A transcriptomic analysis of acute hepatotoxicity induced by aristolochic acid Ⅰ in mice
-
摘要:
目的 探讨马兜铃酸Ⅰ(AAⅠ)致小鼠急性肝损伤的分子机制。 方法 雄性C57BL/6小鼠15只,随机分为正常组(n=6)和处理组(n=9)。处理组小鼠以AAⅠ 20 mg/kg剂量腹腔注射,连续5 d,第6天处死、取材。检测血清ALT、AST,HE染色观察肝组织学变化;两组各随机选择3例肝组织样本提取RNA进行高通量转录组测序。通过生物信息学分析和功能预测筛选出表达显著的差异基因;再采用实时荧光定量PCR (qRT-PCR) 对部分差异基因进行验证。计量资料两组间差异比较采用t检验。 结果 与正常组比较,处理组ALT、AST水平明显升高(t值分别为4.331、4.947,P值均<0.01);处理组肝组织HE染色可见肝小叶结构紊乱,小叶内见点状坏死。通过筛选条件[logFC>2倍且P<0.05]获得差异基因共计1352个,其中上调基因703个,下调基因649个。对这些差异性表达的基因进行GO和KEGG富集分析(P<0.05),小分子分解代谢、中性粒细胞参与的免疫应答、分泌颗粒细胞质囊泡腔、细胞质囊泡腔、细胞外结构组织、细胞外基质组织等相关的GO分类呈显著富集;化学致癌、视黄醇代谢、花生四烯酸代谢、类固醇激素合成、癌症中的转录失调、蛋白质消化和吸收、炎症介质对TRP通道的调节、药物代谢、补体和凝血级联、谷胱甘肽代谢、PPAR信号通路等在KEGG通路中显著富集。聚类分析发现明显下调基因包括Srd5a1、Lipc、Aqp8、Hba-a1、Slco1a1、Pklr等,采用qRT-PCR得以证实(P值均<0.05)。 结论 AAⅠ有明显急性肝毒性,其毒性主要涉及化学致癌、视黄醇代谢、花生四烯酸代谢、类固醇激素合成、癌症中的转录失调等环节。 -
关键词:
- 马兜铃酸 /
- 转录组 /
- 化学性与药物性肝损伤 /
- 小鼠, 近交C57BL
Abstract:Objective To investigate the molecular mechanism of aristolochic acid Ⅰ (AAⅠ) inducing acute hepatotoxicity in mice. Methods A total of 15 male C57BL/6 mice were randomly divided into normal group with 6 mice and treatment group with 9 mice. The mice in the treatment group were given intraperitoneal injection of AAⅠ at a dose of 20 mg/kg for 5 consecutive days and were sacrificed to collect samples on day 6. The serum levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were measured, and HE staining was used to observe liver histological changes; three liver tissue samples were randomly selected from each group, and RNA was extracted for high-throughput transcriptome sequencing. Bioinformatics analysis and functional prediction were used to screen out differentially expressed genes, and quantitative real-time PCR (qRT-PCR) was used for validation. The t-test was used for comparison of continuous data between two groups. Results Compared with the normal group, the treatment group had significant increases in the activities of ALT and AST (t=4.331 and 4.947, both P < 0.01), as well as disordered structure of hepatic lobules and spotted necrosis in hepatic lobules shown by HE staining. A total of 1352 differentially expressed genes were obtained based on the screening conditions of logFC > 2 and P < 0.05, among which there were 703 upregulated genes and 649 downregulated genes. The GO and KEGG enrichment analyses of these differentially expressed genes showed significant enrichment in GO terms (such