Value of the inflammatory markers albumin, C-reactive protein, erythrocyte sedimentation rate, and interleukin-6 in predicting hepatocellular carcinoma with different types of portal vein thrombosis
-
摘要:
目的 比较肝癌合并门静脉血栓(PVBT)与肝癌合并门静脉癌栓(PVTT)患者的生化及常规血液指标,探讨炎症指标在不同类型患者中的表达水平及临床意义。 方法 选取2016年1月—2020年12月于郑州大学第二附属医院诊治的肝癌合并PVBT患者51例(PVBT组),肝癌合并PVTT患者37例(PVTT组),随机选取同期住院的肝癌未合并门静脉栓子患者50例作为对照组。收集比较3组患者的一般临床资料、实验室检查结果等。计数资料组间比较采用χ2检验或Kruskal-Wallis秩和检验; 符合正态分布的计量资料组间比较采用单因素方差分析; 非正态分布的计量资料组间比较采用Kruskal-Wallis秩和检验。绘制ROC曲线评价Alb、CRP、ESR及IL-6在PVBT组与PVTT组的表达水平,计算ROC曲线下面积(AUC),分析各项指标对不同类型栓子的预测价值。 结果 3组一般资料及常规生化指标检查结果差异均无统计学意义(P值均>0.05),而炎症指标Alb、CRP、血沉(ESR)及IL-6在3组间差异均有统计学意义(H值分别为10.207、24.465、8.917、37.584,P值分别为0.006、<0.001、0.012、<0.001),进一步两组间比较提示,PVTT组Alb水平显著低于PVBT组与对照组,CRP、ESR及IL-6水平显著高于PVBT组与对照组,PVBT组CRP、IL-6水平显著高于对照组(P值均<0.05)。ROC曲线提示PVTT组Alb、CRP、ESR及IL-6的AUC分别为0.659、0.826、0.679、0.873,PVBT组AUC分别为0.508、0.635、0.503、0.701。 结论 在肝癌伴PVTT患者中炎症指标IL-6、CRP、ESR高表达,Alb低表达,且预测价值依次递减; 而在肝癌伴PVBT患者中IL-6、CRP、ESR表达相对较低,Alb表达较高,其中IL-6、CRP对PVBT有一定的预测价值,ESR、Alb的预测价值不高。 Abstract:Objective To investigate the biochemical and routine blood parameters in hepatocellular carcinoma (HCC) patients with portal vein blood thrombus (PVBT) or portal vein tumor thrombosis (PVTT), as well as the expression level and clinical significance of inflammatory indices in patients with different types of PVBT. Methods A total of 51 HCC patients with PVBT and 37 HCC patients with PVTT who were diagnosed and treated in The Second Affiliated Hospital of Zhengzhou University from January 2016 to December 2020 were enrolled as PVBT group and PVTT group, respectively, and 50 HCC patients without portal vein thrombosis who were hospitalized during the same period of time were enrolled as control group. General clinical data and laboratory test results were collected from the three groups. The chi-square test or the Kruskal-Wallis rank sum test was used for comparison of categorical data between groups; a one-way analysis of variance was used for comparison of normally distributed continuous data between groups, and the Kruskal-Wallis rank sum test was used for comparison of non-normally distributed continuous data between groups. The receiver operating characteristic (ROC) curve was plotted to investigate the expression levels of albumin (Alb), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and interleukin-6 (IL-6) in the PVBT group and the PVTT group, and the area under the ROC curve (AUC) was calculated to analyze their value in predicting different types of thrombosis. Results There were no significant differences in general data and conventional biochemical parameters between the three groups (all P > 0.05), while there were significant differences in the inflammatory indices Alb, CRP, ESR, and IL-6 between the three groups (H=10.207, 24.465, 8.917, and 37.584, P=0.006, P < 0.001, P=0.012, and P < 0.001), and further analysis between two groups showed that the PVTT group had a significantly lower level of Alb and significantly higher levels of CRP, ESR, and IL-6 than the PVBT group and the control group, and the PVBT group had significantly higher levels of CRP and IL-6 than the control group (all P < 0.05). The ROC curve analysis showed that Alb, CRP, ESR, and IL-6 had an AUC of 0.659, 0.826, 0.679, and 0.873, respectively, in the PVTT group, as well as an AUC of 0.508, 0.635, 0.503, and 0.701, respectively, in the PVBT group. Conclusion HCC patients with PVTT tend to have high expression levels of the inflammatory indices IL-6, CRP, and ESR and a low expression level of Alb, and their predictive value decreases successively, while HCC patients with PVBT tend to have relatively low expression levels IL-6, CRP, and ESR and a relatively high expression level of Alb. IL-6 and CRP have a certain value in predicting PVBT, while ESR and ALB have little predictive value. -
Key words:
- Carcinoma, Hepatocellular /
- Venous Thromboembolism /
- Inflammatory Markers
-
门静脉血栓(portal vein blood thrombus, PVBT)是指发生在门静脉主干和/或门静脉分支的血栓、伴或不伴肠系膜静脉及脾静脉血栓形成[1],有研究[2]表明PVBT的形成可能与全身炎症状态或局部炎症反应有关,且在肝癌患者中的发病率为28%~34.8%。此外,肝癌患者的癌细胞易侵犯肝门静脉进而形成门静脉癌栓(portal vein tumor thrombus,PVTT),PVTT发病无特异性,多伴发于中晚期肝癌患者中,且越来越多的证据表明,感染和炎症可能在PVTT的发生中起重要作用,肝癌一旦合并有PVTT,不仅增加了门静脉阻力,减少门静脉血流,还会使肝功能进一步恶化,预后极差,生存期降低[3-8]。对于肝癌合并PVBT或PVTT形成后患者通常没有临床症状,常在体检或筛查肝癌时偶然发现,本研究旨在分析肝癌合并PVBT/PVTT患者的生化及常规血液指标变化特点,并探讨炎症指标在不同类型患者中的表达水平及临床意义。
1. 资料与方法
1.1 研究对象
选取2016年1月—2020年12月于本院诊治的肝癌合并PVBT患者为PVBT组,肝癌合并PVTT患者为PVTT组,随机选取同期住院的肝癌未合并门静脉栓子患者作为对照组。所有肝癌患者诊断参照欧洲肝病学会发布的肝癌诊疗指南[9],门静脉栓子的诊断及分类参照中华医学会《肝硬化门静脉血栓专家管理共识》[10],并根据2020年美国肝病学会肝脏血管病、门静脉血栓和肝病患者手术相关出血实践指导[11]对所有患者行多普勒超声检查,对于有门静脉阻塞患者进一步行MRI或CT明确PVBT或PVTT的诊断; 无门静脉栓子患者是指本次及既往入院影像学检查未发现门静脉阻塞的患者。根据Child-Pugh分级评估肝病的严重程度。纳入标准:(1) 临床确诊为原发性肝细胞癌; (2)均经彩色多普勒超声、CT血管造影或MRI明确PVBT及PVTT情况; (3)有相关检查资料、住院治疗并有完整临床资料者。排除标准:(1)曾经或正在接受抗凝或抗血小板治疗; (2)脾肠系膜或外周静脉血栓形成; (3)合并其他系统恶性肿瘤; (4)脾切除术后。
