肝移植术后早期并发症风险预测模型的建立与评价
DOI: 10.3969/j.issn.1001-5256.2022.02.027
Establishment and validation of a risk prediction model for early-stage complications after liver transplantation
-
摘要:
目的 探讨影响肝移植受者术后早期并发症发生的危险因素,建立并验证肝移植术后早期并发症风险预测模型。 方法 回顾性收集2016年1月—2018年12月于天津市第一中心医院肝移植科接受原位肝移植234例患者的临床资料。根据肝移植术后是否发生Clavien-Dindo 3级以上并发症,将所有患者分为并发症组(n=97)和无并发症组(n=137),比较2组年龄、性别、BMI、血型、腰大肌厚度/身高值(PMTH)、控制营养状态(CONUT)评分、MELD评分、血清总胆红素、血肌酐、凝血酶原时间国际标准化比值(PT-INR)、血尿素氮、血红蛋白、白细胞计数、血小板计数、术中输注红细胞量、输注冰冻血浆量、失血量、无肝期、手术时间以及供体年龄、BMI、供肝冷缺血时间和供肝热缺血时间等指标。符合正态分布的计量资料2组间比较采用独立样本t检验,非正态分布的计量资料2组间比较采用Mann-Whitney U检验,计数资料2组间比较采用χ2检验。采用单因素分析和二元logistic回归分析法分析肝移植术后早期并发症发生的危险因素,根据Framingham研究中心提供的logistic模型建立积分系统的方法建立肝移植术后并发症风险预测模型。采用一致性指数、受试者工作特征(ROC)曲线、模型校准曲线、Hosmer-Lemeshow检验对模型进行内部验证;采用决策曲线评价模型临床实用性。采用Kaplan-Meier法比较不同风险评分组患者肝移植术后早期并发症发生率。 结果 并发症组患者MELD评分、低PMTH比例、血清总胆红素、血肌酐、血尿素氮、CONUT评分、术中输注红细胞量、术中输注冰冻血浆量均明显高于无并发症组,血红蛋白水平明显低于无并发症组(P值均<0.1)。二元logistc多因素分析结果显示,MELD评分、PMTH、CONUT评分是肝移植术后早期3级以上并发症发生的独立危险因素(OR分别为1.104、2.858、1.481,95%CI分别为1.057~1.154、1.451~5.626、1.287~1.703,P值均<0.05)。将MELD评分、PMTH、CONUT评分纳入预测模型,该预测模型最高分为24分,一致性指数为0.828,ROC曲线下面积为0.812,P<0.001,敏感度为0.792,特异度为0.751,表明该预测模型具有良好的区分度;该预测模型校正曲线接近参考曲线,Hosmer-Lemeshow检验表明该预测模型具有良好的拟合度(χ2=8.525,P=0.382);决策曲线显示大部分患者均能从预测模型中获益,且净获益率较高,表明该预测模型具有良好的临床实用性。根据最佳约登指数0.507,选择11分为截点值,将所有患者分为低风险组(<8分,n=55)、中风险组(8~10分,n=63)、高风险组(11~14分,n=67)、极高风险组(≥15分,n=49),4组术后90 d累积并发症发生率分别为3.6%、28.6%、59.7%、75.5%,并发症发生率随着风险评分的上升而递增(P<0.001)。 结论 MELD评分、PMTH、CONUT评分是肝移植术后早期3级以上并发症发生的独立危险因素,以此建立的风险预测模型对高风险患者具有较高的预测价值。 Abstract:Objective To investigate the risk factors for early-stage complications among liver transplant recipients, and to establish and validate a risk prediction model for early-stage complications after transplantation. Methods A retrospective analysis was performed for the clinical data of 234 patients who underwent orthotopic liver transplantation in Department of Liver Transplantation, Tianjin First Central Hospital, from January 2016 to December 2018. According to the presence or absence of Clavien-Dindo grade ≥Ⅲ complications after liver transplantation, the patients were divided into complication group with 97 patients and non-complication group with 137 patients. The two groups were compared in terms of the indices including age, sex, body mass index (BMI), blood type, psoas muscle thickness/height (PMTH), Controlling Nutritional Status (CONUT) score, Model for End-Stage Liver Disease (MELD) score, total serum bilirubin, serum creatinine, international normalized ratio of prothrombin time, blood urea nitrogen, hemoglobin, white blood cell count, platelet count, amount of intraoperative red blood cell transfusion, amount of frozen plasma transfusion, blood loss, anhepatic phase, time of operation, donor age, donor BMI, cold ischemia time of donor liver, and warm ischemia time of donor liver. The independent samples t-test was used for comparison of normally distributed continuous data between two groups, and the