组LSM、PC/ST、LSM+PC/ST的AUC分别为0.834、0.830、0.903,阳性预测值及阴性预测值分别为80.5%、82.5%,71.4%、83.6%,79.5%、85.0%。PC/ST与LSM+PC/ST预测轻、中、重度EGV的AUC组间比较,差异均有统计学意义(Z值分别为2.66、2.71、2.37,P值分别为0.007、0.007、0.018)。 结论 LSM、PC/ST、LSM+PC/ST对肝硬化合并中重度EGV具有较好的预测价值。 Abstract:Objective To investigate the value of transient elastography (FibroScan) and platelet count-to-spleen thickness (PC/ST) ratio in predicting the degree of esophageal and gastric varices (EGV) in liver cirrhosis. Methods A total of 210 patients with liver cirrhosis who underwent FibroScan, color Doppler ultrasound, electronic gastroscopy, and blood biochemical examination within three days after admission to The Fifth Affiliated Hospital of Zhengzhou University from January 2017 to September 2020 were enrolled, and according to the "gold standard" of gastroscopy, the patients were divided into none, mild, moderate, and severe EGV groups. A one-way analysis of variance or the Kruskal-Wallis H test were used for comparison of continuous data between multiple groups. Receiver operating characteristic (ROC) curves were plotted for liver stiffness measurement (LSM), PC/ST ratio, and LSM+PC/ST, and the diagnostic performance of these ROC curves was compared using the Delong method. Results There were significant differences between the none, mild, moderate, and severe EGV groups in LSM (F=32.00, P < 0.01) and PC/ST ratio (H=49.58, P < 0.01). For the mild EGV group, LSM, PC/ST ratio, and LSM+PC/ST had an area under the ROC curve (AUC) of 0.762, 0.656, and 0.770, respectively, with a positive predictive value of 75.4%, 60.2%, and 82.5%, respectively, and a negative predictive value of 75.0%, 75.8%, and 65.4%, respectively. For the moderate EGV group, LSM, PC/ST ratio, and LSM+PC/ST had an AUC of 0.841, 0.796, and 0.896, respectively, with a positive predictive value of 86.1%, 68.0%, and 74.1%, respectively, and a negative predictive value of 79.7%, 80.5%, and 90.2%, respectively. For the severe EGV group, LSM, PC/ST ratio, and LSM+PC/ST had an AUC of 0.834, 0.830, and 0.903, respectively, with a positive predictive value of 80.5%, 71.4%, and 79.5%, respectively, and a negative predictive value of 82.5%, 83.6%, and 85.0%, respectively. PC/ST ratio and LSM+PC/ST had significantly different AUC in predicting mild, moderate, and severe EGV (Z=2.66, P=0.007; Z=2.71, P=0.007; Z=2.37, P=0.018). Conclusion LSM, PC/ST ratio, and LSM+PC/ST have a good predictive value for moderate-to-severe EGV in liver cirrhosis. -