1.2 研究方法
回顾性查阅所有纳入研究对象的病史、入院时实验室指标及辅助检查结果,包括年龄、性别、肝硬化病因,Child-Pugh分级,记录并分析ALT、AST、Alb、TBil、RBC、PLT、WBC、中性粒细胞百分比(NE%)、淋巴细胞百分比(LY%)、单核细胞百分比(MO%)、CRP、血沉(ESR)、IL-6等实验室检查结果。
1.3 伦理学审查
本研究方案经由郑州大学第二附属医院伦理委员会审批,批号:2021207,纳入患者均签署知情同意书。
1.4 统计学方法
采用SPSS 25.0软件进行数据分析。计数资料组间比较采用χ2检验或Kruskal-Wallis秩和检验; 符合正态分布的计量资料以x±s表示,多组间比较采用单因素方差分析; 非正态分布的计量资料以M(P25~P75)表示,多组间及进一步两组间比较采用Kruskal-Wallis秩和检验。绘制ROC曲线评价Alb、CRP、ESR及IL-6在PVBT组与PVTT组的表达水平,计算ROC曲线下面积(AUC),分析上述指标对不同类型栓子的预测价值。P<0.05为差异有统计学意义。
2. 结果
2.1 一般资料
共纳入PVBT组51例,PVTT组37例,对照组50例。3组患者的性别、年龄、病因、Child-Pugh分级情况比较,差异均无统计学意义(P值均>0.05)(表 1)。
表 1 3组患者一般资料比较指标 PVBT组(n=51) PVTT组(n=37) 对照组(n=50) 统计值 P值 男[例(%)] 32(62.7) 24(64.9) 30(60.0) χ2=0.221 0.896 年龄(岁) 61.06±11.53 62.68±10.62 61.20±10.87 F=0.267 0.766 病因[例(%)] χ2=0.323 0.851 病毒性 39(76.5) 30(81.1) 40(80.0) 非病毒性 12(23.5) 7(18.9) 10(20.0) Child-Pugh分级[例(%)] H=0.708 0.702 A级 24(47.1) 18(48.6) 27(54.0) B级 16(31.4) 11(29.7) 15(30.0) C级 11(21.6) 8(21.6) 8(16.0) 2.2 生化及常规指标比较
ALT、AST、TBil、RBC、PLT、WBC、NE%、LY%、MO%在3组间比较差异均无统计学意义(P值均>0.05),而Alb、CRP、ESR、IL-6组间比较差异均有统计学意义(P值均<0.05)(表 2)。两组间比较, PVTT组Alb显著低于PVBT组和对照组(P值均<0.05);PVTT组ESR、CRP、IL-6显著高于PVBT组和对照组(P值均<0.05);PVBT组CRP、IL-6显著高于对照组(P值均<0.05)。
表 2 3组实验室检查结果比较指标 PVBT组(n=51) PVTT组(n=37) 对照组(n=50) 统计值 P值 ALT(U/L) 56 (38~69) 49(35~72) 39(20~68) H=5.518 0.063 AST(U/L) 82(60~98) 83(68~93) 84(52~94) H=0.532 0.767 Alb(g/L) 33.2(31.0~36.0) 30.7(28.6~33.3)1) 34.9(29.7~38.3)2) H=10.207 0.006 TBil(μmol/L) 27.3(17.6~38.5) 26.4(16.9~36.8) 25.5(16.8~32.1) H=0.429 0.807 RBC(1012/L) 2.95(2.24~3.38) 3.13(2.42~3.64) 3.23(2.58~3.67) H=3.904 0.142 PLT(109/L) 89(74~103) 94(84~109) 106(77~149) H=4.500 0.105 WBC(109/L) 6.63(3.89~8.88) 7.16(4.93~9.16) 7.47(4.01~8.96) H=1.012 0.603 NE% 71.0(64.0~83.6) 73.4(63.8~81.5) 67.9(59.5~80.6) H=1.085 0.581 LY% 21.0(10.7~27.2) 20.0(12.5~28.5) 21.3(11.8~33.2) H=1.648 0.439 MO% 6.02±2.46 5.76±2.41 5.55±2.13 F=0.267 0.604 CRP(mg/L) 10.30(6.30~27.47) 22.80(12.67~33.52)1) 8.31(6.15~13.20)1)2) H=24.465 <0.001 ESR(mm/h) 22(15~36) 31(24~49)1) 22(17~30)2) H=8.917 0.012 IL-6(pg/ml) 7.61(4.64~14.88) 15.60(7.22~25.51)1) 4.28(2.81~7.06)1)2) H=37.584 <0.001 注:与PVBT组比较,1)P<0.05;与PVTT组比较,2)P<0.05。 2.3 Alb、CRP、ESR、IL-6对不同类型门静脉栓子预测价值分析