Mann-Whitney U test was used for comparison of non-normally distributed continuous data between two groups; the chi-square test was used for comparison of categorical data between two groups. Univariate analysis and the binary logistic regression analysis were used to investigate the risk factors for early-stage complications after liver transplantation, and a risk prediction model for complications after liver transplantation was established based on the method for establishing a scoring system using the logistic model provided by Framingham Research Center. Internal validation of the model was performed by C-index, receiver operating characteristic (ROC) curve, calibration curve, and the Hosmer-Lemeshow test, and the decision curve was used to evaluate the clinical applicability of the model. The Kaplan-Meier method was used to compare the incidence rate of early-stage complications after liver transplantation between the patients with different risk scores. Results Compared with the non-complication group, the complication group had significantly higher MELD score, proportion of patients with low PMTH, total serum bilirubin, serum creatinine, blood urea nitrogen, CONUT score, amount of intraoperative red blood cell transfusion, and amount of frozen plasma transfusion, as well as a significantly lower level of hemoglobin (all P < 0.1). The multivariate binary logistic regression analysis showed that MELD score (odds ratio [OR]=1.104, 95% confidence interval [CI]: 1.057-1.154, P < 0.05), PMTH (OR=2.858, 95%CI: 1.451-5.626, P < 0.05), and CONUT score (OR=1.481, 95%CI: 1.287-1.703, P < 0.05) were independent risk factors for grade ≥Ⅲ complications in the early stage after liver transplantation. MELD score, PMTH, and CONUT score were included in a predictive model, and this model had the highest score of 24 points, a C-index of 0.828, an area under the ROC curve of 0.812(P < 0.001), a sensitivity of 0.792, and a specificity of 0.751, suggesting that this predictive model had good discriminatory ability. The calibration curve of this model was close to the reference curve, and the Hosmer-Lemeshow test obtained a chi-square value of 8.528(P=0.382), suggesting that this predictive model had a high degree of fitting. The decision curve showed that most patients were able to benefit from the predictive model and achieved a high net benefit rate, suggesting that this predictive model had good clinical applicability. The score of 11 was selected as the cut-off value according to the optimal Youden index of 0.507, and the patients were divided into low-risk (< 8 points) group with 55 patients, moderate-risk (8-10 points) group with 63 patients, high-risk (11-14 points) group with 67 patients, and extremely high-risk (≥15 points) group with 49 patients. These four groups had a 90-day cumulative incidence rate of early-stage postoperative complications of 3.6%, 28.6%, 59.7%, and 75.5%, respectively, and the incidence rate of complications increased with the increase in risk score (P < 0.001). Conclusion MELD score, PMTH, and CONUT score are independent risk factors for early-stage complications among liver transplant recipients, and the risk prediction model established based on these factors has a high predictive value in high-risk patients. -