as small molecular catabolism, immune response involving neutrophils, cytoplasmic vesicle lumen in secretory granules, cytoplasmic vesicle lumen, extracellular structural organization, and extracellular matrix) and KEGG pathways (such as chemical carcinogenesis, retinol metabolism, arachidonic acid metabolism, steroid hormone biosynthesis, transcriptional dysregulation in cancer, protein digestion and absorption, regulation of TRP channel by inflammatory mediators, drug metabolism, complement and coagulation cascade, glutathione metabolism, and the PPAR signaling pathway). A cluster analysis (P < 0.05) showed that significantly downregulated genes included Srd5a1, Lipc, Aqp8, Hba-a1, Slco1a1, and Pklr, which were validated by qRT-PCR (all P < 0.05). Conclusion AA Ⅰ can lead to significant acute hepatotoxicity, which mainly involves the processes such as chemical carcinogenesis, retinol metabolism, arachidonic acid metabolism, steroid hormone biosynthesis, and transcriptional dysregulation in cancer. -
马兜铃酸(aristolochic acids,AA)是一种硝基菲类化合物,广泛分布在马兜铃属和细辛属植物中[1],已知的AA类化合物主要有4种,分别为AA Ⅰ、Ⅱ、Ⅲ、Ⅳ,其中AA Ⅰ为主要毒性成分且毒性最强,AA Ⅱ次之,二者均是引起肾毒性和基因毒性的主要原因[2-3]。AA具有免疫激活、抗炎、肾毒性、致癌性和致突变性等作用[4],新近研究[5]表明,AA与肝癌密切相关。但AA与肝癌的关系仍有争议,因此对于AAⅠ导致肝损伤的研究还是十分必要的。
转录组学主要从RNA水平研究基因的整体表达情况,是功能基因组学重要的组成部分[6]。随着高通量测序技术的发展,转录组测序技术(RNA-Seq)凭借测序成本低、精度高、覆盖范围广等优点[7],已成为转录组分析的重要方法,为研究基因表达模式、疾病的生物标志物探测等提供了新的手段。本研究采用IIIumina HiSeq X Ten高通量测序平台,对AAⅠ致小鼠肝损伤进行高通量测序,建立相应的转录组数据库,继而进行差异表达分析以及GO、KEGG富集分析,并以实时荧光定量PCR(qRT-PCR)验证本转录表达的可靠性,以进一步明确AAⅠ致肝毒性的分子机制。
1. 材料与方法
1.1 动物
C57BL/6雄性小鼠15只,体质量23~25 g,SPF级,购自上海南方模式生物科技股份有限公司,动物生产许可证号:SCXK(沪)2017-0010;动物使用许可证号:SYXK(沪)2019-0003。按照SPF级动物饲养要求进行饲养。
1.2 试验药物
AAⅠ (批号:3503),购自上海诗丹德标准技术服务有限公司,纯度>98.5 %;羧甲基纤维素钠(批号:F20051103),购自国药集团化学试剂有限公司。
1.3 试剂
ALT试剂盒(批号:20130110)、AST试剂盒(批号:20130411),购自南京建成生物工程研究所;Trizol Reagent (批号:F919KB3054)、总RNA提取试剂盒(批号:B518811),购自上海生工生物工程股份有限公司;反转录试剂盒(批号:AJ92014A)、扩增试剂盒(批号:AJF0343A),购自日本TAKARA公司。
1.4 实验方法
1.4.1 动物分组及模型建立
小鼠适应性喂养1周后,随机分为正常组(n=6)和处理组(n=9)。处理组小鼠以AA Ⅰ 20 mg/kg剂量连续腹腔注射,1次/d,连续5 d,正常组小鼠注射相同容积的0.5%羧甲基纤维素钠混悬液。
1.4.2 药物配置
100 mg羧甲基纤维素钠溶解于20 mL双蒸水中配置成0.5%的混悬液。40 mg AAⅠ加入20 mL 0.5%羧甲基纤维素钠混悬液中制备成2 mg/mL贮存液,超声震荡至完全溶解,4 ℃保存备用。
1.4.3 样本采集
小鼠以3%戊巴比妥钠麻醉、固定,打开腹腔、下腔静脉取血,用于测定血清ALT、AST。肝右叶切割0.5 cm×0.5 cm×0.3 cm大小固定于4%甲醛固定液中,用于HE染色;另取100 mg肝组织存于Trizol中用于提取RNA。
1.4.4 指标测定
按试剂盒说明书测定血清中ALT、AST水平。
1.4.5 肝组织HE染色
肝组织经4%中性甲醛缓冲液固定,自动脱水机脱水,石蜡包埋,4 μm切片,苏木精-伊红染色(HE染色),光学显微镜(Olympus 1×70,Japan)观察并拍照。