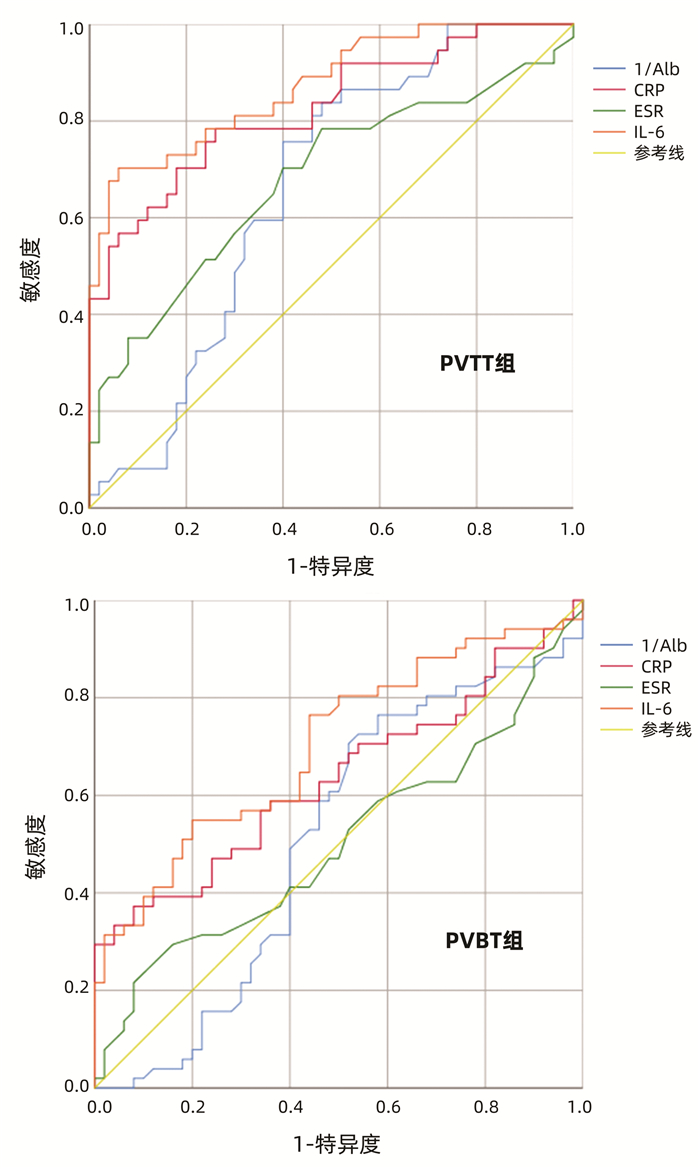
Alb在PVTT组与PVBT组的AUC分别为0.659(95%CI:0.544~0.773) 和0.508(95%CI:0.392~0.624)。CRP在PVTT组与PVBT组的AUC分别为0.826(95%CI:0.737~0.915)和0.635(95%CI:0.526~0.745)。ESR在PVTT组与PVBT组的AUC分别为0.679(95%CI:0.561~0.797)和0.503(95%CI:0.388~0.617。IL-6在PVTT组与PVBT组的AUC分别为0.873(95%CI:0.799~0.947)和0.701(95%CI:0.600~0.803)(图 1)。
3. 讨论
本研究分析了肝癌合并PVBT或PVTT患者的生化及常规血液指标,并探讨了炎症指标Alb、CRP、ESR、IL-6的表达情况。相较于PVBT组和对照组,PVTT组Alb处于较低水平。Alb是格拉斯哥预后评分(GPS)的两种指标之一,GPS也被证实是包括肝细胞癌在内的许多癌症的重要独立预测指标[12],并被认为是代表炎症性疾病和晚期癌症中营养缺乏的象征,可直接影响肝癌细胞的生长以及侵袭性[13],而PVTT作为一种肝细胞癌的肝内转移形式,其侵袭性明显高于无转移者,本研究也表明较低的Alb水平与更具侵袭性的肿瘤参数相关(即PVTT的形成),这与先前报道[7-8]结果一致。此外,在肝细胞癌患者中Alb水平降低是PVTT患者较为重要的危险因素,其对肝细胞癌合并PVTT患者的预测价值(AUC=0.659)较对PVBT的预测价值高(AUC=0.508)。
IL-6是一种促炎细胞因子,可通过不同的信号途径促进细胞增殖、逃避凋亡以及增加癌症的侵袭力[14]。本研究发现,IL-6是预测肝癌栓子类型最重要的细胞因子,对PVTT的预测价值(AUC=0.873)明显高于PVBT患者(AUC=0.701)。有研究[15]表明,较高的血清IL-6水平反映了肿瘤的负荷,与肿瘤的大小、分期和侵袭性(如门静脉侵犯和门静脉癌栓形成)显著相关。此外,肝癌细胞本身可能是IL-6的来源,IL-6通过自分泌机制刺激肿瘤生长,其中,IL-6通过激活核因子-κB和信号转导与转录激活因子3(STAT3)发挥作用,这两个转录因子对肝癌的发生和转移非常重要,这可能促进肿瘤生长和转移[16-19],这与本研究结果一致,这种关联意味着高水平的IL-6可能是侵袭性增加以及对门静脉侵犯的高危因素。然而PVBT组IL-6虽低于PVTT组但仍显著高于对照组(P<0.05),因此推测炎症可能是导致肝硬化肝癌患者门静脉血栓形成的另一原因,晚期肝病患者门静脉压力升高,肠道黏膜屏障被破坏,细菌易位导致炎症的发生,当暴露于局部或全身炎症时,内皮细胞被激活并促进门静脉血栓形成[20]。
CRP是GPS的另一指标,也是一种由肝细胞产生对抗炎症反应的急性期反应物,其水平受促炎细胞因子,特别是IL-6的调节[21]。本研究表明,CRP水平是另一个重要的预测因素,对PVTT的预测价值虽较高(AUC=0.826),但低于IL-6的预测价值(AUC=0.873)。循环CRP的产生主要依赖于IL-6[22],这也就意味着CRP的水平可能与IL-6水平呈正相关,且同样作用于肝癌患者门静脉癌栓形成机制,及影响门静脉血栓形成。然而,其预测价值低于IL-6预测价值,可能是由于循环CRP水平除了跟全身炎症状态相关之外还受高脂、高蛋白饮食、饮酒等因素影响[23-24],具体机制还有待进一步明确。
虽然ESR也常常被认为是炎症指标,但研究结果显示,与其他炎症指标如CRP、IL-6对血栓与癌栓的预测价值不同,PVTT组ESR水平明显高于PVBT组与对照组,但预测价值普遍不高(PVTT组AUC为0.679,PVBT组为0.503)。CRP在肝脏和肝癌细胞中合成,并与IL-1和IL-6以及STAT3相互作用,可影响单核细胞杀瘤活性[25]。相比之下,使用抗凝全血的血沉试验不能反映炎症反应的主动过程,而是炎症过程的被动反映,即是影响血液黏度的纤维蛋白原、血浆蛋白水平,因此,CRP和ESR可能反映了炎症过程的不同方面[8, 26]。
研究[25, 27-28]表明,炎症指数与肝细胞癌侵袭性显著相关,包括PVTT的形成。本研究表明,从炎症水平出发,可以从一定程度上对肝癌患者合并不同的栓子类型做出判断,炎症指标IL-6、CRP、ESR在肝癌伴门静脉癌栓患者中高表达,Alb低表达,且预测价值高, 而在肝癌伴门静脉血栓患者中表达相反,值得进一步行前瞻性研究证实。本研究PVBT及PVTT的诊断均依赖于影像学检查,栓子主要位于门静脉主干,不排除不同分组患者有门静脉分支内微小栓子形成,可能对研究结果造成一定的影响,且本研究样本量偏小,还有待进一步扩大样本量从而提高研究的可靠性。
-
表 1 3组患者一般资料比较
指标 PVBT组(n=51) PVTT组(n=37) 对照组(n=50) 统计值 P值 男[例(%)] 32(62.7) 24(64.9) 30(60.0) χ2=0.221 0.896 年龄(岁) 61.06±11.53 62.68±10.62 61.20±10.87 F=0.267 0.766 病因[例(%)] χ2=0.323 0.851 病毒性 39(76.5) 30(81.1) 40(80.0) 非病毒性 12(23.5) 7(18.9) 10(20.0) Child-Pugh分级[例(%)] H=0.708 0.702 A级 24(47.1) 18(48.6) 27(54.0) B级 16(31.4) 11(29.7) 15(30.0) C级 11(21.6) 8(21.6) 8(16.0) 表 2 3组实验室检查结果比较