Key words:
- Liver Transplantation /
- Spostoperative Complications /
- Risk Factors /
- Forecasting /
- Models, Statistical
-
肝移植是治疗终末期肝病的最终手段,成功的肝移植将延长患者生存期。但由于肝移植受者术前普遍基础条件差,且手术过程复杂,导致术后并发症复杂多样,从而影响术后效果。目前,国内鲜有直接预测肝移植受者术后早期并发症发生的相关研究。研究[1-3]发现,肌肉减少症与肝移植术后并发症相关,腰大肌厚度/身高值(psoas muscle thickness per height,PMTH)可以通过脐平面的腰大肌横向厚度与患者的身高比值来诊断肌肉减少症,其计算方法简便,可用于预测肝移植等待名单中的患者死亡率[4]。控制营养状态(controlling nutritional status,CONUT)评分是一种评估患者术前营养状态的工具,其由血清白蛋白、血清总胆固醇、总淋巴细胞计数计算得出[5]。研究[6-8]表明,CONUT评分与原发性肝癌(以下简称为肝癌)肝切除患者的预后相关。本文旨在通过肝移植受者术前PMTH、CONUT评分结合术前、术中相关危险因素建立肝移植术后早期并发症发生的风险预测模型,用于指导肝移植围手术期治疗。
1. 资料与方法
1.1 研究对象
回顾性收集2016年1月—2018年12月于天津市第一中心医院肝移植科接受原位肝移植患者的临床资料。纳入标准:(1)受者年龄18~65岁;(2)供者为公民逝世后器官捐献;(3)肝癌肝移植受者符合米兰标准;(4) 受者术前2个月内行腹部CT检查;(5)受者术前1周内行血清总胆固醇检查。排除标准:(1)2次、多次或者挽救性肝移植受者;(2)术前合并严重肺炎受者;(3)术前合并心脑血管疾病受者;(4)边缘供肝(包括高龄供肝、脂肪肝供肝、劈离式肝移植供肝、病毒性肝炎供肝、高钠血症供肝、血流动力学不稳定,以及术前存在潜在供者来源性感染供肝等);(5)联合其他器官移植受者。
1.2 分组标准
按照Clavien-Dindo分级[9]将肝移植术后并发症分为5级,仅统计3级及以上并发症。3级并发症为需要行手术、内镜或介入干预的并发症,包括血管并发症(如门静脉狭窄、门静脉血栓、肝动脉血栓、脾肾分流、脾动脉盗血综合征等)、胆道并发症(如胆漏、胆汁引流不畅、胆管狭窄)以及其他并发症(如需要行穿刺的胸腹腔积液、腹腔出血,需要放置微导管引流的T管出口漏、鼻肠管置入等);4级并发症以单一器官或多器官衰竭为主,包括呼吸衰竭、心力衰竭、肝衰竭、肾衰竭并需要予以气管切开、体外肺膜氧合、人工肝、透析等治疗;5级并发症包括因感染性或失血性休克、重症肺炎、心力衰竭、肝衰竭、肾衰竭、颅内出血等原因导致受者死亡。随访受者术后90 d内并发症发生情况,根据是否发生3级以上并发症分为并发症组和无并发症组。
1.3 相关变量的测定与计算
1.3.1 PMTH测定与二分类变量划分
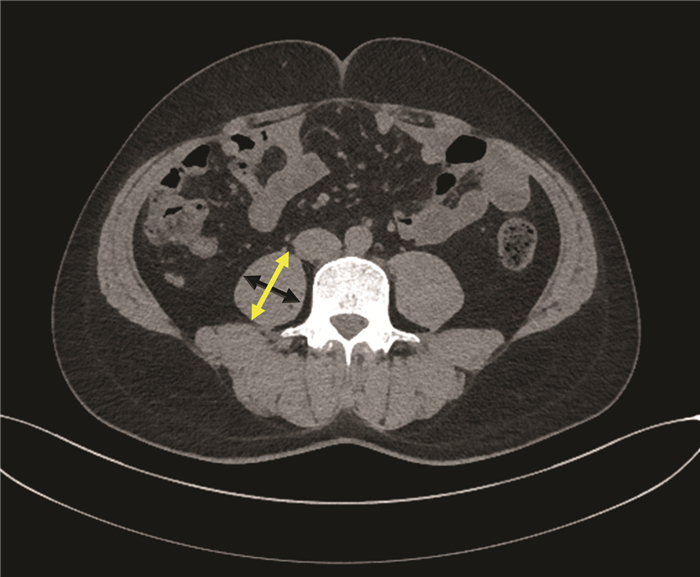
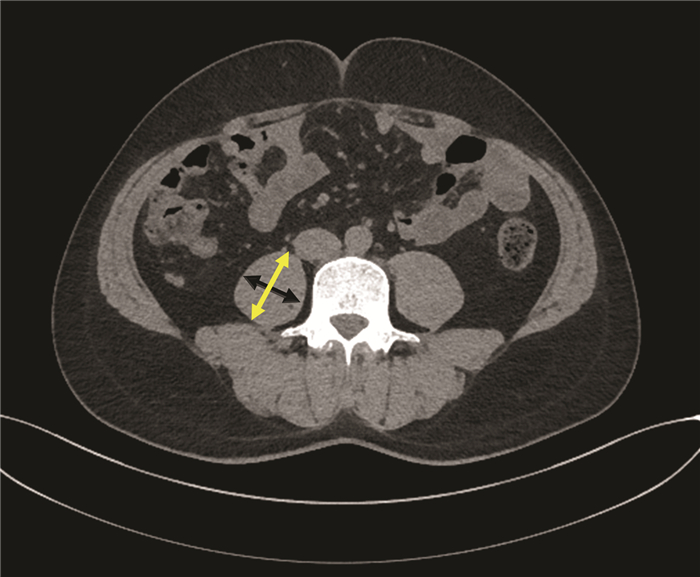
肝移植受者术前2个月内,采用美国GE Revolution 64排CT仪行腹部CT平扫或者增强CT,在CT图像上手动测量脐平面右侧腰大肌轴向和横向厚度。轴向腰大肌厚度为腰大肌在轴向视图上的最大直径,横向腰大肌厚度为在横向视图上垂直于轴向的直径(图 1),横向腰大肌厚度/患者身高=PMTH。PMTH与性别相关,且低PMTH与肝硬化患者的病死率相关[4]。根据男性患者术后90 d内死亡情况绘制受试者工作特征(ROC)曲线,曲线下面积(AUC)为0.699(P=0.003),95%CI: 0.625~0.766,敏感度为0.692,特异度为0.701,根据最佳约登指数0.394,选取截点值为17.9cm/m2,以PMTH≥17.9 cm/m2为高PMTH,以PMTH<17.9 cm/m2为低PMTH。根据女性患者术后90 d内死亡情况绘制ROC曲线,AUC为0.750(P=0.012),95%CI: 0.621~0.853,敏感度为0.833,特异度为0.778,根据最佳约登指数0.611 1,选取截点值为14.1 cm/m2,以PMTH≥14.1 cm/m2为高PMTH,以PMTH<14.1 cm/m2为低PMTH。因此,通过截点值将连续变量PMTH划分为二分类变量。
1.3.2 CONUT评分
采用受者术前最近一次血清白蛋白、血清总胆固醇、总淋巴细胞数检测结果计算CONUT评分,计算方法:血清白蛋白≥35.0 g/L计0分,30.0~34.9 g/L计2分,25.0~29.9 g/L计4分,<25.0 g/L计6分;血清总胆固醇≥180 mg/dL计0分,140~179 mg/dL计1分,100~139 mg/dL计2分,<100 mg/dL计3分;总淋巴细胞计数≥1.60×109/L计0分,1.20~1.59×109/L计1分,0.80~1.19×109/L计2分,<0.80×109/L计3分。
1.3.3 其他相关指标
收集受者年龄、性别、体质量指数(BMI)、血型、MELD评分[MELD评分=9.57×ln血肌酐(mg/dL)+3.78×ln血清总胆红素(mg/dL)+11.2×ln国际标准化比值+6.4×病因(胆汁淤积性和酒精性肝硬化为0,其他为1)]、血清总胆红素、血肌酐、凝血酶原时间国际标准化比值(PT-INR)、血尿素氮、血红蛋白、白细胞计数、血小板计数、术中输注红细胞量、输注冰冻血浆量、失血量、无肝期、手术时间以及供体年龄、BMI、供肝冷缺血时间(cold ischemia time,CIT)和供肝热缺血时间(warm ischemia time,WIT)等资料。
1.4 伦理学审查
本研究方案经由天津市第一中心医院伦理委员会审批,批号:2019N179KY,所纳入患者均签署知情同意书。
1.5 统计学方法