1.4.6 肝组织转录组测序
提取肝组织总RNA,采用Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara) 进行质量检测;Qubit 2.0 RNA检测试剂盒测定RNA浓度,包括mRNA分离/片段化、双链DNA合成、末端修复、末端dA-Tailing、桥头连接、连接产物纯化和片段大小分选及文库扩增等;利用Qubit 2.0 DNA检测试剂盒精确定量回收的DNA,按1∶ 1比例定量混合,使用IIIumina HiSeq X Ten测序仪进行测序,产生150 bp的双端数据。建库测序由上海欧易生物医学科技有限公司完成。
1.4.7 基因质量控制
使用Trimmomatic (Version 0.36)软件进行质控并去除接头,在此基础上过滤掉低质量碱基以及N碱基,最终得到高质量的cleanreads。利用hisat2 (Version 2.2.1.0)软件将Clean Reads与小鼠参考基因组(从NCBI中下载参考基因组,基因组版本为GRCm38.p6)进行序列比对,获取在参考基因组或基因上的位置信息,以及测序样品特有的序列特征信息。各样本的有效数据量分布在5.99~6.83 G,Q30碱基分布在93.97%~95.01%,平均GC含量为47.90 %。通过将reads比对到参考基因组上,得到各个样本的基因组比对情况,比对率为92.6%~98.23%。
1.4.8 差异表达基因筛选
导出各样本测序结果,通过R语言软件的“Affy”“limma”包对数据分析差异表达基因,将筛选条件设为P.Value<0.05、adj.P.Value<0.05、logFC>2,获取上调和下调的差异基因。Pheatmap包绘制热图和火山图。
1.4.9 差异表达基因的GO和KEGG富集分析
得到差异表达基因后,采用R语言的“clusterProfiler”“org.Mm.eg.db”“enrichplot”“colorspace”“stringi”“ggplot2”包对差异表达基因进行GO和KEGG富集分析。设置筛选条件为P.Value Cutoff<0.05和Q.Value Cutoff<0.05,并计算生物学过程(biological process, BP)、分子功能(molecular function, MF)、细胞组分(cellular component, CC)功能上的基因富集数,最后将排名前20的GO生物过程和KEGG通路,绘制成气泡图。
1.4.10 差异表达基因的qRT-PCR验证
基于转录组差异基因结果,选择变化最显著基因进行qRT-PCR验证。使用NCBI在线引物设计软件Primer-Blast设计引物,由上海生工生物工程有限公司合成(基因名称、引物序列见表 1)。Trizol提取肝组织总RNA,采用试剂盒进行逆转录,按照SYBR Premix Ex Taq Ⅱ荧光染料法实时定量试剂盒说明进行cDNA扩增,每个样品重复3次。以β-actin为内参,采用2-△△CT法进行分析定量。
表 1 基因引物序列基因 正向引物(5′-3′) 反向引物(5′-3′) β-actin TGACGAGGCCCAGAGCAAGA ATG GGCACAGTGTGGGTGAC Srd5a1 CTACTGGATGCGCTAGTCTAC TCAGAAACATAGCTAGCAGGAC Lipc CTACAAACACATTGCAGAGCAT GTTGCAAGGAGTCAATGAAGAG Aqp8 GCTCCGCTCTCTTCATCTTCATCG GAAGTGTCCACCGCTGATGTTCC Hba-a1 ACCACCAAGACCTACTTCCCTCAC CAGAGCATCGGCGACCTTCTTG Slco1a1 TGGATTTATCACTGGGAGCTTT AGGAAATGAGGTGATGCCATTA Pklr GGCTCAGAAGATGATGATTGGA ATCGCATGTTGCATCTTTACAG 1.5 伦理学审查
所有操作均由上海南方模式生物科技股份有限公司实验动物管理和使用委员会(IACUC)审批通过,批号:IACUC号2018-0026。
1.6 统计学方法
实验结果以SPSS 21.0分析软件进行统计分析。计量资料以x ±s表示,两组间差异比较采用t检验。P<0.05为差异具有统计学意义。
2. 结果
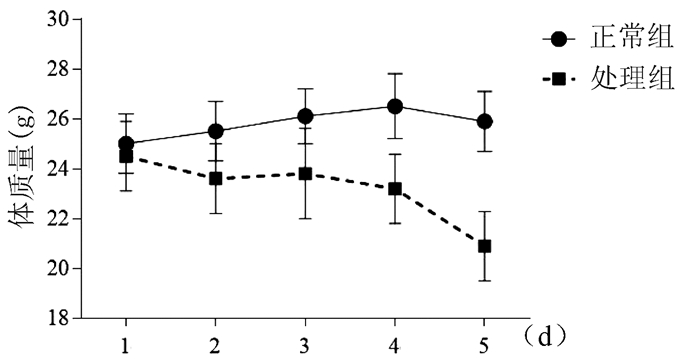
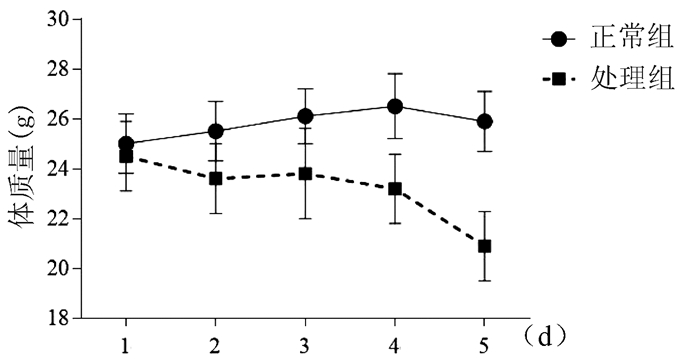
2.1 小鼠体质量变化
正常组小鼠体质量逐渐增加,AAⅠ染毒过程中,处理组小鼠体质量逐步下降,造模至第4天,体质量下降明显(图 1)。
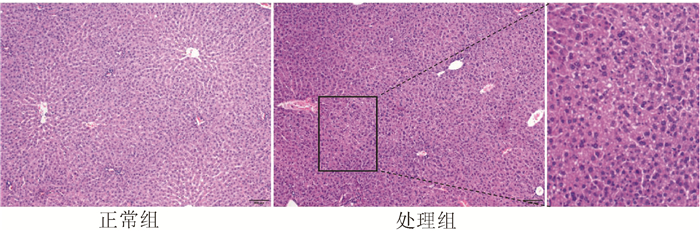
2.2 小鼠肝功能以及肝组织学的比较
与正常组相比,处理组小鼠血清ALT、AST水平分别升高7倍和4倍(表 2),差异均有统计学意义(P值均<0.01)。HE染色可见,正常组肝组织小叶结构完整,肝细胞排列整齐、无坏死;处理组肝小叶结构紊乱、肝细胞胞浆疏松、小叶内可见点状坏死(图 2)。表明20 mg/kg AA Ⅰ给药5 d可造成明显肝损伤。
表 2 两组小鼠血清ALT、AST水平的比较组别 动物数(只) ALT(U/L) AST(U/L) 正常组 6 13.95±1.56 13.44±2.05 处理组 9 102.15±34.10 53.62±22.73 t值 4.331 4.947 P值 0.002 0.001 2.3 差异表达基因的筛选结果