指标 PVBT组(n=51) PVTT组(n=37) 对照组(n=50) 统计值 P值 ALT(U/L) 56 (38~69) 49(35~72) 39(20~68) H=5.518 0.063 AST(U/L) 82(60~98) 83(68~93) 84(52~94) H=0.532 0.767 Alb(g/L) 33.2(31.0~36.0) 30.7(28.6~33.3)1) 34.9(29.7~38.3)2) H=10.207 0.006 TBil(μmol/L) 27.3(17.6~38.5) 26.4(16.9~36.8) 25.5(16.8~32.1) H=0.429 0.807 RBC(1012/L) 2.95(2.24~3.38) 3.13(2.42~3.64) 3.23(2.58~3.67) H=3.904 0.142 PLT(109/L) 89(74~103) 94(84~109) 106(77~149) H=4.500 0.105 WBC(109/L) 6.63(3.89~8.88) 7.16(4.93~9.16) 7.47(4.01~8.96) H=1.012 0.603 NE% 71.0(64.0~83.6) 73.4(63.8~81.5) 67.9(59.5~80.6) H=1.085 0.581 LY% 21.0(10.7~27.2) 20.0(12.5~28.5) 21.3(11.8~33.2) H=1.648 0.439 MO% 6.02±2.46 5.76±2.41 5.55±2.13 F=0.267 0.604 CRP(mg/L) 10.30(6.30~27.47) 22.80(12.67~33.52)1) 8.31(6.15~13.20)1)2) H=24.465 <0.001 ESR(mm/h) 22(15~36) 31(24~49)1) 22(17~30)2) H=8.917 0.012 IL-6(pg/ml) 7.61(4.64~14.88) 15.60(7.22~25.51)1) 4.28(2.81~7.06)1)2) H=37.584 <0.001 注:与PVBT组比较,1)P<0.05;与PVTT组比较,2)P<0.05。 -
[1] JANSSEN HL. Changing perspectives in portal vein thrombosis[J]. Scand J Gastroenterol Suppl, 2000, 232: 69-73. http://europepmc.org/abstract/med/11232496 [2] VIOLI F, CORAZZA GR, CALDWELL SH, et al. Portal vein thrombosis relevance on liver cirrhosis: Italian venous thrombotic events registry[J]. Intern Emerg Med, 2016, 11(8): 1059-1066. DOI: 10.1007/s11739-016-1416-8. [3] VILLA E, CAMMÀ C, MARIETTA M, et al. Enoxaparin prevents portal vein thrombosis and liver decompensation in patients with advanced cirrhosis[J]. Gastroenterology, 2012, 143(5): 1253-1260. e4. DOI: 10.1053/j.gastro.2012.07.018. [4] VIOLI F, FERRO D. Clotting activation and hyperfibrinolysis in cirrhosis: Implication for bleeding and thrombosis[J]. Semin Thromb Hemost, 2013, 39(4): 426-433. DOI: 10.1055/s-0033-1334144. [5] VIOLI F. Should the term coagulopathy in cirrhosis be abandoned?[J]. JAMA Intern Med, 2015, 175(5): 862-863. DOI: 10.1001/jamainternmed.2015.90. [6] WANG JT, ZHAO HY, LIU YL. Portal vein thrombosis[J]. Hepatobiliary Pancreat Dis Int, 2005, 4(4): 515-518. [7] ZHANG ZM, LAI EC, ZHANG C, et al. The strategies for treating primary hepatocellular carcinoma with portal vein tumor thrombus[J]. Int J Surg, 2015, 20: 8-16. DOI: 