采用SPSS 20.0软件进行统计学分析。数据正态性检验采用Shapiro-Wilk检验。正态分布的计量资料以x±s表示,2组间比较采用独立样本t检验。非正态分布的计量资料以M(P25~P75)表示,2组间比较采用Mann-Whitney U检验。计数资料2组间比较采用χ2检验或Fisher确切概率法。采用AUC以及最佳约登指数选取PMTH的截点值。单因素分析时,以P<0.1为差异有统计学意义,将其纳入二元logistic回归,采用逐步法进行分析。采用bootstrap法(抽样1000次)进行内部验证,采用一致性指数、AUC检验模型的区分度,校正曲线及Hosmer-Lemeshow检验显示模型的拟合度,临床决策曲线检验模型的临床实用性。并发症发生率比较采用Kaplan-Meier法,以P<0.05为差异有统计学意义。
2. 结果
2.1 一般资料
共纳入肝移植受者234例,其中并发症组97例,无并发症组137例。并发症组中,男73例,女24例,年龄(50±9)岁,原发病包括乙型肝炎肝硬化24例,自身免疫性肝硬化8例,酒精性肝硬化14例,非酒精性脂肪性肝病2例,隐源性肝硬化1例,肝癌合并肝硬化25例(合并乙型或丙型肝炎),肝衰竭20例,其他3例;无并发症组中,男101例,女36例,年龄(49±10)岁,原发病包括乙型肝炎肝硬化43例,丙型肝炎肝硬化2例,自身免疫性肝硬化18例,酒精性肝硬化10例,非酒精性脂肪性肝病4例,隐源性肝硬化2例,肝癌合并肝硬化36例(合并乙型或丙型肝炎),肝衰竭19例,其他3例。两组原发病分布差异比较,差异无统计学意义(P=0.403)。根据PMTH测定结果,男性患者中高PMTH 117例,低PMTH 57例;女性患者中高PMTH 43例,低PMTH 17例。
2.2 建立预测模型
2.2.1 单因素分析
并发症组患者MELD评分、低PMTH比例、血清总胆红素、血肌酐、血尿素氮、CONUT评分、术中输注红细胞量、术中输注冰冻血浆量均明显高于无并发症组,血红蛋白水平明显低于无并发症组(P值均<0.1)。其他差异均无统计学意义(P值均>0.1)(表 1)。
表 1 肝移植术后早期并发症危险因素单因素分析指标 并发症组(n=97) 无并发症组(n=137) 统计值 P值 受者信息 年龄(岁) 50±9 49±10 t=0.780 0.354 性别[例(%)] χ2=0.070 0.791 男 73(75) 101(74) 女 24(25) 36(26) BMI (kg/m2) 24.0(21.7~27.0) 23.5(21.2~26.0) Z=-0.944 0.345 血型不相容[例(%)] 5(5.2) 8(5.8) χ2=0.051 1.000 MELD评分 20(16~24) 13(10~20) Z=5.499 <0.001 PMTH[例(%)] χ2=14.381 <0.001 低 44(45) 30(22) 高 53(55) 107(78) 血清总胆红素(μmol/L) 123.0(72.1~379.2) 29.9(18.0~115.6) Z=6.792 <0.001 血肌酐(μmol/L) 68.0(51.0~118.5) 61.0(50.0~69.5) Z=2.371 0.018 PT-INR 1.50(1.18~2.03) 1.45(1.19~1.97) Z=0.228 0.820 血尿素氮(mmol/L) 5.3(3.7~11.8) 4.7(3.5~5.8) Z=2.560 0.010 血红蛋白(g/L) 100±25 107±29 t=-2.097 0.044 白细胞计数(×109/L) 4.9(3.4~6.8) 4.6(3.1~6.8) Z=0.985 0.324 血小板计数(×109/L) 93(47~152) 117(54~170) Z=-1.441 0.150 ConUT评分(分) 7(6~8) 5(3~7) Z=6.308 0.004 手术情况 术中输注红细胞量(U) 10(8~12) 8(6~10) Z=2.456 0.014 术中输注冰冻血浆量(mL) 2000(1600~2350) 2000(1400~2000) Z=2.171 0.030 术中失血量(mL) 2000(1500~2450) 1800(1500~2400) Z=1.365 0.172 无肝期(min) 45(35~50) 45(40~50) Z=-0.051 0.959 手术时间(h) 8.0±1.6 7.8±1.5 t=1.144 0.200 供者信息 年龄(岁) 41±9 42±10 t=-0.904 0.939 BMI(kg/m2) 24.1(21.4~27.4) 23.8(22.1~26.4) Z=-0.255 0.799 CIT(h) 7.3(6.4~7.9) 6.8(6.2~7.8) Z=1.138 0.255 WIT(min) 5.8±1.7 5.7±1.5 t=0.237 0.182 2.2.2 预测模型的推导与建立
二元logistc多因素分析(采用逐步法分析,由于有报道[10]称CIT、WIT为肝移植术后胆道并发症的危险因素,遂将二者均纳入)结果显示,MELD评分、低PMTH比例、CONUT评分为肝移植术后3级及以上并发症发生的独立危险因素(P值均<0.05) (表 2)。笔者根据Framingham研究中心提供的logistic模型建立积分系统的方法[11],以0.393为常量,将此常量设定为1分,设定变量CONUT评分截距为1,基于MELD评分,PMTH、血清白蛋白、血清总胆固醇以及总淋巴细胞计数建立了一个简单的肝移植术后并发症风险预测模型(表 3),该预测模型最高分为24分。
表 2 肝移植术后早期并发症危险因素多因素分析危险因素 β值 P值 OR 95%CI MELD评分 0.099 0.002 1.104 1.057~1.154 PMTH 1.050 <0.001 2.858 1.451~5.626 ConUT评分 0.393 <0.001 1.481 1.287~1.703 表 3 肝移植术后早期并发症风险预测模型危险因素 得分 MELD评分 <10分 0 10~19分 3 20~29分 6 ≥30分 9 PMTH 高 0 低 3 血清白蛋白 ≥35.0 g/L 0 30.0~34.9 g/L 2 25.0~29.9 g/L 4 <25.0 g/L 6 血清总胆固醇 ≥180 g/L 0 140~179 g/L 1 100~139 g/L 2 <100 g/L 3 总淋巴细胞计数 ≥1.60×109/L 0 1.20×109/L~1.59 ×109/L 1 0.80×109/L~1.19×109/L 2 <0.8×109/L 3 2.3 预测模型的内部验证与评价
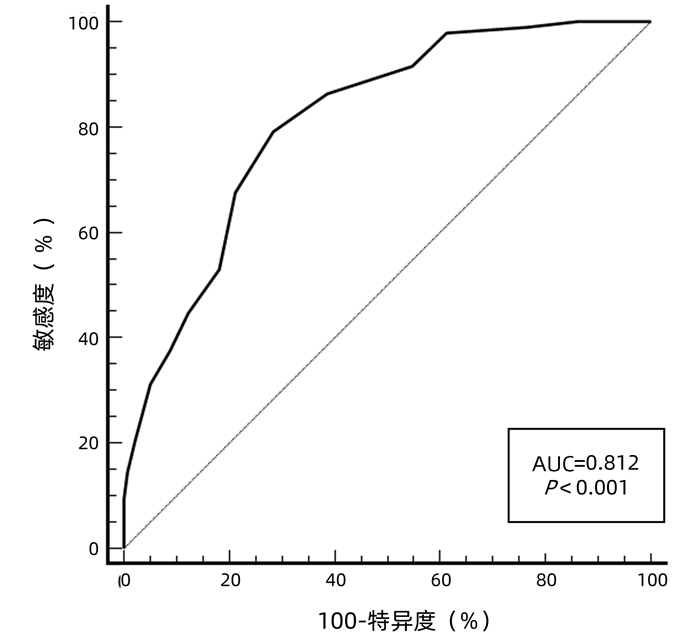
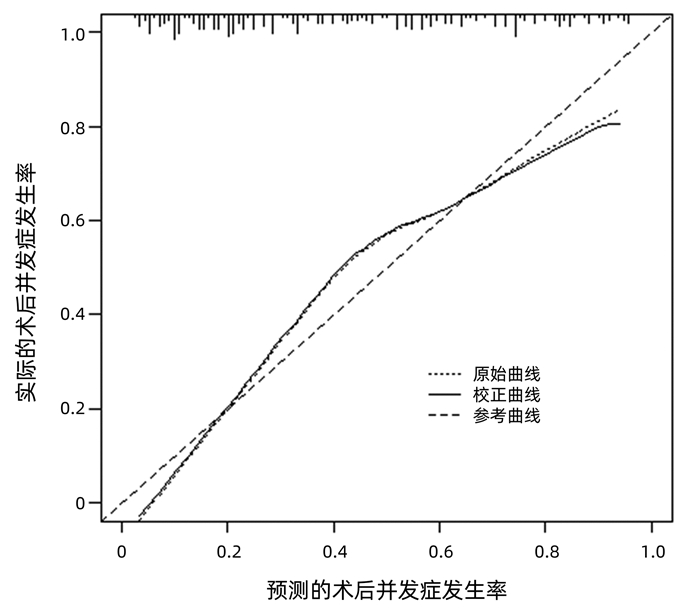
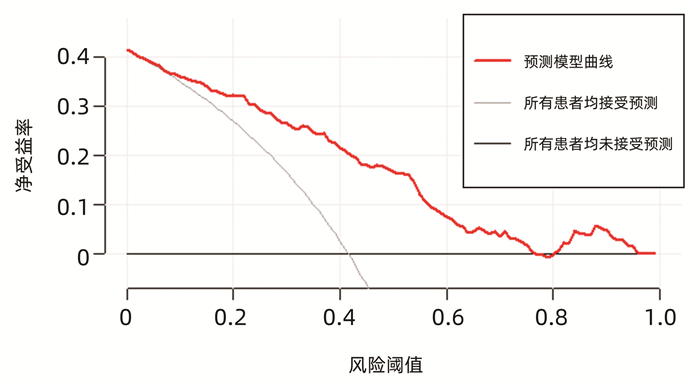
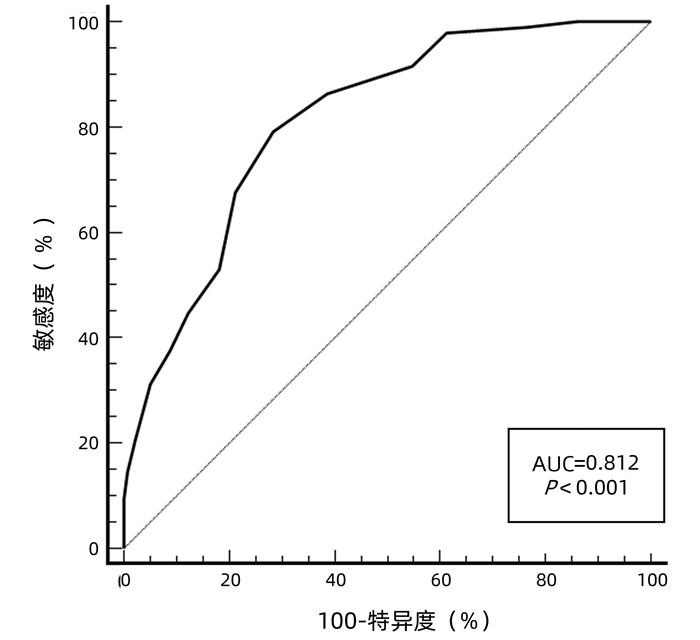
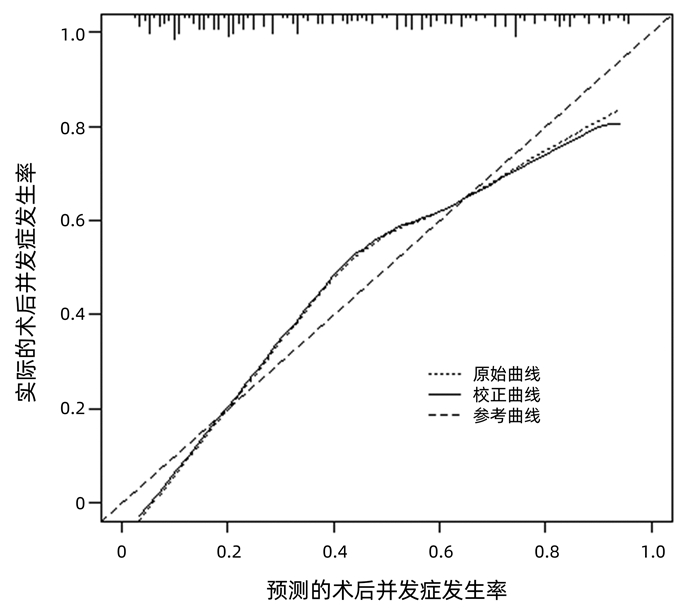
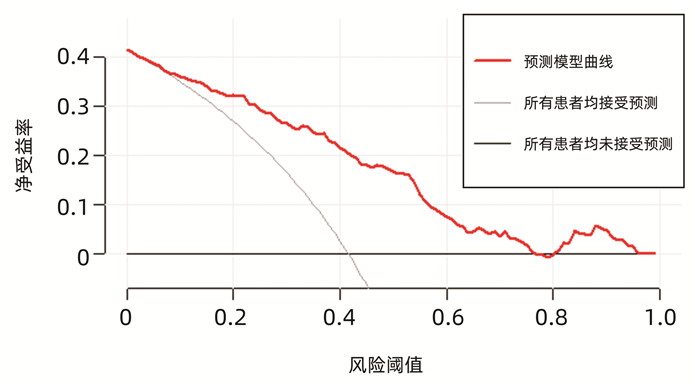
运用bootstrap法(抽样1000次)对该预测模型进行内部验证,其一致性指数为0.828,95%CI: 0.826~0.829。AUC=0.812,P<0.001(图 2),95%CI: 0.756~0.860,敏感度为0.792,特异度为0.751,最佳约登指数为0.507,表明该预测模型具有良好的区分度。该预测模型校正曲线接近参考曲线(图 3),Hosmer-Lemeshow检验表明该预测模型具有良好的拟合度(χ2=8.525,P=0.382)。决策曲线显示大部分患者均能从预测模型中获益,且净获益率较高(图 4),表明该预测模型具有良好的临床实用性。
2.4 风险评分分组及并发症发生率
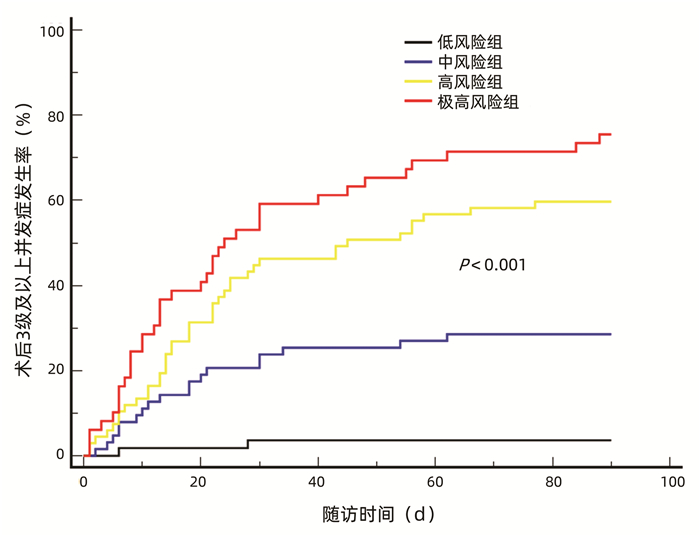
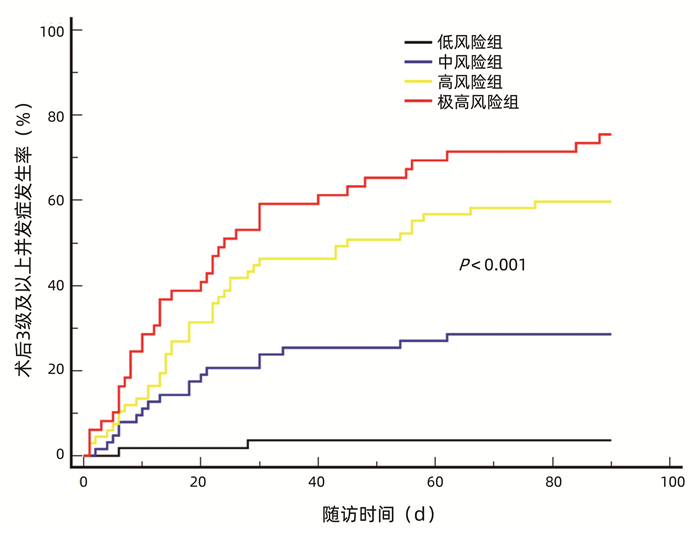
根据最佳约登指数,选择11分为截点值,并根据受者术后实际并发症发生情况,将风险评分进一步划分为低风险组(<8分,n=55),中风险组(8~10分,n=63)、高风险组(11~14分,n=67)、极高风险组(≥15分,n=49)。根据4组3级以上并发症发生率分别制作Kaplan-Meier曲线,结果显示,术后90 d,低风险组、中风险组、高风险组、极高风险组累积并发症发生率分别为3.6%、28.6%、59.7%、75.5%,并发症发生率随着风险评分的上升而递增(P<0.001)(图 5)。
3. 讨论
近年来,肝移植技术不断完善发展,但对术后各类并发症的控制仍不理想,严重影响受者和移植肝的长期存活[12]。据报道[13-14],营养状态是影响肝移植受者预后的因素之一。目前,临床常用的营养状态评估工具主要包括BMI、营养风险筛查2002(nutrition risk screening 2002,NRS2002)、皇家自由医院-营养优先排序工具(Royal Free Hospital-nutritional prioritizing tool,RFH-NPT)、上臂围、肌量和肌肉功能测定等[13, 15-16]。由于部分终末期肝病患者存在大量腹腔积液,难以精确地测量其体质量,并且部分患者由于长期卧床,缺少术前体质量数据,因此,使用BMI对肝移植术前患者进行营养评估缺乏准确性。而NRS2002和RFH-NPT均需要评估一段时间内患者体质量和进食量变化[17-18],实际统计时往往掺杂患者的主观因素,且对于依从性较差的患者往往难以获取相关数据;此外,部分患者因为病情严重,短期内亟需行肝移植术,难以完成长期检测,影响了NRS2002、RFH-NPT的临床应用。上臂围是非常客观的营养评估指标,其不受液体潴留以及周围水肿的影响,操作方法简单、易行,有学者[19-20]推荐其作为慢性肝病的筛查指标,但其并不是我国肝移植患者的基本检测项目,且因测量者不同,测量结果可能存在一定误差。测量全身的骨骼肌含量是诊断肌肉减少症的金标准,但是测量工作复杂、繁琐,需要通过测量第3腰椎水平面的骨骼肌指数(skeletal muscle index,SMI)并依赖相关软件实现对骨骼肌含量的计算[21-23]。
笔者团队在设计建立肝移植术后早期并发症风险预测模型前,全面分析了数种营养相关指标,并结合临床实际,最终选定纳入CONUT评分、PMTH、血清白蛋白、总淋巴细胞计数、血清总胆固醇、MELD评分作为参数。CONUT评分的计算需要的3个指标(血清白蛋白、血清总胆固醇、总淋巴细胞计数)均与临床紧密相关,且为患者定期检查项目,容易及时获得数据,计算方法简单。而相比于需要计算第3腰椎水平面的SMI,PMTH仅需要在CT图像上测量右侧腰大肌的横向厚度,其过程方便、简洁。研究[24]表明,在肝硬化患者中,PMTH与第3腰椎水平面的SMI高度相关,是肝硬化患者死亡的独立危险因素。腰大肌的横向厚度与性别、年龄、人种相关,因此,笔者根据本研究数据确定出截点值,即男性患者PMTH<17.9 cm/m2诊断为低PMTH,女性患者PMTH<14.1 cm/m2诊断为低PMTH。本研究结果显示,低PMTH受者术后3级以上并发症发生风险明显增加,究其原因可能是由于骨骼肌含量减少导致氨基酸存储降低,从而不能为细胞修复提供足量的氨基酸,进而导致血管、胆道相关并发症以及伤口不愈合[25];再者,骨骼肌可以释放谷氨酰胺激活淋巴细胞,从而提高机体免疫力[26-28],而骨骼肌减少使受者免疫力下降,同时,受者呼吸肌肌肉含量减少,一定程度上引起术后咳痰能力下降,导致肺相关并发症发生[29]。而受者白蛋白、总淋巴细胞计数水平降低将导致机体免疫力降低,使受者术后易发肺炎、伤口感染等并发症。在终末期肝病患者中,由于肝功能受损,导致肝细胞合成胆固醇能力下降,而胆固醇是细胞膜的组成成分,发挥着维持细胞结构和功能的作用,胆固醇的减少可能会使细胞修复功能受损,导致术后相关并发症[30]。另据报道[10],较长的供肝冷、热缺血时间是术后胆道并发症发生的危险因素。然而,本研究中未见其与术后早期3级以上并发症的发生存在相关性,可能是由于本中心供肝的冷、热缺血时间较短,一直处于安全的时间范围内[31]。
本研究的局限性包括:(1)纳入的女性患者较少,在选取女性患者PMTH截点值时可能存在一定程度上的偏倚,影响结果的准确性;(2)受者PMTH值为术前2个月内,血清总胆固醇值为术前1周内,部分病情较重患者的PMTH以及血清总胆固醇会随着病情变化而改变,在一定程度上影响模型的准确性;(3)该预测模型仅完成内部验证,尚未开展外部验证,笔者希望今后可以联合各个移植中心,扩大样本量,进一步开展临床可行性验证。
综上所述,术前MELD评分、PMTH和CONUT评分是肝移植术后3级以上并发症发生的独立危险因素,本研究纳入MELD评分、PMTH、血清白蛋白、血清总胆固醇、总淋巴细胞计数作为参数,建立并验证了我国18~65岁人群中初次行原位肝移植的患者术后90 d内并发症发生的风险预测模型,该预测模型能够有效识别高风险患者,进而指导临床医师加强对高风险患者围手术期的管理。
-
表 1 肝移植术后早期并发症危险因素单因素分析
指标 并发症组(n=97) 无并发症组(n=137) 统计值 P值 受者信息 年龄(岁) 50±9 49±10 t=0.780 0.354 性别[例(%)] χ2=0.070 0.791 男 73(75) 101(74) 女 24(25) 36(26) BMI (kg/m2) 24.0(21.7~27.0) 23.5(21.2~26.0) Z=-0.944 0.345 血型不相容[例(%)] 5(5.2) 8(5.8) χ2=0.051 1.000 MELD评分 20(16~24) 13(10~20) Z=5.499 <0.001 PMTH[例(%)] χ2=14.381 <0.001 低 44(45) 30(22) 高 53(55) 107(78) 血清总胆红素(μmol/L) 123.0(72.1~379.2) 29.9(18.0~115.6) Z=6.792 <0.001 血肌酐(μmol/L) 68.0(51.0~118.5) 61.0(50.0~69.5) Z=2.371 0.018 PT-INR 1.50(1.18~2.03) 1.45(1.19~1.97) Z=0.228 0.820 血尿素氮(mmol/L) 5.3(3.7~11.8) 4.7(3.5~5.8) Z=2.560 0.010 血红蛋白(g/L) 100±25 107±29 t=-2.097 0.044 白细胞计数(×109/L) 4.9(3.4~6.8) 4.6(3.1~6.8) Z=0.985 0.324 血小板计数(×109/L) 93(47~152) 117(54~170) Z=-1.441 0.150 ConUT评分(分) 7(6~8) 5(3~7) Z=6.308 0.004 手术情况 术中输注红细胞量(U) 10(8~12) 8(6~10) Z=2.456 0.014 术中输注冰冻血浆量(mL) 2000(1600~2350) 2000(1400~2000) Z=2.171 0.030 术中失血量(mL) 2000(1500~2450) 1800(1500~2400) Z=1.365 0.172 无肝期(min) 45(35~50) 45(40~50) Z=-0.051 0.959 手术时间(h) 8.0±1.6 7.8±1.5 t=1.144 0.200 供者信息 年龄(岁) 41±9 42±10 t=-0.904 0.939 BMI(kg/m2) 24.1(21.4~27.4) 23.8(22.1~26.4) Z=-0.255 0.799 CIT(h) 7.3(6.4~7.9) 6.8(6.2~7.8) Z=1.138 0.255 WIT(min) 5.8±1.7 5.7±1.5 t=0.237 0.182 表 2 肝移植术后早期并发症危险因素多因素分析
危险因素 β值 P值 OR 95%CI MELD评分 0.099 0.002 1.104 1.057~1.154 PMTH 1.050 <0.001 2.858 1.451~5.626 ConUT评分 0.393 <0.001 1.481 1.287~1.703 表 3 肝移植术后早期并发症风险预测模型
危险因素 得分 MELD评分 <10分 0 10~19分 3 20~29分 6 ≥30分 9 PMTH 高 0 低 3 血清白蛋白 ≥35.0 g/L 0 30.0~34.9 g/L 2 25.0~29.9 g/L 4 <25.0 g/L 6 血清总胆固醇 ≥180 g/L 0 140~179 g/L 1 100~139 g/L 2 <100 g/L 3 总淋巴细胞计数 ≥1.60×109/L 0 1.20×109/L~1.59 ×109/L 1 0.80×109/L~1.19×109/L 2 <0.8×109/L 3 -
[1] HOU JC, ZHENG H, QIANG Z, et al. Impact of psoas muscle index on early postoperative mortality and complications after liver transplantation[J]. Chin J Surg, 2018, 56(5): 374-378. DOI: 10.3760/cma.j.issn.0529-5815.2018.05.010.侯建存, 郑虹, 强喆, 等. 腰大肌指数对肝移植早期预后及并发症的影响[J]. 中华外科杂志, 2018, 56(5): 374-378. DOI: 10.3760/cma.j.issn.0529-5815.2018.05.010. [2] SAIMAN Y, SERPER M. Frailty and sarcopenia in patients pre- and post-liver transplant[J]. Clin Liver Dis, 2021, 25(1): 35-51. DOI: 10.1016/j.cld.2020.08.004. [3] LEE J, JEONG WK, KIM JH, et al. Serial Observations of muscle and fat mass as prognostic factors for deceased donor liver transplantation[J]. Korean J Radiol, 2021, 22(2): 189-197. DOI: 10.3348/kjr.2019.0750. [4] HUGUET A, LATOURNERIE M, DEBRY PH, et al. The psoas muscle transversal diameter predicts mortality in patients with cirrhosis on a waiting list for liver transplantation: A retrospective cohort study[J]. Nutrition, 2018, 51-52: 73-79. DOI: 10.1016/j.nut.2018.01.008. [5] IGNACIO de ULÍBARRI J, GONZÁLEZ-MADROÑO A, de VILLAR NG, et al. CONUT: A tool for controlling nutritional status. First validation in a hospital population[J]. Nutr Hosp, 2005, 20(1): 38-45. [6] KURODA D, SAWAYAMA H, KURASHIGE J, et al. Controlling Nutritional Status (CONUT) score is a prognostic marker for gastric cancer patients after curative resection[J]. Gastric Cancer, 2018, 21(2): 204-212. DOI: 10.1007/s10120-017-0744-3. [7] PRAVISANI R, MOCCHEGIANI F, ISOLA M, et al. Controlling Nutritional Status score does not predict patients' overall survival or hepatocellular carcinoma recurrence after deceased donor liver transplantation[J]. Clin Transplant, 2020, 34(3): e13786. DOI: 10.1111/ctr.13786. [8] FUKAMI Y, SAITO T, OSAWA T, et al. Preoperative controlling nutritional status plus tumor burden score for the assessment of prognosis after curative liver resection for hepatocellular carcinoma[J]. Med Princ Pract, 2021, 30(2): 131-137. DOI: 10.1159/000514031. [9] DINDO D, DEMARTINES N, CLAVIEN PA. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey[J]. Ann Surg, 2004, 240(2): 205-213. DOI: 10.1097/01.sla.0000133083.54934.ae. [10] LYU GY. Risk factors for biliary complications after liver transplantation from donation after cardiac death[J]. J Clin Hepatol, 2015, 31(12): 2027-2030. DOI: 10.3969/j.issn.1001-5256.2015.12.009.吕国悦. 心脏死亡供肝受体肝移植术后胆道并发症的危险因素探讨[J]. 临床肝胆病杂志, 2015, 31(12): 2027-2030. DOI: 10.3969/j.issn.1001-5256.2015.12.009. [11] SULLIVAN LM, MASSARO JM, SR DRB. Presentation of multivariate data for clinical use: The Framingham Study risk score functions[J]. Stat Med, 2004, 23(10): 1631-1660. DOI: 10.1002/sim.1742. [12] Branch of Organ Transplantation of Chinese Medical Association. Diagnosis and treatment specification for postoperative complications after liver transplantation in China (2019 edition)[J]. Organ Transpl, 2021, 12(2): 129-133. DOI: 10.3969/j.issn.1674-7445.2021.02.002.中华医学会器官移植学分会. 中国肝移植术后并发症诊疗规范(2019版)[J]. 器官移植, 2021, 12(2): 129-133. DOI: 10.3969/j.issn.1674-7445.2021.02.002. [13] Chinese Society of Hepatology, Chinese Medical Association, Chinese Society of Gastroenterology, Chinese Medical Association. Clinical guidelines on nutrition in end-stage liver disease[J]. J Clin Hepatol, 2019, 35(6): 1222-1230. DOI: 10.3969/j.issn.1001-5256.2019.06.010.中华医学会肝病学分会, 中华医学会消化病学分会. 终末期肝病临床营养指南[J]. 临床肝胆病杂志, 2019, 35(6): 1222-1230. DOI: 10.3969/j.issn.1001-5256.2019.06.010. [14] DOIJ, MORO A, FUJIKI M, et al. Nutrition support in liver transplantation and postoperative recovery: The effects of vitamin D level and vitamin D supplementation in liver transplantation[J]. Nutrients, 2020, 12(12): 3677. DOI: 10.3390/nu12123677. [15] TABERNA DJ, NAVAS-CARRETERO S, MARTINEZ JA. Current nutritional status assessment tools for metabolic care and clinical nutrition[J]. Curr Opin Clin Nutr Metab Care, 2019, 22(5): 323-328. DOI: 10.1097/MCO.0000000000000581. [16] MARESCHAL J, ACHAMRAH N, NORMAN K, et al. Clinical value of muscle mass assessment in clinical conditions associated with malnutrition[J]. J Clin Med, 2019, 8(7): 1040. DOI: 10.3390/jcm8071040. [17] CAMPOS DEL PORTILLO R, PALMA MIILA S, GARCÍA VÁQUEZ N, et al. Assessment of nutritional status in the healthcare setting in Spain[J]. Nutr Hosp, 2015, 31(Suppl 3): 196-208. DOI: 10.3305/nh.2015.31.sup3.8767. [18] BORHOFEN SM, GERNER C, LEHMANN J, et al. The royal free hospital-nutritional prioritizing tool is an independent predictor of deterioration of liver function and survival in cirrhosis[J]. Dig Dis Sci, 2016, 61(6): 1735-1743. DOI: 10.1007/s10620-015-4015-z. [19] European Association for the Study of the Liver. EASL clinical practice guidelines on nutrition in chronic liver disease[J]. J