食管胃底静脉曲张(esophageal and gastric varices, EGV)是肝硬化常见的并发症,EGV破裂出血发生率为5%~15%, 短期病死率达15% ~ 20%[1]。早期发现并预测EGV的程度,对其进行相关干预可以有效降低致死率[2]。目前胃镜检查仍是诊断EGV的“金标准”,但其作为侵入性检查有诱发出血风险,且患者依从性差,难以对EGV患者进行临床观察[3]。近年已有多种无创血清学模型探讨其预测价值[4-5]。本研究将探讨FibroScan检测肝硬度值(LSM)和血小板计数与脾脏厚径比率(PC/ST)对肝硬化EGV程度的预测价值。
1. 资料与方法
1.1 研究对象
选取2017年1月—2020年9月在本院住院并临床诊断为肝硬化的患者,纳入标准:18岁<年龄<80岁,根据症状、体征、影像或肝穿刺病理诊断为肝硬化,不限病因,均接受电子胃镜、肝脾超声、FibroScan、血清生化指标检查。排除标准:肝脏肿瘤,血液病或其他原因导致的脾大、脾切除、血小板减少症、布加综合征,曾出现EGV破裂出血,胃镜下套扎或硬化治疗,曾行经颈静脉肝内门体分流术,因腹水过多或ALT过高影响LSM,6个月内曾使用抑制或促进骨髓造血或白蛋白、血小板的药物,既往或入院时有消化道出血的患者。所有患者的EGV程度诊断均符合《肝硬化门静脉高压食管胃静脉曲张出血的防治指南》[6]。
1.2 研究方法
采集患者入院次日清晨空腹血液,由检验科专业技术人员按照操作手册进行操作,记录患者性别、年龄、病因诊断、血液生化指标。
1.2.1 超声检查
使用ToshibaPW6000(购自日本东芝公司)、GELOGIQ9彩色超声诊断仪(购自美国GE公司),由两位专业超声医师操作完成,记录脾脏厚径。
1.2.2 肝硬度检测
LSM检测采用来自法国(Echosens公司)FibroScan 502瞬时弹性扫描仪,由经过规范培训且至少200次检测经验的专业技术人员独立完成,患者检查前禁食2 h以上,操作参照FibroScan用户手册,检测结果取10次有效激发的中位数[7]。
1.2.3 电子胃镜检查
采用日本奥林巴斯XQ260型胃镜进行检查,由两位年资超过5年的经验丰富的内镜医师检查,食管静脉曲张程度按照《肝硬化门静脉高压食管胃静脉曲张出血的防治指南》[6]将其分为无静脉曲张组、轻度静脉曲张组、中度静脉曲张组、重度静脉曲张组。
1.3 伦理学审查
本研究通过郑州大学第五附属医院医学伦理委员会审核批准,批号:Y2021002。
1.4 统计学方法
应用SPSS 25.0软件进行统计学分析。计量资料进行正态性和方差齐性检验,符合正态分布采用x ±s表示,多组间比较采One-Way ANOVA检验,方差齐则使用Bonferroni法,不齐使用Tamhane法;非正态分布采用M(P25~P75)表示,组间比较采用Kruskal-Wallis H检验。计算受试者工作特征曲线(ROC曲线)下面积(AUC),评估各个模型诊断价值并确定界值,计算敏感度、特异度、阳性预测值、阴性预测值,使用MedCalc软件DeLong法比较各指标ROC曲线的诊断性能。P<0.05为差异有统计学意义。
2. 结果
2.1 一般情况
共纳入肝硬化患者210例,其中乙型肝炎肝硬化156例,丙型肝炎肝硬化13例,自身免疫性肝硬化8例,酒精性肝硬化23例,不名原因肝硬化10例;男113例,女97例,平均(56.00±11.06)岁;无静脉曲张组60例,轻度静脉曲张组61例,显著静脉曲张组89例(中度45例、重度44例)。
2.2 无、轻、中、重度静脉曲张分别与LSM、PC/ST的关系
结果显示LSM与PC/ST,中、重度静脉曲张组与无曲张和轻度曲张组相比,差异均有统计学意义(P值均<0.01)(表 1)。
表 1 LSM、PC/ST与食管静脉曲张程度的关系分组 例数 LSM(kPa) PC/ST 无静脉曲张组 60 16.60±5.03 2.74(1.82~5.01) 轻度静脉曲张组 61 21.07±4.64 2.06(1.45~2.93) 中度静脉曲张组 45 25.25±6.791)2) 1.44(0.91~2.00)1)2) 重度静脉曲张组 44 28.53±9.711)2) 1.07(0.72~1.75)1)2) 统计值 F=32.00 H=49.58 P值 <0.01 <0.01 注:与无食管静脉曲张组相比,1) P<0.01;与轻度食管静脉曲张组相比,2) P<0.01。 2.3 LSM、PC/ST、LSM+PC/ST对各组食管静脉曲张程度的预测价值比较