10.1016/j.ijsu.2015.05.009. [8] AKKIZ H, CARR BI, BAG HG, et al. Serum levels of inflammatory markers CRP, ESR and albumin in relation to survival for patients with hepatocellular carcinoma[J]. Int J Clin Pract, 2021, 75(2): e13593. DOI: 10.1111/ijcp.13593. [9] European Association for the Study of the Liver. EASL clinical practice guidelines: Management of hepatocellular carcinoma[J]. J Hepatol, 2018, 69(1): 182-236. DOI: 10.1016/j.jhep.2018.03.019. [10] Hepatobiliary Disease Study Group, Chinese Society of Gastroenterology, Chinese Medical Association. Consensus for management of portal vein thrombosis in liver cirrhosis(2020, Shanghai)[J]. J Clin Hepatol, 2020, 36(12): 2667-2674. DOI: 10.3969/j.issn.1001-5256.2020.12.007.中华医学会消化病学分会肝胆疾病学组. 肝硬化门静脉血栓管理专家共识(2020年, 上海)[J]. 临床肝胆病杂志, 2020, 36(12): 2667-2674. DOI: 10.3969/j.issn.1001-5256.2020.12.007. [11] NORTHUP PG, GARCIA-PAGAN JC, GARCIA-TSAO G, et al. Vascular liver disorders, portal vein thrombosis, and procedural bleeding in patients with liver disease: 2020 practice guidance by the American Association for the Study of Liver Diseases[J]. Hepatology, 2021, 73(1): 366-413. DOI: 10.1002/hep.31646. [12] LAURSEN I, BRIAND P, LYKKESFELDT AE. Serum albumin as a modulator on growth of the human breast cancer cell line, MCF-7[J]. Anticancer Res, 1990, 10(2A): 343-351. http://www.tandfonline.com/servlet/linkout?suffix=CIT0004&dbid=8&doi=10.1080%2F07357900701407947&key=2346307 [13] BAǦIRSAKÇI E, ŞAHIN E, ATABEY N, et al. Role of albumin in growth inhibition in hepatocellular carcinoma[J]. Oncology, 2017, 93(2): 136-142. DOI: 10.1159/000471807. [14] JOHNSON C, HAN Y, HUGHART N, et al. Interleukin-6 and its receptor, key players in hepatobiliary inflammation and cancer[J]. Transl Gastrointest Cancer, 2012, 1(1): 58-70. DOI: 10.3978/j.issn.2224-4778.2011.11.02. [15] JANG JW, OH BS, KWON JH, et al. Serum interleukin-6 and C-reactive protein as a prognostic indicator in hepatocellular carcinoma[J]. Cytokine, 2012, 60(3): 686-693. DOI: 10.1016/j.cyto.2012.07.017. [16] GRIVENNIKOV SI, GRETEN FR, KARIN M. Immunity, inflammation, and cancer[J]. Cell, 2010, 140(6): 883-899. DOI: 10.1016/j.cell.2010.01.025. [17] BUDHU A, WANG XW. The role of cytokines in hepatocellular carcinoma[J]. J Leukoc Biol, 2006, 80(6): 1197-1213. DOI: 10.1189/jlb.0506297. [18] KARIN M, GRETEN FR. NF-kappaB: Linking inflammation and immunity to cancer development and progression[J]. Nat Rev Immunol, 2005, 5(10): 749-759. DOI: 10.1038/nri1703. [19] HE G, KARIN M. NF-κB and STAT3-key players in liver inflammation and cancer[J]. Cell Res, 2011, 21(1): 159-168. DOI: 10.1038/cr.2010.183. [20] HUANG X, FAN X, ZHANG R, et al. Systemic inflammation and portal vein thrombosis in cirrhotic patients with gastroesophageal varices[J]. Eur J Gastroenterol Hepatol, 2020, 32(3): 401-405. DOI: 10.1097/MEG.0000000000001526. [21] CASTELL JV, GÓMEZ-LECHÓN MJ, DAVID M, et al. Acute-phase response of human hepatocytes: Regulation of acute-phase protein synthesis by interleukin-6[J]. Hepatology, 1990, 12(5): 1179-1186. DOI: 10.1002/hep.1840120517. [22] MCKEOWN DJ, BROWN DJ, KELLY A, et al. The relationship between circulating concentrations of C-reactive protein, inflammatory cytokines and cytokine receptors in patients with non-small-cell lung cancer[J]. Br J Cancer, 2004, 91(12): 1993-1995. DOI: 10.1038/sj.bjc.6602248. [23] GALLAND L. Diet and inflammation[J]. Nutr Clin Pract, 2010, 25(6): 634-640. DOI: 10.1177/0884533610385703. [24] BELL S, MEHTA G, MOORE K, et al. Ten-year alcohol consumption typologies and trajectories of C-reactive protein, interleukin-6 and interleukin-1 receptor antagonist over the following 12 years: A prospective cohort study[J]. J Intern Med, 2017, 281(1): 75-85. DOI: 10.1111/joim.12544. [25] CARR BI, AKKIZ H, GUERRA V, et al. C-reactive protein and hepatocellular carcinoma: Analysis of its relationships to tumor factors[J]. Clin Pract (Lond), 2018, 15(Spec Issue): 625-634. DOI: 10.4172/clinical-practice.1000409. [26] BATLIVALA SP. Focus on diagnosis: The erythrocyte sedimentation rate and the C-reactive protein test[J]. Pediatr Rev, 2009, 30(2): 72-74. DOI: 10.1542/pir.30-2-72. [27] PINATO DJ, NORTH BV, SHARMA R. A novel, externally validated inflammation-based prognostic algorithm in hepatocellular carcinoma: The prognostic nutritional index (PNI)[J]. Br J Cancer, 2012, 106(8): 1439-1445. DOI: 10.1038/bjc.2012.92. [28] LI X, CHEN ZH, XING YF, et al. Platelet-to-lymphocyte ratio acts as a prognostic factor for patients with advanced hepatocellular carcinoma[J]. Tumour Biol, 2015, 36(4): 2263-2269. DOI: 10.1007/s13277-014-2833-9. -




 PDF下载 ( 2273 KB)
PDF下载 ( 2273 KB)

 下载:
下载:

 下载:
下载:



