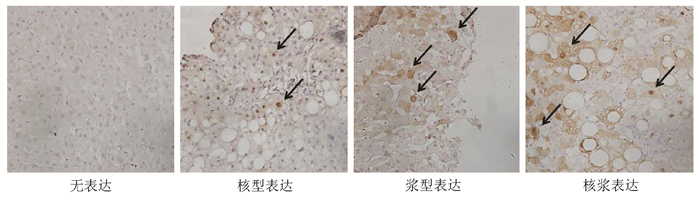
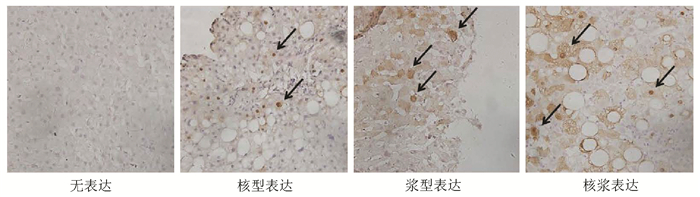
HBcAg在肝细胞中的表达及其与抗病毒疗效的关系
DOI: 10.3969/j.issn.1001-5256.2021.06.017
Expression of HBcAg in hepatocytes and its association with the efficacy of antiviral therapy
-
摘要:
目的 研究HBcAg在肝细胞中的表达对血清抗-HBc水平和核苷类药物抗病毒治疗后HBeAg血清学转换的影响。 方法 收集南方医院和番禺区中心医院2015年1月—2018年6月101例接受核苷类药物抗病毒治疗的慢性乙型肝炎患者的血清样本和肝组织石蜡切片,酶联免疫吸附测定血清中的抗-HBc定量,免疫组化检测肝内HBcAg表达。通过GEO数据库(GSE96851)分析获得HBcAg阳性肝炎活动患者肝内差异基因表达。计量资料两组间比较采用两独立样本t检验, 多组间比较采用多个独立样本非参数Kruskal-Wrallis H检验,进一步两两比较采用Dunnett法。计数资料多组间比较采用χ2检验。 结果 HBcAg在肝细胞中,根据表达类型可分为无表达型、核型、浆型和核浆型,根据表达量可分为Ⅰ、Ⅱ、Ⅲ和Ⅳ级。抗病毒96周的HBeAg血清学转换在无表达型、核型、浆型和核浆型患者的比例分别为5.88%、16.67%、22.73%、24.24%(χ2=4.753,P=0.037);抗病毒96周的HBeAg血清学转换在Ⅰ、Ⅱ、Ⅲ和Ⅳ级患者的比例分别为5.88%、13.04%、27.59%、26.67%(χ2=6.580,P=0.016)。血清IgM型抗-HBc、总抗-HBc在无表达型、核型、浆型和核浆型患者中差异均有统计学意义(H值分别为9.760、21.46,P值分别为0.021<0.001);血清IgM型抗-HBc、总抗-HBc在Ⅰ、Ⅱ、Ⅲ和Ⅳ级患者中差异均有统计学意义(H值分别为18.80、26.03,P值均<0.001)。肝内差异基因分析显示,HBcAg阳性急性肝衰竭患者,肝内抗体相关基因表达上调。 结论 肝细胞内HBcAg在胞浆表达类型及表达水平与血清抗-HBc有关,其检测有利于预测核苷类药物抗病毒治疗的效果。 Abstract:Objective To investigate the effect of the expression of HBcAg in hepatocytes on the serum level of HBcAb and seroconversion of HBeAg after antiviral therapy with nucleos(t)ide analogues (NUCs). Methods Serum samples and liver tissue paraffin sections were collected from 101 chronic hepatitis B (CHB) patients who received antiviral therapy with NUCs in Nanfang Hospital and Panyu Central Hospital from January 2015 to June 2018. ELISA was used to measure the serum level of HBcAb, and immunohistochemistry was used to measure the expression of HBcAg in the liver. The GEO database (GSE96851) was analyzed to obtain differentially expressed genes in the liver of patients with HBcAg-positive hepatitis. The two-independent-samples t test was used for comparison of continuous data between two groups; the multiple-independent-samples nonparametric Kruskal-Wallis H test was used for comparison