通过R语言中的Affy包去除原始数据中系统误差,得到标准化的基因表达数据。通过R语言中的Limma包对肝损伤组织和正常组织标准化之后的数据进行差异表达分析,最终1352个基因达到筛选标准(P<0.05且|logFC|>2),其中有703个上调基因,649个下调基因。利用R语言中的pheatmap包绘制差异显著的前20个上调基因和前20下调基因的热图,直观的显示出差异表达基因(附录1)。
热图(附录1a)显示出多个基因在两组中的表达水平。与正常组相比,处理组主要有以下基因发生差异性表达:Srd5a1、Lipc、Aqp8、Hba-a1、Slco1a1、Pklr、keg1、Nudt7等。其中,下调基因最明显前6位分别为Srd5a1、Lipc、Aqp8、Hba-a1、Slco1a1、Pklr。火山图(附录1b)用于展示基因表达差异的分布,其中横轴为FC的对数,越偏离中心FC越大;纵轴为差异显著性P值的负对数,值越大表明差异越显著。
2.4 差异表达基因的GO功能富集分析
为进一步了解差异表达基因的功能,陈述其在分子水平上的作用,针对差异表达基因进行GO注释(附录2)。Gene Ratio表明差异基因中与该Term相关的基因数与差异基因总数的比值,圆圈大小代表基因的多少,qvalue为对P值进行统计学检验的q值,qvalue值越小说明富集程度越明显。结果表明,小分子分解代谢(small moleculle catabolic process)、中性粒细胞参与的免疫应答(neutrophil activation involved inimmune respond)、分泌颗粒细胞质囊泡腔(secretory granule lumen)、细胞质囊泡腔(cytoplasmic vesicle lumen)、细胞外结构组织(extracellular structure organization)、细胞外基质组织(extracellular matrix organization)等相关的GO分类呈显著富集。
2.5 差异表达基因的KEGG富集分析
对正常组和处理组变化最明显的40个差异基因进行KEGG富集分析(附录3),其中富集因子(enrichment Score, ES)代表基因的富集程度,X轴为富集分数,气泡越大的条目包含的差异蛋白编码基因数目越多,气泡颜色由紫-蓝- 绿-黄-红变化,其富集P值越小,显著程度越大。
结果表明,化学致癌(Chemical carcinogenesis)、视黄醇代谢(Retinol metabolism)、花生四烯酸代谢(Arachidonic acid metabolism)、类固醇激素合成(Steroid hormone biosynthesis)、癌症中的转录失调(Transcriptional misregulation in cancers)、蛋白质消化和吸收(Protein digestion and absorption)、炎症介质对TRP通道的调节(Inflammatory mediator regulation of TRP channels)、药物代谢(Drug metabolism)、补体和凝血级联(Complement and coagulation cascades)、谷胱甘肽代谢(Glutathione metabolism)、PPAR信号通路(PPAR signaling pathway)等在KEGG通路中显著富集。
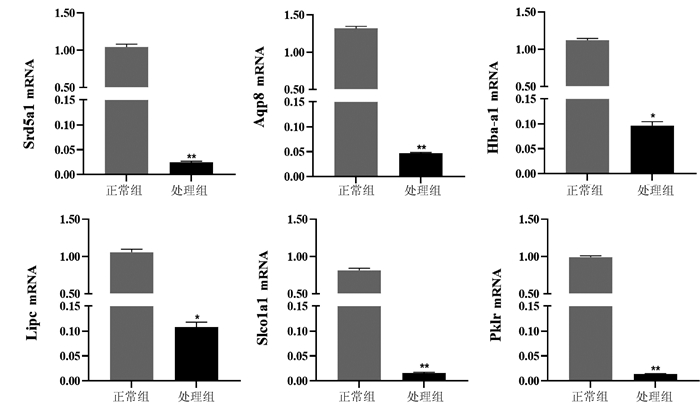
2.6 差异表达基因的qRT-PCR验证
在表达的差异基因中挑选5个差异基因,以β-actin为内参,采用qRT-PCR验证RNA-seq的可靠性(图 3)。结果发现,与正常组相比,处理组Srd5a1、Lipc、Aqp8、Hba-a1、Slco1a1、Pklr 6个基因表达水平显著减少(P值均<0.05)。
3. 讨论
自从20世纪90年代确认AA具有肾毒性以来[8],已有部分药材被禁用;但由于部分含AA药物的不可替代性,仍然应用于临床。研究证实AA可导致肿瘤的发生。AA和马兜铃药材已被世界卫生组织国际癌症研究机构、美国食品药品监督管理局等列为Ⅰ类致癌物[9-10]。近年来关于AA与肝癌的研究[11]屡见不鲜,然而,AA的肾脏毒性已被学界广泛认可,但是AA引起的肝损伤,尤其是AA能诱发肝癌的说法有着很大的争议,其具体机制还需进一步探讨。
AAⅠ灌胃5 d,小鼠血清ALT、AST分别升高7倍和4倍,且肝组织病理结果显示肝小叶结构紊乱、肝细胞胞浆疏松、小叶内可见点状坏死,这提示AAⅠ可引起小鼠急性肝损伤。进一步提取肝组织RNA进行高通量转录组测序,发现正常组与处理组的差异基因数目为1352个;其中,表达上调的基因有703个,下调的基因有649个。差异基因中下调最明显前6位分别为Srd5a1、Lipc、Aqp8、Hba-a1、Slco1a1、Pklr。再采用qRT-PCR检测,与正常组相比,处理组Srd5a1、Lipc、Aqp8、Hba-a1、Slco1a1、Pklr的基因表达均明显降低,与转录组测序结果一致。
Srd5a(5-α-Reductase)是类固醇5α-还原酶,能将睾酮还原为双氢睾酮,从而调节激素水平和肿瘤的发生与发展。SRD5A具有3种亚型分别为Srd5a1、Srd5a2和Srd5a3[12]。Srd5a1广泛分布于人体,如皮肤、肝脏、肾脏、免疫组织和神经组织等,它与多种系统疾病有关。Srd5a1的异常会造成局部或全身雄激素水平紊乱。近年来有研究报道,Srd5a1在前列腺癌[12]、乳腺癌[13]、非小细胞肺癌[14]和肝癌[15]中均表达升高。肝脂肪酶(LIPC, 又称HL),由肝实质细胞合成,可以水解脂蛋白中的甘油三酯和磷脂,从而促进它们的清除和代谢[16]。LIPC基因位于15号染色体长臂(15q21),全长35 kb, 由9个外显子和8个内含子构成, 编码由449个氨基酸组成的蛋白质[17]。LIPC的多态性及基因突变可导致血脂异常。Homanics等[18]发现,小鼠的LIPC缺乏会产生轻度血脂异常,包括总胆固醇、磷脂和高密度脂蛋白胆固醇的增加。水通道蛋白-8(AQP8)是哺乳动物水通道蛋白家族的成员之一,能促进水和某些小溶质在生物膜上的运动[19],其在消化系统中广泛表达,包括小肠、结肠、胰腺和肝脏等[20]。AQP8在肝细胞中具有广泛的生理功能,其表达缺乏易引起肝功能紊乱。Jablonski等[21]研究证实肝细胞癌的发生与AQP8表达降低和质膜水渗透性降低有关。Slco1a1,又名OATP1,是属于溶质载体超家族(solute carrier, SLC)的一种跨膜蛋白[22],OATP1的表达在肝炎、肝硬化中表达下调[23-24]。PKLR是丙酮酸激酶(pyruvate kinase,PK)的基因编码。PK是催化糖酵解的关键酶,用于催化磷酸烯醇式丙酮酸生成丙酮酸,同时使ADP磷酸化而生成ATP[23]。PK有4种亚型。其中的PKM2已经被证实在肝癌细胞中显著增加,在糖酵解的调控中起关键作用[25]。有研究[26-27]报道,PKLR主要通过增加内源性抗氧化剂谷胱甘肽来促进肿瘤核心的细胞存活,负调节PKM2的糖酵解活性,并介导结肠癌在肝脏定植。上述基因在AAⅠ引起的急性肝损伤中鲜有报道。
差异基因中,上调最为明显的为LCN2、MT1和MT2。LCN2是一个25 kDa的小分子、分泌性蛋白[28]。在LPS和ConA诱导的肝损伤模型中明显升高[29]。在胆管炎症或肝脏炎症时,血液和肝脏中LCN2水平明显升高[30],同时也与肝衰竭和全身炎症反应相关,是慢加急性肝衰竭及预后的生物标志物[31]。MT1及MT2是金属硫蛋白(MTs)的亚型,在细胞、组织均有表达,以肝、肾最为丰富[32]。研究[33]报道MT对急性化学性肝损伤有一定的保护作用。在由镉、四氯化碳或对乙酰氨基酚引起的急性肝毒性中,可通过清除氧自由基对肝脏起到保护作用。
本研究通过高通量测序技术,从基因转录水平初步揭示了AAⅠ致急性肝毒性机制。并采用qPCR验证了下调最明显的6个基因,提示转录组学的可靠性。本研究中转录组学揭示的AAⅠ肝毒性的基因及相关信号通路有待进一步深入研究。

-
表 1 基因引物序列
基因 正向引物(5′-3′) 反向引物(5′-3′) β-actin TGACGAGGCCCAGAGCAAGA ATG GGCACAGTGTGGGTGAC Srd5a1 CTACTGGATGCGCTAGTCTAC TCAGAAACATAGCTAGCAGGAC Lipc CTACAAACACATTGCAGAGCAT GTTGCAAGGAGTCAATGAAGAG Aqp8 GCTCCGCTCTCTTCATCTTCATCG GAAGTGTCCACCGCTGATGTTCC Hba-a1 ACCACCAAGACCTACTTCCCTCAC CAGAGCATCGGCGACCTTCTTG Slco1a1 TGGATTTATCACTGGGAGCTTT AGGAAATGAGGTGATGCCATTA Pklr GGCTCAGAAGATGATGATTGGA ATCGCATGTTGCATCTTTACAG 表 2 两组小鼠血清ALT、AST水平的比较
组别 动物数(只) ALT(U/L) AST(U/L) 正常组 6 13.95±1.56 13.44±2.05 处理组 9 102.15±34.10 53.62±22.73 t值 4.331 4.947 P值 0.002 0.001 -
[1] MICHL J, INGROUILLE MJ, SIMMONDS MS, et al. Naturally occurring aristolochic acid analogues and their toxicities[J]. Nat Prod Rep, 2014, 31(5): 676-693. DOI: 10.1039/c3np70114j. [2] KUMAR V, POONAM, PRASAD AK, et al. Naturally occurring aristolactams, aristolochic acids and dioxoaporphines and their biological activities[J]. Nat Prod Rep, 2003, 20(6): 565-583. DOI: 10.1039/b303648k. [3] ZHANG H, LIU R, AN Z, et al. Aristolactam-type alkaloids and aristolochic acids from Aristolochia moupinensis and Aristolochia cathcartii[J]. Biochem Syst Ecol, 2016, 65: 198-201. DOI: 10.1016/j.bse.2016.02.028. [4] JELAKOVIĆ B, KARANOVIĆ S, VUKOVIĆ-LELA I, et al. Aristolactam-DNA adducts are a biomarker of environmental exposure to aristolochic acid[J]. Kidney Int, 2012, 81(6): 559-567. DOI: 10.1038/ki.2011.371. [5] GUO MZ, YANG S, ZHAO LL. Transcriptome analysis method based on RNA-Seq[J]. Computer Science, 2020, 47(z2): 35-39. DOI: 10.11896/jsjkx.200600057.郭茂祖, 杨帅, 赵玲玲. 基于RNA-Seq的转录组分析方法[J]. 计算机科学, 2020, 47(z2): 35-39. DOI: 10.11896/jsjkx.200600057. [6] NG A, POON SL, HUANG MN, et al. Aristolochic acids and their derivatives are widely implicated in liver cancers in Taiwan and throughout Asia[J]. Sci Transl Med, 2017, 9(412): eaan6446. DOI: 10.1126/scitranslmed.aan6446. [7] JIANG Z, ZHOU X, LI R, et al. Whole transcriptome analysis with sequencing: Methods, challenges and potential solutions[J]. Cell Mol Life Sci, 2015, 72(18): 3425-3439. DOI: 10.1007/s00018-015-1934-y. [8] VANHERWEGHEM JL, DEPIERREUX M, TIELEMANS C, et al. Rapidly progressive interstitial renal fibrosis in young women: Association with slimming regimen including Chinese herbs[J]. Lancet, 1993, 341(8842): 387-391. DOI: 10.1016/0140-6736(93)92984-2. [9] JADOT I, DECLÈVES AE, NORTIER J, et al. An integrated view of aristolochic acid nephropathy: Update of the literature[J]. Int J Mol Sci, 2017, 18(2). DOI: 10.3390/ijms18020297. [10] BALACHANDRAN P, WEI F, LIN RC, et al. Structure activity relationships of aristolochic acid analogues: Toxicity in cultured renal epithelial cells[J]. Kidney Int, 2005, 67(5): 1797-1805. DOI: 10.1111/j.1523-1755.2005.00277.x. [11] LU ZN, LUO Q, ZHAO LN, et al. The mutational features of aristolochic acid-induced mouse and human liver cancers[J]. Hepatology, 2020, 71(3): 929-942. DOI: 10.1002/hep.30863. [12] LI J, DING Z, WANG Z, et al. Androgen regulation of 5α-reductase isoenzymes in prostate cancer: Implications for prostate cancer prevention[J]. PLoS One, 2011, 6(12): e28840. DOI: 10.1371/journal.pone.0028840. [13] LEWIS MJ, WIEBE JP, HEATHCOTE JG. Expression of progesterone metabolizing enzyme genes (AKR1C1, AKR1C2, AKR1C3, SRD5A1, SRD5A2) is altered in human breast carcinoma[J]. BMC Cancer, 2004, 4: 27. DOI: 10.1186/1471-2407-4-27. [14] KAPP FG, SOMMER A, KIEFER T, et al. 5-alpha-reductase type I (SRD5A1) is up-regulated in non-small cell lung cancer but does not impact proliferation, cell cycle distribution or apoptosis[J]. Cancer Cell Int, 2012, 12(1): 1. DOI: 10.1186/1475-2867-12-1. [15] DOWMAN JK, HOPKINS LJ, REYNOLDS GM, et al. Loss of 5α-reductase type 1 accelerates the development of hepatic steatosis but protects against hepatocellular carcinoma in male mice[J]. Endocrinology, 2013, 154(12): 4536-4547. DOI: 10.1210/en.2013-1592. [16] ANDRÉS-BLASCO I, HERRERO-CERVERA A, VINUÉ Á, et al. Hepatic lipase deficiency produces glucose intolerance, inflammation and hepatic steatosis[J]. J Endocrinol, 2015, 227(3): 179-191. DOI: 10.1530/JOE-15-0219. [17] DOOLITTLE MH, WONG H, DAVIS RC, et al. Synthesis of hepatic lipase in liver and extra hepatic tissues[J]. J Lipid Res, 1987, 28(11): 1326-1334. DOI: 10.1016/S0022-2275(20)38591-6 [18] HOMANICS GE, DE SILVA HV, OSADA J, et al. Mild dyslipidemia in mice following targeted inactivation of the hepatic lipase gene[J]. J Biol Chem, 1995, 270(7): 2974-2980. DOI: 10.1074/jbc.270.7.2974. [19] MARINELLI RA, LEHMANN GL, SORIA LR, et al. Hepatocyte aquaporins in bile formation and cholestasis[J]. Front Biosci (Landmark Ed), 2011, 16: 2642-2652. DOI: 10.2741/3877. [20] MATSUZAKI T, TAJIKA Y, ABLIMIT A, et al. Aquaporins in the digestive system[J]. Med Electron Microsc, 2004, 37(2): 71-80. DOI: 10.1007/s00795-004-0246-3. [21] JABLONSKI EM, MATTOCKS MA, SOKOLOV E, et al. Decreased aquaporin expression leads to increased resistance to apoptosis in hepatocellular carcinoma[J]. Cancer Lett, 2007, 250(1): 36-46. DOI: 10.1016/j.canlet.2006.09.013. [22] YANG YJ, LIU L, XU M, et al. Significance of regulating liver transporters in the prevention and treatment of liver diseases[J]. West China J Pharma Sci, 2020, 35(3): 316-324. DOI: 10.13375/j.cnki.wcjps.2020.03.018.杨玉洁, 刘蕾, 徐苗, 等. 调控肝脏转运体对肝脏疾病防治的意义[J]. 华西药学杂志, 2020, 35(3): 316-324. DOI: 10.13375/j.cnki.wcjps.2020.03.018. [23] WANG H, CHEN H, OUYANG ZM, et al. Correlation of the protein expression of organic anion transport polypeptide 1 and multidrug resistance-associated protein 2 on the surface of hepatocyte membrane with signal intensity on Gd -EOB -DTPA -enhanced magnetic resonance imaging in dogs with acute liver failure[J]. J Clin Hepatol, 2020, 36(8): 1788-1793. DOI: 10.3969/j.issn.1001-5256.2020.08.022.王昊, 陈好, 欧阳中敏, 等. 急性肝衰竭犬模型肝细胞膜表面OATP1及MRP2蛋白表达与钆塞酸二钠增强MRI信号强度的相关性分析[J]. 临床肝胆病杂志, 2020, 36(8): 1788-1793. DOI: 10.3969/j.issn.1001-5256.2020.08.022. [24] GEIER A, KIM SK, GERLOFF T, et al. Hepatobiliary organic anion transporters are differentially regulated in acute toxic liver injury induced by carbon tetrachloride[J]. J Hepatol, 2002, 37(2): 198-205. DOI: 10.1016/s0168-8278(02)00108-3. [25] MAZUREK S. Pyruvate kinase type M2: A key regulator