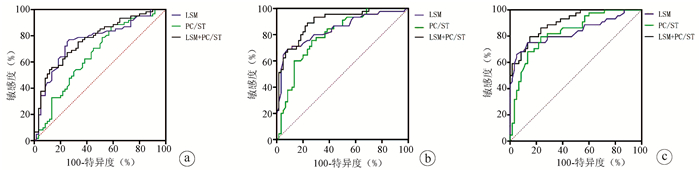
ROC曲线分析显示:LSM、PC/ST、LSM+PC/ST在轻度静脉曲张组的AUC分别为0.762、0.656、0.770(图 1a),阳性预测值及阴性预测值分别为75.4%、75.0%,60.2%、75.8%,82.5%、65.4%(表 2);LSM、PC/ST、LSM+PC/ST在中度静脉曲张组的AUC分别为0.841、0.796、0.896(图 1b),阳性预测值及阴性预测值分别为86.1%、79.7%,68.0%、80.5%,74.1%、90.2%(表 2);LSM、PC/ST、LSM+PC/ST在重度静脉曲张组的AUC分别为0.834、0.830、0.903(图 1c);阳性预测值及阴性预测值分别为80.5%、82.5%,71.4%、83.6%,79.5%、85.0%(表 2);轻度静脉曲张组中,LSM的截断值为18.9,敏感度及特异度为75.4%、75.0%, PC/ST的截断值为3.70,敏感度及特异度为86.9%、41.7%;中度静脉曲张组中,LSM的截断值为22.5,敏感度及特异度为68.9%、91.7%,PC/ST的截断值为1.95,敏感度及特异度为75.6%、73.3%;重度静脉曲张组中,LSM的截断值为21.5,敏感度及特异度为75.0%、86.7%,PC/ST的截断值为1.81,敏感度及特异度为79.5%、76.7%;提示LSM、PC/ST及LSM+PC/ST在中重度静脉曲张组中都有较好的预测价值(表 2)。
表 2 LSM、PC/ST、LSM+PC/ST对各组食管静脉曲张程度的预测价值分组 AUC 截断值 敏感度(%) 特异度(%) 95% CI 阳性预测值(%) 阴性预测值(%) 轻度静脉曲张组 LSM 0.762 18.9 75.4 75.0 0.675~0.849 75.4 75.0 PC/ST 0.656 3.70 86.9 41.7 0.558~0.754 60.2 75.8 LSM+PC/ST 0.7701) 54.1 88.3 0.687~0.854 82.5 65.4 中度静脉曲张组 LSM 0.841 22.5 68.9 91.7 0.762~0.922 86.1 79.7 PC/ST 0.796 1.95 75.6 73.3 0.711~0.880 68.0 80.5 LSM+PC/ST 0.8961) 88.9 76.7 0.836~0.956 74.1 90.2 重度静脉曲张组 LSM 0.834 21.5 75.0 86.7 0.748~0.920 80.5 82.5 PC/ST 0.830 1.81 79.5 76.7 0.752~0.909 71.4 83.6 LSM+PC/ST 0.9031) 79.5 85.0 0.848~0.959 79.5 85.0 注:与PC/ST相比,1) P<0.05。 2.4 PC/ST、LSM+PC/ST预测不同食管静脉曲张程度的AUC比较
LSM+PC/ST与PC/ST AUC在轻、中、重度静脉曲张组面积差分别为0.114(95%CI:0.032~0.198)、0.101(95%CI:0.028~0.174)、0.073(95% CI:0.013~ 0.134)。PC/ST与LSM+PC/ST预测轻、中、重度食管静脉曲张的AUC组间比较差异均具有统计学意义(Z值分别为2.66、2.71、2.37, P值分别为0.007、0.007、0.018)(表 2)。
3. 讨论
2005年,Castéra等[8]首次提出了FibroScan和血清学标志物(APRI或FibroTest) 联合诊断肝纤维化的思想,最终有75% 的患者避免肝穿刺,王帅等[9]分别比较了LSM、APRI、LSM+APRI在轻中重EGV患者中的AUC,提示LSM+APRI在肝硬化伴EGV中显示出较好的预测效果,既往研究[4]表明PC/ST对预测食管静脉曲张有较好的预测价值,多种实验室参数相互配合构建联合指标的研究很常见,比如ABP标准[10]、LSM-血小板[11]、脾体积-LSM-实验室参数[12]等。本研究探索了LSM、PC/ST及LSM+PC/ST在不同程度EGV中的预测价值。
LSM已被提议作为诊断食管静脉曲张的一种非侵入性替代标志物[13],但是LSM作为单一测试仍然有限,因为用于预测静脉曲张存在的截断值变化很大,范围在13.9~21.5 kPa[14],美国胃肠病学会在对LSM是否能排除高风险食管静脉曲张的证据等级中提出[15],对于任何病因引起的疑似代偿性肝硬化的成人,LSM<19.5(±2)kPa时,可以准确地排除存在高危EGV。本研究结果中,随着曲张程度的加重,LSM逐渐增大,但中、重度组之间差异无统计学意义,中、重度食管静脉曲张的LSM与无、轻度相比,差异均具有统计学意义,LSM在轻、中、重组中的截断值分别为18.9、22.5、21.5 kPa,其中LSM在中重度曲张中的AUC面积达到0.83以上,有较好的预测效果,且截断值大于美国胃肠学会提出的截断值,可能原因有以下两点:(1)本研究病例病因大多数为乙型肝炎,导致未能显示出病因的多样性;(2)高风险EGV因素只纳入一部分,导致结果稍有偏倚。本研究表明,LSM能够区别无、轻、中重度食管静脉曲张,当LSM≥22.5 kPa时,常提示处于中、重度食管静脉曲张。