of continuous data between multiple groups, and Dunnett method was used for further comparisons; the chi-square test was used for comparison of categorical data between groups. Results The expression pattern of HBcAg in hepatocytes was classified as absent expression, nuclear expression, cytoplasmic expression, and nuclear/cytoplasmic expression, and according to expression level, HBcAg expression was classified as grades Ⅰ, Ⅱ, Ⅲ, and Ⅳ expression. HBeAg seroconversion rates after 96 weeks of antiviral therapy were 5.88%, 16.67%, 22.73%, and 24.24%, respectively, in the patients with absent expression, nuclear expression, cytoplasmic expression, and nuclear/cytoplasmic expression (χ2=4.753, P=0.037), and HBeAg seroconversion rates after 96 weeks of antiviral therapy were 5.88%, 13.04%, 27.59%, and 26.67%, respectively, in the patients with grade Ⅰ, Ⅱ, Ⅲ, and Ⅳ expression (χ2=6.580, P=0.016). There were significant differences in the serum levels of HBcAb-IgM and total HBcAb between the patients with absent expression, nuclear expression, cytoplasmic expression, and nuclear/cytoplasmic expression of HBcAg (HBcAb-IgM: H=9.760, P=0.021; total HBcAb: H=21.46, P < 0.001), and there were also significant differences in the serum levels of HBcAb-IgM and total HBcAb between the patients with grade Ⅰ, Ⅱ, Ⅲ, and IV expression of HBcAg (HBcAb-IgM: H=18.80, P < 0.001; total HBcAb: H=26.03, P < 0.001). The analysis of differentially expressed genes in the liver showed that the expression of antibody-related genes was upregulated in the liver of patients with HBcAg-positive acute liver failure. Conclusion The expression pattern and level of HBcAg in the cytoplasm of hepatocytes are associated with serum HBcAb, and the measurement of HBcAg may help to predict the efficacy of antiviral therapy with NUCs. -
慢性乙型肝炎(CHB)是肝硬化和肝细胞癌(HCC)发生的主要原因[1]。核苷类药物可以有效抑制HBV复制,但需要长期治疗,且停用后复发风险高,因此寻找预测抗病毒疗效的指标成为CHB治疗的重要临床问题[2-3]。HBeAg血清学转换作为HBeAg阳性的CHB患者抗病毒疗效反应的重要替代终点之一,在临床研究中得到广泛认可[4-5]。
近年来,不断有研究发现血清抗-HBc定量对于预测CHB患者抗病毒疗效具有重要意义,发生HBeAg血清学转换的患者其治疗前血清抗-HBc定量明显升高[6-7]。另外,核苷类药物抗病毒停药时高血清抗-HBc定量与停药后复发率降低有关[8]。抗-HBc的产生由HBV中的HBcAg刺激机体免疫细胞产生,而HBcAg在肝内分布特点以及其对免疫细胞的刺激作用的相关研究报道仍然相对缺乏。因此,深入探索HBcAg表达与HBeAg血清学转换之间的关系,将有助于CHB患者临床疗效的预测,对CHB治疗新靶点研发具有指导意义。
1. 资料与方法
1.1 一般资料
纳入2015年1月—2018年6月在南方医院和番禺区中心医院采用核苷类药物行抗病毒治疗的101例CHB患者作为研究对象,并对所有患者随访96周。患者诊断符合《慢性乙型肝炎防治指南(2015年更新版)》[1],且为HBeAg阳性,并排除了合并甲、丙、丁和戊型肝炎,自身免疫性肝病。排除未经抗病毒治疗或者使用了干扰素治疗方案的患者。
1.2 观察指标
实验所用的血清为临床检测项目剩余标本。采用美国Beckman全自动生化分析仪检测患者血清中的生化指标(ALT、AST、TBil); 采用全自动实时荧光定量PCR检测血清HBV DNA(试剂购自广州达安基因诊断中心); 采用Abbott i2000化学发光免疫分析仪进行HBV血清标志物中的各项指标的检测。
1.3 肝脏病理和HBcAg免疫组化染色
所有患者均接受B超引导下的肝穿刺活检,采用10%甲醛固定,石蜡包埋,切片制作由病理科医生负责。5 μm石蜡切片在65 ℃中脱蜡2 h后水化,PBS缓冲液充分洗涤后在枸橼酸钠缓冲液中进行高压抗原修复,3% H2O2浸泡10 min,PBS缓冲液洗涤,10% BSA常温封闭1 h,加入兔抗人HBcAg抗原抗体[基因科技(上海)股份有限公司,货号GB058607],4 ℃孵育16 h,PBS洗涤后加入羊抗兔HRP,DAB进行显色。HBcAg免疫组化结果通过Image J软件进行半定量分析,HBcAg表达量在0~5%为Ⅰ级,5%~25%为Ⅱ级,25%~50%为Ⅲ级,大于50%为Ⅳ级。
1.4 GEO数据库分析
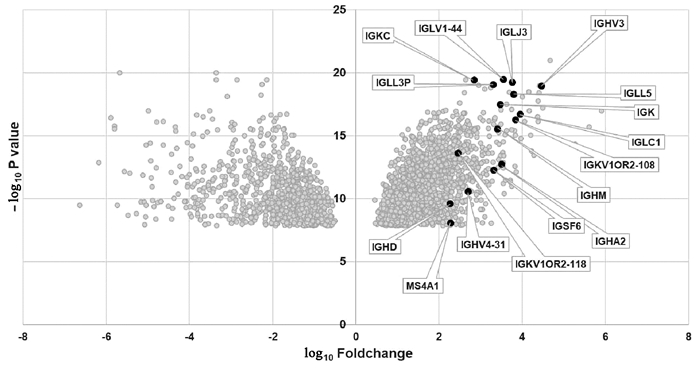
通过GEO2R网上在线分析工具(www.ncbi.nlm.nih.gov/geo/geo2r)分析GSE96851数据集中的差异基因表达。该数据库中10例肝移植供者肝脏组织和7例血管瘤患者切除肝组织为对照组,急性肝衰竭患者组织4例为实验组(其中3例标本有4个重复检测数据,1例标本有5个重复检测数据,共17个测序数据)。差异基因的筛选标准为|logFC (fold change)|≥2且调整后的P值<0.05。火山图使用EXCEL作图。
1.5 酶联免疫吸附试验(ELISA)
干燥采血管收集患者2 ml全血,离心后留取血清标本。采用北京万泰生物药业股份有限公司的总核心抗体定量和IgM型核心抗体ELISA试剂盒,严格按照说明书操作步骤进行操作,对患者血清中的核心抗体进行检测。使用美国博腾公司酶标仪Gen5,读取450 nm检测值,使用分光光度(optical density, OD)值进行比较分析。
1.6 伦理学审查
本研究方案经由广州市番禺区中心医院伦理委员会审批,批号:PYRC-2020-020,所纳入患者均签署知情同意书。
1.7 统计学方法
所有数据采用SPSS 20.0统计软件及Graphpad Prism 5.0进行处理和构图。符合正态分布的计量资料用x±s表示,两组间比较采用两独立样本t检验; 不符合正态分布的计量资料多组间比较采用多个独立样本非参数Kruskal-Wrallis H检验,进一步两两比较采用Dunnett法。计数资料多组间比较采用χ2检验。P<0.05为差异有统计学意义。
2. 结果
2.1 CHB患者基本信息
101例患者在接受96周抗病毒治疗后,有17例患者发生HBeAg血清学转换。HBeAg血清学转换和未转换患者在基线时,年龄、AST、TBil、HBV DNA载量和HBsAg定量均无统计学差异(P值均>0.05),但是发生HBeAg转换的患者ALT水平较高(P=0.035)(表 1)。