of the metabolic budget system in tumor cells[J]. Int J Biochem Cell Biol, 2011, 43(7): 969-980. DOI: 10.1016/j.biocel.2010.02.005. [26] LIU WR, TIAN MX, YANG LX, et al. PKM2 promotes metastasis by recruiting myeloid-derived suppressor cells and indicates poor prognosis for hepatocellular carcinoma[J]. Oncotarget, 2015, 6(2): 846-861. DOI: 10.18632/oncotarget.2749. [27] NGUYEN A, LOO JM, MITAL R, et al. PKLR promotes colorectal cancer liver colonization through induction of glutathione synthesis[J]. J Clin Invest, 2016, 126(2): 681-694. DOI: 10.1172/JCI83587. [28] KJELDSEN L, JOHNSEN AH, SENGELØV H, et al. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase[J]. J Biol Chem, 1993, 268(14): 10425-10432. [29] BORKHAM-KAMPHORST E, van de LEUR E, ZIMMERMANN HW, et al. Protective effects of lipocalin-2 (LCN2) in acute liver injury suggest a novel function in liver homeostasis[J]. Biochim Biophys Acta, 2013, 1832(5): 660-673. DOI: 10.1016/j.bbadis.2013.01.014. [30] CHEN F, HE JL, ZHENG M, et al. Complementary laboratory indices for predicting the disease status of patients with hepatitis B virus infection[J]. J Viral Hepat, 2013, 20(8): 566-574. DOI: 10.1111/jvh.12067. [31] ARIZA X, GRAUPERA I, COLL M, et al. Neutrophil gelatinase-associated lipocalin is a biomarker of acute-on-chronic liver failure and prognosis in cirrhosis[J]. J Hepatol, 2016, 65(1): 57-65. DOI: 10.1016/j.jhep.2016.03.002. [32] VAŠÁK M, MELONI G. Chemistry and biology of mammalian metallothioneins[J]. J Biol Inorg Chem, 2011, 16(7): 1067-1078. DOI: 10.1007/s00775-011-0799-2. [33] NAGAMINE T, NAKAJIMA K. Significance of metallothionein expression in liver disease[J]. Curr Pharm Biotechnol, 2013, 14(4): 420-426. DOI: 10.2174/1389201011314040006. 期刊类型引用(6)
1. 程庆兵,汪海宣,郑正,袁杰,杨彬,王玮,张亚中. 超高效液相色谱-串联质谱法测定丹桂香颗粒中马兜铃酸Ⅰ. 化学分析计量. 2024(11): 52-57 .  百度学术
百度学术2. 沈婷婷,朱哿瑞,王帆,孙鑫,黄恺,彭渊,陶艳艳,刘成海. 基因表达谱分析非酒精性脂肪性肝病中谷胱甘肽转移酶的作用. 临床肝胆病杂志. 2023(01): 89-96 .  本站查看
本站查看3. 王义翠,刘延鑫,林华忠,魏炳琦,刘曹露,周茜如,赵梦瑶,徐华明. 四氯化碳诱导慢性肝损伤模型小鼠的转录组学分析. 黑龙江畜牧兽医. 2023(05): 65-69 .  百度学术
百度学术4. 朱哿瑞,皮亚妮,王静,黄恺,彭渊,陈高峰,刘成海,陶艳艳. 黄芪提取物调节IL-6/STAT3信号通路治疗马兜铃酸Ⅰ诱导肝肾损伤小鼠模型的效果观察. 临床肝胆病杂志. 2023(08): 1903-1910 .  本站查看
本站查看5. 庞逸辉,李岳,张宪. 高效液相色谱法测定杜仲壮骨丸中马兜铃酸A. 化学分析计量. 2022(06): 50-53 .  百度学术
百度学术6. 赵晨阳,吴倩,张云波,李刚,那晓琳. 苯并[b]荧蒽亚急性经口染毒的大鼠肝脏毒性以及转录组改变. 环境卫生学杂志. 2022(11): 777-784+789 .  百度学术
百度学术其他类型引用(4)
-




 PDF下载 ( 2591 KB)
PDF下载 ( 2591 KB)

 下载:
下载:



 下载:
下载:


 百度学术
百度学术