Hepatol, 2019, 70(1): 172-193. DOI: 10.1016/j.jhep.2018.06.024. [20] ENDO K, SATO T, KAKISAKA K, et al. Calf and arm circumference as simple markers for screening sarcopenia in patients with chronic liver disease[J]. Hepatol Res, 2021, 51(2): 176-189. DOI: 10.1111/hepr.13589. [21] YAO J, ZHOU X, YUAN L, et al. Prognostic value of the third lumbar skeletal muscle mass index in patients with liver cirrhosis and ascites[J]. Clin Nutr, 2020, 39(6): 1908-1913. DOI: 10.1016/j.clnu.2019.08.006. [22] GADDUCCI A, COSIO S. The prognostic relevance of computed tomography-assessed skeletal muscle index and skeletal muscle radiation attenuation in patients with gynecological cancer[J]. Anticancer Res, 2021, 41(1): 9-20. DOI: 10.21873/anticanres.14747. [23] MURRAY TÉ, WILLIAMS D, LEE MJ. Osteoporosis, obesity, and sarcopenia on abdominal CT: A review of epidemiology, diagnostic criteria, and management strategies for the reporting radiologist[J]. Abdom Radiol (NY), 2017, 42(9): 2376-2386. DOI: 10.1007/s00261-017-1124-5. [24] GU DH, KIM MY, SEO YS, et al. Clinical usefulness of psoas muscle thickness for the diagnosis of sarcopenia in patients with liver cirrhosis[J]. Clin Mol Hepatol, 2018, 24(3): 319-330. DOI: 10.3350/cmh.2017.0077. [25] LIGHTFOOT A, MCARDLE A, GRIFFITHS RD. Muscle in defense[J]. Crit Care Med, 2009, 37(10 Suppl): S384-S390. DOI: 10.1097/CCM.0b013e3181b6f8a5. [26] NEWSHOLME P. Why is L-glutamine metabolism important to cells of the immune system in health, postinjury, surgery or infection?[J]. J Nutr, 2001, 131(9 Suppl): 2515S-2522S; discussion 2523S-2524S. DOI: 10.1093/jn/131.9.2515S. [27] ROGERI PS, GASPARINI SO, MARTINS GL, et al. Crosstalk between skeletal muscle and immune system: Which roles do IL-6 and glutamine play?[J]. Front Physiol, 2020, 11: 582258. DOI: 10.3389/fphys.2020.582258. [28] BATATINHA H, BIONDO LA, LIRA FS, et al. Nutrients, immune system, and exercise: Where will it take us?[J]. Nutrition, 2019, 61: 151-156. DOI: 10.1016/j.nut.2018.09.019. [29] OKAZAKI T, SUZUKAMO Y, MIYATAKE M, et al. Respiratory muscle weakness as a risk factor for pneumonia in older people[J]. Gerontology, 2021, 67(5): 581-590. DOI: 10.1159/000514007. [30] NARWAL V, DESWAL R, BATRA B, et al. Cholesterol biosensors: A review[J]. Steroids, 2019, 143: 6-17. DOI: 10.1016/j.steroids.2018.12.003. [31] HAN S, KIM G, LEE SK, et al. Comparison of the tolerance of hepatic ischemia/reperfusion injury in living donors: Macrosteatosis versus microsteatosis[J]. Liver Transpl, 2014, 20(7): 775-783. DOI: 10.1002/lt.23878. 期刊类型引用(4)
1. 李正优,李荷,周俊. 预见性护理结合舒适护理干预在肝移植术后患者中的应用. 西藏医药. 2024(06): 123-125 .  百度学术
百度学术2. 李珊珊,王媛,高春辉. 早期下床活动对肝移植术后患者胃肠功能及下肢血流的影响. 国际移植与血液净化杂志. 2023(01): 41-44 .  百度学术
百度学术3. 张欣悦,王海刚,王昊,杨传伟. 他克莫司治疗1例肝移植术后患者的药学监护. 中国医药导报. 2023(14): 183-187 .  百度学术
百度学术4. 种庚,彭晓春,吴若林,赵红川,邵敏. 成人肝移植术后早期严重并发症相关危险因素分析. 肝胆外科杂志. 2022(06): 454-459 .  百度学术
百度学术其他类型引用(0)
-




 PDF下载 ( 2853 KB)
PDF下载 ( 2853 KB)


 下载:
下载:




 下载:
下载:
 百度学术
百度学术