在非侵入性预测EGV方法中,血小板、LSM、超声检查三者之间联合预测肝硬化EGV的诊断价值较为常见,研究最多的参数为血小板计数/脾脏长径,在截断值为909时,可以使55%的患者避免内镜检查[14]。Kim等[16]建立了脾长径与血小板比率评分标准(LSPS),LSPS>5.5时有90.3%的概率为高风险EGV,但是关于PC/ST的相关研究较少。此外多项研究[3, 17-18]表明,血小板计数、脾脏厚度是EGV发生的独立危险因素,且与食管静脉曲张程度具有相关性。韩莹等[19]结果表明PC/ST可以区分有无EGV,AUC为0.743,敏感度68.8%,特异度75%,尽管诊断价值较低,但是给研究者提供了探索的空间,本研究将在此基础上对EGV程度进一步分组研究。
本研究显示PC/ST能够区分无、轻、中重度食管静脉曲张,且在轻度曲张中敏感度为86.9%,但AUC仅为0.656,诊断价值低,而在重度曲张中,截断值为1.81时,AUC面积0.830,敏感度79.5%,特异度76.7%,LSM+PC/ST的AUC达到0.903,敏感度79.5%,特异度85.0%,且在轻、中、重度静脉曲张组中分别与单一的PT/ST AUC相比,差异均具有统计学意义,由此,可以认为LSM、PC/ST、LSM+PC/ST对肝硬化中重度EGV有较好的预测价值,而且超声、血液实验参数作为常规入院检查,获取比较方便,对于无法进行胃镜检查的肝硬化患者,该项指标能给予临床医生相应的判断价值,当PC/ST≤1.81或LSM≥22.5 kPa时,提示有中重度食管静脉曲张。由于本研究中的病例大多数为乙型肝炎患者,不能作为各类肝病病因的代表,可能导致结果有所偏差,而且数据量较小,为回顾性、单中心、横向研究,需要多中心数据进一步临床验证。
-
表 1 LSM、PC/ST与食管静脉曲张程度的关系
分组 例数 LSM(kPa) PC/ST 无静脉曲张组 60 16.60±5.03 2.74(1.82~5.01) 轻度静脉曲张组 61 21.07±4.64 2.06(1.45~2.93) 中度静脉曲张组 45 25.25±6.791)2) 1.44(0.91~2.00)1)2) 重度静脉曲张组 44 28.53±9.711)2) 1.07(0.72~1.75)1)2) 统计值 F=32.00 H=49.58 P值 <0.01 <0.01 注:与无食管静脉曲张组相比,1) P<0.01;与轻度食管静脉曲张组相比,2) P<0.01。 表 2 LSM、PC/ST、LSM+PC/ST对各组食管静脉曲张程度的预测价值
分组 AUC 截断值 敏感度(%) 特异度(%) 95% CI 阳性预测值(%) 阴性预测值(%) 轻度静脉曲张组 LSM 0.762 18.9 75.4 75.0 0.675~0.849 75.4 75.0 PC/ST 0.656 3.70 86.9 41.7 0.558~0.754 60.2 75.8 LSM+PC/ST 0.7701) 54.1 88.3 0.687~0.854 82.5 65.4 中度静脉曲张组 LSM 0.841 22.5 68.9 91.7 0.762~0.922 86.1 79.7 PC/ST 0.796 1.95 75.6 73.3 0.711~0.880 68.0 80.5 LSM+PC/ST 0.8961) 88.9 76.7 0.836~0.956 74.1 90.2 重度静脉曲张组 LSM 0.834 21.5 75.0 86.7 0.748~0.920 80.5 82.5 PC/ST 0.830 1.81 79.5 76.7 0.752~0.909 71.4 83.6 LSM+PC/ST 0.9031) 79.5 85.0 0.848~0.959 79.5 85.0 注:与PC/ST相比,1) P<0.05。 -
[1] de FRANCHIS R. Revising consensus in portal hypertension: Report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension[J]. J Hepatol, 2010, 53(4): 762-768. DOI: 10.1016/j.jhep.2010.06.004. [2] de FRANCHIS R, DELL'ERA A. Invasive and noninvasive methods to diagnose portal hypertension and esophageal varices[J]. Clin Liver Dis, 2014, 18(2): 293-302. DOI: 10.1016/j.cld.2013.12.002. [3] ZHANG F, LIU T, GAO P, et al. Predictive value of a noninvasive serological hepatic fibrosis scoring system in cirrhosis combined with oesophageal varices[J]. Can J Gastroenterol Hepatol, 2018, 2018: 7671508. DOI: 10.1155/2018/7671508. [4] DUAH A, NKRUMAH KN, TACHI K. Non-invasive markers as predictors of oesophageal varices in cirrhotic patient in a teaching hospital in Ghana[J]. Ghana Med J, 2019, 53(2): 142-149. DOI: 10.4314/gmj.v53i2.9. [5] LI N, ZHENG SQ, ZHAO SS. Application of FibroScan, APRI, FIB-4 and GPR in prediction of esophageal varices in patients with liver cirrhosis[J]. J Pract Hepatol, 2020, 23(4): 560-563. DOI: 10.3969/j.issn.1672-5069.2020.04.027.李娜, 郑少秋, 赵守松. FibroScan联合多种预测模型预测肝硬化患者食管静脉曲张程度应用价值探讨[J]. 实用肝脏病杂志, 2020, 23(4): 560-563. DOI: 10.3969/j.issn.1672-5069.2020.04.027. [6] Chinese Society of Hepatology, Chinese Medical Association; Chinese Society of Gastroenterology, Chinese Medical Association; Chinese Society of Endoscopy, Chinese Medical Association. Guidelines for the diagnosis and treatment of esophageal and gastric variceal bleeding in cirrhotic portal hypertension[J]. J Clin Hepatol, 2016, 32(2): 203-219. DOI: 10.3969/j.issn.1001-5256.2016.02.002.中华医学会肝病学分会, 中华医学会消化病学分会, 中华医学会内镜学分会. 肝硬化门静脉高压食管胃静脉曲张出血的防治指南[J]. 临床肝胆病杂志, 2016, 32(2): 203-219. DOI: 10.3969/j.issn.1001-5256.2016.02.002. [7] YU YC, HOU JL. Recommendations for the use of transient elastography in chronic viral hepatitis: Introduction to the Australian Liver Association expert consensus in 2014[J]. J Clin Hepatol, 2015, 31(4): 490-494. DOI: 10.3969/J.issn.1001-5256.2015.04.004.于乐成, 侯金林. 《2014年澳大利亚肝病学会专家共识: 瞬时弹性成像在慢性病毒性肝炎中的应用建议》简介[J]. 临床肝胆病杂志, 2015, 31(4): 490-494. DOI: 10.3969/j.issn.1001-5256.2015.04.004. [8] CASTÉRA L, VERGNIOL J, FOUCHER J, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C[J]. Gastroenterology, 2005, 128(2): 343-350. DOI: 10.1053/j.gastro.2004.11.018. [9] WANG S, ZHANG W, ZHANG F, et al. Correlation between FibroScan, APRI detecting the degree of esophageal and gastric varices of liver cirrhosis[J]. Chin J Hepatol, 2018, 26(5): 342-346. DOI: 10.3760/cma.j.issn.1007-3418.2018.05.006.王帅, 张威, 张帆, 等. FibroScan和APRI对肝硬化食管胃底静脉曲张程度的预测价值[J]. 