表 1 CHB患者基本资料指标 HBeAg转换(n=17) HBeAg未转换(n=84) 统计值 P值 男/女 10/7 64/20 χ2=2.177 年龄(岁) 33.7±8.5 30.2±9.6 t=1.323 0.189 ALT (U/L) 85.6±13.3 73.0±22.1 t=2.136 0.035 AST(U/L) 51.3±20.2 45.3±22.6 t=0.963 0.338 TBil (μmol/L) 5.2±2.8 6.7±3.1 t=1.752 0.083 HBV DNA (log10 IU/ml) 5.81±1.81 5.94±1.53 t=0.295 0.768 HBsAg(IU/ml) 3.76±0.65 3.93±0.45 t=1.257 0.212 2.2 CHB患者肝内HBcAg的表达类型和方式
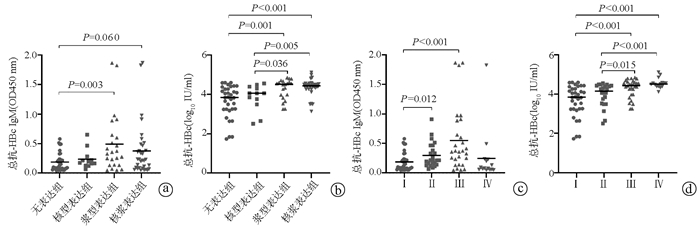
通过对101例患者肝穿活检石蜡标本进行HBcAg免疫组化染色,结果发现CHB患者肝内HBcAg表达主要分成4个类型,分别为无表达型、核型表达(HBcAg表达于肝细胞核)、浆型表达(HBcAg表达于肝细胞胞浆)和核浆表达(HBcAg同时表达于肝细胞核和胞浆)(图 1)。
2.3 HBcAg表达类型、病理分级与HBeAg血清学转换的关系
按照HBcAg在肝内不同的表达类型分组,96周核苷类抗病毒治疗后,HBeAg血清学转换率在不同组间存在显著差异(χ2=4.753,P=0.037)。按照不同HBcAg分级分组,HBeAg血清学转换率同样在不同组间存在显著差异(χ2=6.580,P=0.016)(表 2)。
表 2 不同HBcAg表达类型、分级的患者HBeAg血清学转换情况HBcAg表达类型 例数 HBeAg血清学转换[例(%)] HBcAg分级 例数 HBeAg血清学转换[例(%)] 无表达组 34 2(5.88) Ⅰ级 34 2(5.88) 核型表达组 12 2(16.67) Ⅱ级 23 3(13.04) 浆型表达组 22 5(22.73) Ⅲ级 29 8(27.59) 核浆表达组 33 8(24.24) Ⅳ级 15 4(26.67) 2.4 抗-HBc定量在HBcAg不同类型患者的差异
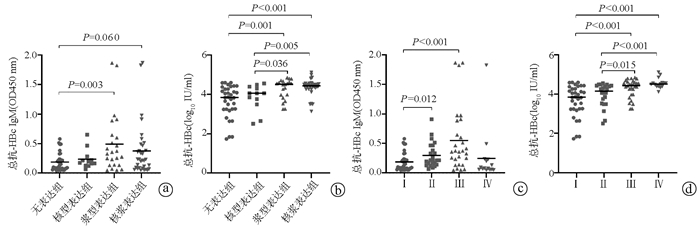
ELISA结果显示,血清IgM型抗-HBc、总抗-HBc在无表达型、核型、浆型和核浆型患者差异均有统计学意义(H=9.760,P=0.021;H=21.46,P<0.001);血清IgM型抗-HBc、总抗-HBc在Ⅰ、Ⅱ、Ⅲ和Ⅳ级患者差异均有统计学意义(H=18.80,P<0.001;H=26.03,P<0.001)。组间进一步比较,无表达组血清IgM抗-HBc表达明显低于浆型表达组(P=0.003)(图 2a); 而在总抗-HBc表达上,无表达组低于浆型表达组(P=0.001)和核浆表达组(P<0.001),核型表达组低于浆型表达组(P=0.036)和核浆表达组(P=0.005)(图 2b)。HBcAg病理Ⅱ、Ⅲ级患者IgM型抗-HBc水平高于Ⅰ级患者,Ⅲ、Ⅳ级的患者,其总抗-HBc水平高于Ⅰ级和Ⅱ级患者(P值均<0.05)(图 2c、d)。
2.5 HBcAg阳性的肝炎活动患者肝内差异基因表达
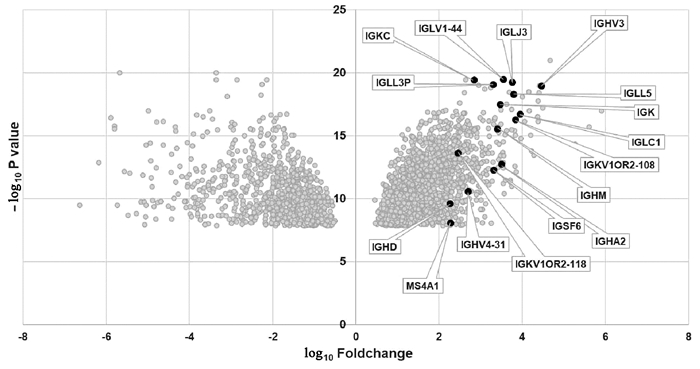
通过GSE96851基因表达芯片分析,发现HBcAg阳性的急性肝衰竭患者相对于对照组患者,肝内B细胞基因MS4A1(即CD20分子)和抗体相关基因(IGHA2、IGHD、IGHM、IGHV3、IGHV4-31、IGK、IGKC、IGKV1OR2-108、IGKV1OR2-118、IGLC1、IGLJ3、IGLL3P、IGLL5、IGLV1-44、IGSF6)有明显上调表达。结果提示肝炎活动患者,肝内B细胞活化及抗体产生等免疫反应明显激活(图 3)。
3. 讨论
据统计我国目前约有CHB患者3000万,且每年约有30万人死于乙型肝炎相关肝硬化、肝癌及终末期肝病,带来沉重的经济负担[1]。目前核苷类药物治疗仅能有效抑制HBV的复制,而难以达到临床治愈,探索并挖掘预测临床疗效的指标和分子机制,具有重要的临床意义。在本研究中,对101例HBeAg阳性的CHB患者进行长期随访,发现抗病毒基线时CHB患者肝内HBcAg表达具有不同的表达类型及表达强度,浆型和核浆表达患者在核苷类抗病毒治疗后HBeAg血清学转换率较高,HBcAg表达强度为Ⅲ级患者HBeAg血清学转换率也较高。
已有动物实验[9-10]表明,B淋巴细胞在HBV感染的控制中发挥重要作用,而CHB患者体内HBV特异性B淋巴细胞功能明显受到抑制或者损害,以至不能产生特异性抗体[11]。HBcAg具有强免疫原性,有利于机体免疫细胞的活化[12],然而其主要在肝细胞内表达而极少分泌到外周血中,从而影响其对免疫的调节作用,与以往报道类似[13-15]。其中,浆型和核浆型表达患者,以及肝内HBcAg表达越高的患者,其血清中总抗-HBc和IgM型抗-HBc明显升高,提示肝脏炎症损伤时,HBcAg暴露或者释放到血液循环中,激活了免疫细胞。因此,HBcAg在肝细胞的表达类型与表达水平,可能是影响患者免疫功能和抗病毒疗效的因素之一。
近年来,不断有文献[16-17]报道血清抗-HBc定量检测可以作为CHB患者接受核苷类或者干扰素治疗后预测HBeAg血清学转换的独立危险因素,然而其中的相关机制尚未完全阐明。既往临床实践表明,HBcAg在血清中难以被检测,主要原因在于其被HBsAg包裹而无法暴露,更难以直接刺激免疫细胞。有趣的是,本研究发现肝内HBcAg由细胞核向细胞胞浆表达的转变中,与血清总抗-HBc和IgM型抗-HBc定量有明显关系,推测HBV感染患者在肝炎波动时,肝细胞损伤后HBcAg由胞核向胞浆甚至是胞膜外暴露,激活机体免疫反应(尤其是B细胞反应产生HBV特异性抗体),这在一定程度上解释了抗-HBc定量预测CHB患者抗病毒治疗后发生HBeAg血清学转换的免疫学机制。
综上所述,HBcAg在肝细胞中的检测对CHB患者核苷类药物抗病毒疗效预测具有重要作用。
-
表 1 CHB患者基本资料
指标 HBeAg转换(n=17) HBeAg未转换(n=84) 统计值 P值 男/女 10/7 64/20 χ2=2.177 年龄(岁) 33.7±8.5 30.2±9.6 t=1.323 0.189 ALT (U/L) 85.6±13.3 73.0±22.1 t=2.136 0.035 AST(U/L) 51.3±20.2 45.3±22.6 t=0.963 0.338 TBil (μmol/L) 5.2±2.8 6.7±3.1 t=1.752 0.083 HBV DNA (log10 IU/ml) 5.81±1.81 5.94±1.53 t=0.295 0.768 HBsAg(IU/ml) 3.76±0.65 3.93±0.45 t=1.257 0.212 表 2 不同HBcAg表达类型、分级的患者HBeAg血清学转换情况
HBcAg表达类型 例数 HBeAg血清学转换[例(%)] HBcAg分级 例数 HBeAg血清学转换[例(%)] 无表达组 34 2(5.88) Ⅰ级 34 2(5.88) 核型表达组 12 2(16.67) Ⅱ级 23 3(13.04) 浆型表达组 22 5(22.73) Ⅲ级 29 8(27.59) 核浆表达组 33 8(24.24) Ⅳ级 15 4(26.67) -
[1] Chinese Society of Hepatology and Chinese Society of Infectious Diseases Chinese, Medical Association. The guideline of prevention and treatment for chronic hepatitis B: A 2015 update[J]. J Clin Hepatol, 2015, 31(12): 1941-1960. DOI: 10.3969/j.issn.1001-5256.2015.12.002.中华医学会肝病学分会, 中华医学会感染病学分会. 慢性乙型肝炎防治指南(2015年更新版)[J]. 临床肝胆病杂志, 2015, 31(12): 1941-1960. DOI: 10.3969/j.issn.1001-5256.2015.12.002. [2] YEO YH, HO HJ, YANG HI, et al. Factors associated with rates of HBsAg seroclearance in adults with chronic HBV infection: A systematic review and Meta-analysis[J]. Gastroenterology, 2019, 156(3): 635-646. e9. DOI: 10.1053/j.gastro.2018.10.027. [3] LOK AS, MCMAHON BJ. Chronic hepatitis B: Update 2009[J]. Hepatology, 2009, 50(3): 661-662. DOI: 10.1002/hep.23190. [4] LIAW YF, KAO JH, PIRATVISUTH T, et al. Erratum to: Asian-Pacific consensus statement on the management of chronic hepatitis B: A 2012 update[J]. Hepatol Int, 2012, 6(4): 809-810. DOI: 10.1007/s12072-012-9386-z. [5] LIAW YF, LAU GK, KAO JH, et al. Hepatitis B e antigen seroconversion: A critical event in chronic hepatitis B virus infection[J]. Dig Dis Sci, 2010, 55(10): 2727-2734. DOI: 10.1007/s10620-010-1179-4. [6] FAN R, SUN J, YUAN Q, et al. Baseline quantitative hepatitis B core antibody titre alone strongly predicts HBeAg seroconversion across chronic hepatitis B patients treated with peginterferon or nucleos(t)ide analogues[J]. Gut, 2016, 65(2): 313-320. DOI: 10.1136/gutjnl-2014-308546. [7] YUAN Q, SONG LW, LIU CJ, et al. Quantitative hepatitis B core antibody level may help predict treatment response in chronic hepatitis B patients[J]. Gut, 2013, 62(1): 182-184. DOI: 10.1136/gutjnl-2012-302656. [8] CHI H, LI Z, HANSEN BE, et al. Serum level of antibodies against hepatitis B core protein is associated with clinical relapse after discontinuation of nucleos(t)ide analogue therapy[J]. Clin Gastroenterol Hepatol, 2019, 17(1): 182-191. e1. DOI: 10.1016/j.cgh.2018.05.047. [9] WANG X, DONG Q, LI Q, et al. Dysregulated response of follicular helper t cells to hepatitis B surface antigen promotes HBV persistence in mice and associates with outcomes of patients[J]. Gastroenterology, 2018, 154(8): 2222-2236. DOI: 10.1053/j.gastro.2018.03.021. [10] LIU XJ, ZHANG Z. Functional cure of chronic hepatitis B from the perspective of specific immune cells for hepatitis B virus[J]. J Clin Hepatol, 2020, 36(5): 977-979. DOI: 10.3969/j.issn.1001-5256.2020.05.004.刘小菊, 张政. 从HBV特异性免疫细胞角度谈慢性乙型肝炎功能性治愈[J]. 临床肝胆病杂志, 2020, 36(5): 977-979. DOI: 10.3969/j.issn.1001-5256.2020.05.004. [11] SALIMZADEH L, LE BERT N, DUTERTRE CA, et al. PD-1 blockade partially recovers dysfunctional virus-specific B cells in chronic hepatitis B infection[J]. J Clin Invest, 2018, 128(10): 4573-4587. DOI: 10.1172/JCI121957. [12] MILICH DR, MCLACHLAN A. The nucleocapsid of hepatitis B virus is both a T-cell-independent and a T-cell-dependent antigen[J]. Science, 1986, 234(4782): 1398-1401. DOI: 10.1126/science.3491425. [13] CHU CM, LIAW YF. Intrahepatic distribution of hepatitis B surface and core antigens in chronic hepatitis B virus infection. Hepatocyte with cytoplasmic/membranous hepatitis B core antigen as a possible target for immune hepatocytolysis[J]. Gastroenterology, 1987, 92(1): 220-225. DOI: 10.1016/0016-5085(87)90863-8. [14] CHU CM, LIAW YF. Immunohistological study of intrahepatic expression of hepatitis B core and e antigens in chronic type B hepatitis[J]. J Clin Pathol, 1992, 45(9): 791-795. DOI: 10.1136/jcp.45.9.791. [15] SAITO T, KAMIMURA T, ISHIBASHI M, et al. Electron microscopic study of hepatitis B virus-associated antigens on the infected liver cell membrane in relation to analysis of immune target antigens in chronic hepatitis B[J]. Gastroenterol Jpn, 1992, 27(6): 734-744. DOI: 10.1007/BF02806526. [16] CHEN HS, WU JF, SU TH, et al. Baseline level of hepatitis B core antibody predicts spontaneous hepatitis B e antigen (HBeAg) seroconversion in HBeAg-positive children with a normal alanine aminotransferase level[J]. Hepatology, 2019, 70(6): 1903-1912. DOI: 10.1002/hep.30788. [17] VANWOLLEGHEM T, GROOTHUISMINK Z, KREEFFT K, et al. Hepatitis B core-specific memory B cell responses associate with clinical parameters in patients with chronic HBV[J]. J Hepatol, 2020, 73(1): 52-61. DOI: 10.1016/j.jhep.2020.01.024. 期刊类型引用(1)
1. 李剧,花艳艳,韩亚男. 自身抗体及肝功能检测在自身免疫性肝病与病毒性肝炎鉴别诊断中的价值. 中国医药指南. 2022(10): 58-61 .  百度学术
百度学术其他类型引用(0)
-




 PDF下载 ( 2138 KB)
PDF下载 ( 2138 KB)

 下载:
下载:



 下载:
下载:
 百度学术
百度学术