中华肝脏病杂志, 2018, 26(5): 342-346. DOI: 10.3760/cma.j.issn.1007-3418.2018.05.006. [10] KEW GS, CHEN ZJ, YIP AW, et al. Identifying patients with cirrhosis who might avoid screening endoscopy based on serum albumin and bilirubin and platelet counts[J]. Clin Gastroenterol Hepatol, 2021, 19(1): 199-201. e2. DOI: 10.1016/j.cgh.2019.11.015. [11] BERGER A, RAVAIOLI F, FARCAU O, et al. Including ratio of platelets to liver stiffness improves accuracy of screening for esophageal varices that require treatment[J]. Clin Gastroenterol Hepatol, 2021, 19(4): 777-787. e17. DOI: 10.1016/j.cgh.2020.06.022. [12] YANG LB, XU JY, TANTAI XX, et al. Non-invasive prediction model for high-risk esophageal varices in the Chinese population[J]. World J Gastroenterol, 2020, 26(21): 2839-2851. DOI: 10.3748/wjg.v26.i21.2839. [13] KARATZAS A, KONSTANTAKIS C, AGGELETOPOULOU I, et al. Non-invasive screening for esophageal varices in patients with liver cirrhosis[J]. Ann Gastroenterol, 2018, 31(3): 305-314. DOI: 10.20524/aog.2018.0241. [14] SAMI SS, HARMAN D, RAGUNATH K, et al. Non-invasive tests for the detection of oesophageal varices in compensated cirrhosis: Systematic review and meta-analysis[J]. United European Gastroenterol J, 2018, 6(6): 806-818. DOI: 10.1177/2050640618767604. [15] SINGH S, MUIR AJ, DIETERICH DT, et al. American Gastroenterological Association institute technical review on the role of elastography in chronic liver diseases[J]. Gastroenterology, 2017, 152(6): 1544-1577. DOI: 10.1053/j.gastro.2017.03.016. [16] KIM BK, HAN KH, PARK JY, et al. A liver stiffness measurement-based, noninvasive prediction model for high-risk esophageal varices in B-viral liver cirrhosis[J]. Am J Gastroenterol, 2010, 105(6): 1382-1390. DOI: 10.1038/ajg.2009.750. [17] ZHANG X, GUO Y, YANG J. Evaluation ofesophageal and gastric varices by liver and splenic stiffness measurement by elastography in patients with hepatitis B liver cirrhosis[J]. J Pract Hepatol, 2021, 24(1): 95-98. DOI: 10.3969/j.issn.1672-5069.2021.01.025.张昕, 郭悦, 杨军. 瞬时弹性成像测定肝脾硬度值评估乙型肝炎肝硬化患者食管静脉曲张初步研究[J]. 实用肝脏病杂志, 2021, 24(1): 95-98. DOI: 10.3969/j.issn.1672-5069.2021.01.025. [18] LIN XY, HU XM, CHEN YP. Value of noninvasive liver fibrosis markers in predicting high-risk gastroesophageal varices in patients with liver cirrhosis[J]. J Clin Hepatol, 2020, 36(8): 1742-1746. DOI: 10.3969/j.issn.1001-5256.2020.08.013.林小钰, 胡晓敏, 陈永鹏. 无创肝纤维化指标对肝硬化患者高风险胃食管静脉曲张的预测价值[J]. 临床肝胆病杂志, 2020, 36(8): 1742-1746. DOI: 10.3969/j.issn.1001-5256.2020.08.013. [19] HAN Y, LIU YM, LIAO HY, et al. Non-invasive diagnostic indicators for predicting esophagealgastric varices in patients with hepatitis B-related cirrhosis[J]. Chin J Hepatol, 2012, 20(2): 141-143. DOI: 10.3760/cma.j.issn.1007-3418.2012.02.016.韩莹, 刘燕敏, 廖慧钰, 等. 无创伤诊断指标对乙型肝炎肝硬化患者伴食管胃底静脉曲张风险的评估[J]. 中华肝脏病杂志, 2012, 20(2): 141-143. DOI: 10.3760/cma.j.issn.1007-3418.2012.02.016. 期刊类型引用(15)
1. 汪思远,雷淼,赵文. 病毒性肝炎肝硬化食管静脉曲张无创评估方法的预测价值比较. 齐齐哈尔医学院学报. 2024(02): 118-123 .  百度学术
百度学术2. 果鑫园,崔婷. 基于能谱CT成像的脾脏血流参数在CPH患者GOV程度中的评估价值. 中国CT和MRI杂志. 2024(04): 106-108 .  百度学术
百度学术3. 郭声,罗颖敏,曾艺军,包龙远,魏应凤. 肝脾硬度联合门静脉宽度与酒精性肝硬化食管胃底静脉曲张严重程度的相关性分析. 中国当代医药. 2024(16): 30-33+41 .  百度学术
百度学术4. 王影,胡海峰,李璐,刘慧临,张英慧,邓佳佳. 超声剪切波弹性成像联合血小板计数在肝硬化食管胃底静脉曲张中的应用价值. 影像研究与医学应用. 2024(16): 167-169+173 .  百度学术
百度学术5. 邱素玲,郭裕婷. 二维超声剪切波弹性成像及频散成像在诊断代偿期肝硬化高风险食管胃静脉曲张中的临床价值. 中国医疗器械信息. 2024(17): 80-82 .  百度学术
百度学术6. 靳晓燕,魏红冬,王文刚,要志军,许文胜. 基于肝脾多模态超声构建肝硬化食管胃底静脉曲张破裂出血风险Nomogram无创预测模型. 河北医科大学学报. 2024(10): 1156-1162 .  百度学术
百度学术7. 王静. 超声剪切波弹性成像技术在评估肝硬化程度评估中的应用价值及相关参数分析. 影像研究与医学应用. 2024(22): 30-32+35 .  百度学术
百度学术8. 牛丽娜,宋贺卫,赵金库,李鑫贺,钟浩义,徐强,王晓忠. 门静脉流速、血小板计数与脾脏长径比值对乙型肝炎肝硬化食管胃底静脉曲张的预测价值. 肝脏. 2024(10): 1225-1229 .  百度学术
百度学术9. 中华医学会消化内镜学分会食管胃静脉曲张内镜诊断与治疗学组. 肝硬化门静脉高压食管胃静脉曲张内镜下硬化治疗专家共识(2022, 长沙). 中华消化内镜杂志. 2023(01): 1-11 .  百度学术
百度学术10. 刘水澎,张国顺. 彩色多普勒超声检测门静脉、脾静脉血流动力学在肝硬化食管胃底静脉曲张患者中的临床应用研究. 中国实验诊断学. 2023(04): 436-439 .  百度学术
百度学术11. 商宁,黄秀香,田楠楠,陈美玲,叶迎宾,张嫄. 原发性胆汁性胆管炎的肝脏硬度值与脾静动脉血流学参数的相关性分析. 生物医学工程与临床. 2023(03): 286-291 .  百度学术
百度学术12. 程晓静. 肝瞬时弹性成像指标、脾脏厚度、门静脉内径及FIB4、APRI与肝硬化食管静脉曲张程度的相关性. 中外医学研究. 2023(14): 75-78 .  百度学术
百度学术13. 黄柏盛,邓文婷,区蓝芯,张莹洁,方梦冰,黎胜,施梅姐,萧焕明. 吲哚菁绿清除试验对乙型肝炎肝硬化患者发生食管胃底静脉曲张的预测价值. 中西医结合肝病杂志. 2023(10): 869-872 .  百度学术
百度学术14. 卢慧玲. 三项联合检测对肝硬化合并食管胃底静脉曲张破裂出血风险的预测价值. 当代临床医刊. 2023(05): 36-38 .  百度学术
百度学术15. 韩才均,黄媛,粘彬,朴美花. 血管性血友病因子抗原与白蛋白比值和糖萼素指数评分对乙型肝炎肝硬化食管静脉曲张的预测价值. 临床肝胆病杂志. 2022(12): 2750-2754 .  本站查看
本站查看其他类型引用(7)
-




 PDF下载 ( 2392 KB)
PDF下载 ( 2392 KB)

 下载:
下载:

 下载:
下载:
 百度学术
百度学术


